
Pirfenidone inhibits cell fibrosis in connective tissue disease-associated interstitial lung disease by targeting the TNF-α/STAT3/KL6 pathway
Introduction
Connective tissue disease (CTD) is an autoimmune disease involving multiple organs and systems that is characterized by damage to connective tissue. CTD may be involved in different tissues or organs and cause different degrees of damage. However, almost all involve the respiratory system. Lung interstitial damage secondary to CTD is called connective tissue disease-associated interstitial lung disease (CTD-ILD) (1). The main clinical manifestations of CTD-ILD patients are hypoxemia, restrictive ventilation dysfunction, and even life-threatening conditions (2). Furthermore, CTD patients are prone to lung infection or pneumonia due to long-term use of immunosuppressive therapy, such as glucocorticoids. Challenges remain in the diagnosis and treatment of CTD-ILD in clinical practice because of the lack of specificity of laboratory test indicators.
Pirfenidone (PF) is a new type of antifibrotic drug that has been broadly adopted for pulmonary fibrosis treatment in recent years. It can inhibit collagen synthesis by acting on tumor necrosis factor-α (TNF-α), thereby reducing the level of fibrosis and inhibiting the formation of scars (3). Tumor necrosis factor (TNF) is a cytokine that can directly kill tumor cells, and it is a key mediator of acute and chronic systemic inflammatory reactions and a core cytokine that regulates the inflammatory response of the immune system. A study has shown that the level of TNF-α is elevated in the alveolar lavage fluid of patients with CTD-ILD (4), indicating that TNF-α is key in CTD-ILD pathogenesis. Signal transducer and activator of transcription 3 (STAT3) is a member of the STAT protein family. It was originally identified as an acute phase response factor in the inflammatory response and can induce the transcription of target genes. STAT3 is also closely related to autoimmune diseases caused by autoimmune system disorders. As a transcription activator, STAT3 can regulate gene expression, cell growth and apoptosis (5). Uninterrupted activation of STAT3 can lead to high expression of genes closely related to cell differentiation, proliferation, and apoptosis, thus resulting in the occurrence of a variety of autoimmune diseases. STAT3 is a downstream molecule of TNF-α, affecting the process of pulmonary fibrosis in idiopathic pulmonary fibrosis (IPF). Meanwhile, Krebs von den Lungen-6 (KL-6) is a glycoprotein belonging to the MUC1 family of mucins, and its serum level is positively correlated with the degree of pulmonary fibrosis. Research by Solomon et al. demonstrated that serum KL-6 levels can be used as a molecular marker for CTD-ILD and has achieved good clinical results in Japan (6). Furthermore, studies have shown that serum KL-6 has high diagnostic sensitivity and specificity for the diagnosis of interstitial pneumonia with confirmed CTD (7); CTD-ILD patients who have been treated with pirfenidone have shown decreased levels of serum KL-6 and improved clinical indications (8,9). Based on this background, we hypothesize that pirfenidone administration might be an effective method for treating CTD-ILD patients. Furthermore, pirfenidone can inhibit cell fibrosis via the TNFα/STAT3/KL-6 pathway. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-41/rc).
Methods
Study design
Pirfenidone has a certain effect on improving pulmonary fibrosis in CTD-ILD patients, but no final conclusion on this is available. Therefore, this study subsequently validated and discussed the mechanism of pirfenidone through cell experiments.
Reagents
The rat type II lung epithelial cell line RLE-6TN (ATCC Cell Bank); bleomycin (Zhejiang Hisun Pharmaceutical Co., Ltd., certificate number: National Medicine H20055883); Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α (Abcam, ab46070), KL-6 (Abcam, ab100772), and E-cadherin (Yaki Bio, China, IH-1519R); a reverse transcription kit (Takara, China); a qPCR kit (Takara, China); antibodies targeting KL-6, (ab84597, Abcam, 1:1,000), STAT3 (ab68153, Abcam, 1:1,000), phosphorylated signal transducer and activator of transcription 3 (p-STAT3, sc-293059, Santa Cruz, 1:1,000), α-smooth muscle actin (α-SMA, ab232784, Abcam, 1:1,000), and vimentin (ab92547, Abcam, 1:1,000); goat anti-rabbit IgG H&L (HRP) (ab97051, Abcam, 1:5,000), and an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) kit (Biyuntian Bio, C0009S) were used in this study.
CTD-ILD patient inclusion
From 2018 to 2020, patients initially diagnosed with CTD-ILD in our hospital were included. The diagnostic criteria were (I) interstitial lung disease diagnosed according to ILD diagnostic criteria issued by the European Respiratory Society (10) and (II) connective tissue disease diagnosed according to the 2012 SICCA guidelines (11). The patients were divided into the study group (pirfenidone treatment) and control group (without pirfenidone treatment) based on whether they received pirfenidone treatment.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Cangzhou Central Hospital (No. 2016-098-01). Patient’s informed consent was waived in view of the retrospective nature of the study.
Clinical information
Pulmonary function tests, including forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC, peak expiratory flow (PEF) and changes in arterial partial pressure (PaO2), were performed before and after treatment, and the results were recorded. ELISAs to detect TNF-α, STAT3, and KL-6 expression in blood samples were performed before and after treatment.
Cell culture
RLE-6TN cells were cultured in a constant-temperature incubator at 37 ℃ with 95% humidity and 5% CO2 for 24 hours. Then, the cells were cultured in serum-free Dulbecco’s modified eagle medium (DMEM) for 6 h under starvation to synchronize the cell cycle. The serum-free DMEM was then discarded. After that, DMEM containing 10% fetal bovine serum and 1% double-antibody was added to the wells of all cell culture plates.
Drug treatment
Based on different interventions, the cells were sorted into the nontreated group (NTC), bleomycin model group (MC), pirfenidone treatment model group (PMC), infliximab group (TNF-α inhibitor) (IPMC), Stattic group (STAT3 inhibitor) and TNF-α/Stattic (STAT3 inhibitor) group.
Bleomycin model group: Bleomycin was diluted with DMEM, and the final concentration was adjusted to 3.5 µM. The culture medium was changed after 48 hours of treatment.
Pirfenidone treatment model group: cells were treated with bleomycin and pirfenidone at final concentrations of 3.5 and 54.05 µM, respectively; the culture medium was replaced after 48 hours.
TNF-α group: cells were treated with bleomycin, pirfenidone, and TNF-α at final concentrations of 3.5 mg/L, 54.05 µM and 3 mg/L, respectively, and the culture medium was changed after 48 hours.
Stattic group: cells were treated with bleomycin, pirfenidone and statticone at final concentrations of 3.5 mg/L, 54.05 µM and 4 mg/L, respectively.
TNF-α/Stattic combined group: cells were treated with bleomycin, pirfenidone, TNF-α, and Stattic at final concentrations of 3.5 mg/L, 54.05 µM, 3 mg/L, and 4 mg/L, respectively; the culture medium was changed after 48 hours.
Testing indicators
MTT assay
A MTT assay was used to evaluate the cellular activity of cells in each group. The cells were inoculated on 96-well plates (3×103 cells/well), with 3 parallel samples in each group, and a zero group with only culture medium without cells was set at the same time. The cells were treated according to different groups 24 hours after seeding, and MTT testing was performed 48 hours after treatment. Then, 0.1 mg/ml MTT solution diluted by DMEM was added to each well, and the cells were incubated at 37 ℃ for 4 hours. The absorbance (A) was measured at 570 nm, and the data were assessed using a ThermoFisher Multiskan FC microplate reader. Cell survival rate (%) = treatment group A/control group A × 100%; the test was repeated 3 times.
Western blotting (WB)
After drug treatment, the cells were lysed to extract total protein, and the protein was quantified using the Bradford method. The protein extraction kit was purchased from Amyjet Biotechnology Co., Ltd. (product number AMJ-KT0007). The Bradford protein concentration determination kit was purchased from Biyuntian Biotechnology Co., Ltd. (product number P0006).
A 50 mg protein sample was mixed with loading buffer at a volume ratio of 4:1. After boiling and denaturing, the protein sample was separated in a preprepared gel via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE). After electrophoresis, we transferred the proteins to a polyvinylidene fluoride (PVDF) membrane and used tris-buffered saline and tween 20 (TBST) containing 5% milk powder to block the protein at room temperature for 1 hour. The membrane was incubated with primary antibodies targeting KL-6 (1:1,000), STAT3 (1:1,000), p-STAT3 (1:1,000), α-SMA (1:1,000), or vimentin (1:1,000) overnight at 4 ℃; after washing three times with TBST, the membrane was incubated with secondary antibody for 1 hour at room temperature. An Invitrogen E-Gel imager was used for imaging, and ImageJ was used for image processing.
ELISA
TNF-α and E-cadherin ELISA kits were adopted to detect the expression of TNF-α and E-cadherin in the NTC, MC, PMC, Stattic and TNF-α/Stattic combined groups. After the cells were processed according to the different treatment conditions, they were collected by centrifugation, resuspended in 200 µL cytolytic buffer and allowed to rest for 30 minutes. After centrifugation at 1,000 r/min for 10 min, 20 µL of supernatant was added to the ELISA plate. Then, we added 80 µL of immunoreaction reagent to each well and incubated the plate for 2 h at room temperature. We removed the supernatant and washed the wells three times. After adding 100 µL buffer, we allowed the plate to stand for 30 min. Substrate buffer was used as the blank control group, and the absorbance was measured at a wavelength of 405 nm.
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was adopted to detect the expression of STAT3 and BL-6 in the NTC, MC, PMC, Stattic and TNF-α/Stattic combined groups. Total RNA was extracted from the cells using the TRIzol method. We removed 2 µL of the dissolved liquid to determine the concentration and purity of RNA in the solution using a microspectrophotometer. Then, a Takara reverse transcription kit was used for reverse transcription of RNA into cDNA. Meanwhile, a cDNA reaction system was established using a qPCR kit, and reactions were conducted at 95 ℃ for 10 minutes, followed by 95 ℃ for 30 seconds and 60 ℃ for 30 seconds for 40 cycles. A dissolution curve analysis was performed, and the final data were used for statistical analysis of the target gene expression level in each sample using the 2-∆∆CT method.
Statistical analyses
We used SPSS 24.0 software for statistical analysis of the data. The measurement data, including the clinical data, age, disease course, and pulmonary function test results, are expressed as the mean ± standard deviation and were analyzed using a t-test. Count data, including patient gender, are expressed as n (%) and were analyzed using a chi-squared test. Differences in ELISA and Western blotting data from the cell experiments were analyzed via Analysis of Variance (ANOVA), and the groups with differences were tested in pairs afterward. If the P value was less than 0.05, then it was considered statistically significant. We used GraphPad Prism 8.0 to draw box plots.
Results
Comparative analysis of clinical patient baseline data
According to the inclusion and exclusion criteria, fifty patients diagnosed with CTD-ILD were included in this study, and no cases of death occurred during treatment. A comparison of the baseline data of the two groups of patients is shown in Table 1. The results showed that the two groups of patients had no significant differences in terms of CTD disease type, sex, age, disease course, pretreatment routine blood tests, infection index, immune index, lung ventilation function, or diffusion function (P<0.05).
Table 1
| Indicator | Control group (n=20) | Study group (n=20) | χ2/t | P value |
|---|---|---|---|---|
| CTD type | 0.089 | 0.923 | ||
| Sjogren’s syndrome (SS) | 8 | 10 | ||
| Systemic lupus erythematosus (SLE) | 5 | 4 | ||
| Polymyositis (PM) | 3 | 4 | ||
| Systemic sclerosis (SSc) | 3 | 2 | ||
| Undifferentiated connective tissue disease (UCTD) | 1 | 0 | ||
| Gender (M/F) | 11/9 | 12/8 | 0.102 | 0.62 |
| Age (year) | 65.8±9.2 | 67.1±10.4 | 0.672 | 0.784 |
| Diagnostic period (month) | 15.4±20.9 | 13.8±22.3 | 0.980 | 0.834 |
| Routine blood parameters | ||||
| Leukocyte (×109/L) | 8.76±5.32 | 9.03±4.20 | 1.035 | 0.642 |
| Red blood cells (×1012/L) | 3.93±0.80 | 3.87±0.92. | 0.982 | 0.840 |
| Hemoglobin (g/L) | 117±26 | 113±32 | 1.108 | 0.552 |
| CRP (mg/L) | 45.03±10.17 | 43.87±12.14 | 1.086 | 0.628 |
| IgG (g/L) | 15.12±8.15 | 15.60±7.84 | 0.849 | 0.816 |
| IgA (g/L) | 2.83±1.56 | 2.79±1.52 | 0.946 | 0.829 |
| IgM (g/L) | 31.03±10.11 | 30.82±8.84 | 0.878 | 0.819 |
| Ventilatory dysfunction | ||||
| Minor | 7 | 6 | 0.136 | 0.934 |
| Medium | 8 | 9 | ||
| Severe | 5 | 5 | ||
| Diffuse dysfunction | ||||
| Minor | 8 | 8 | 0.20. | 0.904 |
| Medium | 8 | 9 | ||
| Severe | 4 | 3 | ||
| FVC | 1.21±0.35 | 1.15±0.27 | 0.672 | 0.543 |
| FEV1 | 0.75±0.13 | 0.74±0.11 | 0.873 | 0.744 |
| FEV1/FVC | 38.02±3.54 | 40.15±1.43 | 0.574 | 0.673 |
| PEF | 2.73±0.56 | 2.78±0.89 | 0.875 | 0.836 |
| Concomitant drug | 0.233 | 0.806 | ||
| Antibiotic | 5 | 4 | ||
| Bronchodilator | 3 | 4 |
No significant differences were found in the baseline data between the two groups of patients, and they were comparable. CTD, connective tissue disease; SS, Sjogren’s syndrome; SLE, systemic lupus erythematosus; PM, Polymyositis; SSc, systemic sclerosis; UCTD, undifferentiated connective tissue disease; CRP, C-reactive protein; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; PEF, peak expiratory flow.
Changes in serum ELISA test results and lung ventilation function in the two groups of patients
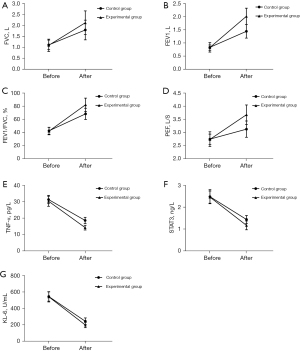
Pulmonary ventilation function assessments in the two groups of patients demonstrated that the pulmonary ventilation function of the patients in the control group and study group was significantly restored after treatment. Specifically, FVC, FEV1, FEV1/FVC, and PEF were all significantly improved compared with the values before the treatment (P<0.05, Figure 1A-1D). The comparison of the pulmonary function results of the two groups after treatment demonstrated that the pulmonary ventilation function in the study group was better than that in the control group. Specifically, FVC, FEV1, FEV1/FVC, and PEF were increased significantly in the study group compared with in the control group (P<0.05, Figure 1A-1D). The ELISA test results of patients in the two groups demonstrated that the expression levels of serum TNF-α, STAT3, and KL-6 in the control group and the study group were noticeably lower after treatment than those before treatment (P<0.05, Figure 1E-1G). The expression levels of TNF-α, STAT3 and KL-6 in the study group after treatment were significantly lower than those in the control group (Figure 1E-1G).
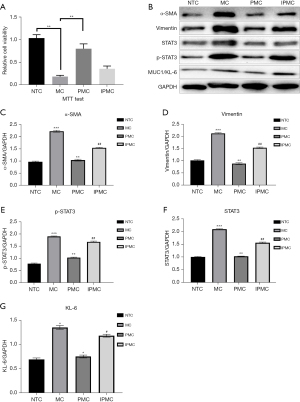
Pirfenidone inhibits bleomycin-induced damage to RLE-6TN cells through TNF-α
After the corresponding treatment of each group of cells, the results of the MTT assays demonstrated that cell viability was significantly reduced after bleomycin induction, cell viability was significantly restored after the addition of pirfenidone, and cell viability was further inhibited after the addition of TNF-α (P<0.05, Figure 2A). WB was used to detect fibrosis degree indicators (vimentin and α-SMA) in each group of cells, and the results demonstrated that the expression of vimentin and α-SMA in the cells increased significantly after bleomycin induction (P<0.05, Figure 2B). When pirfenidone was added, the expression of vimentin and α-SMA decreased significantly (P<0.05). When pirfenidone and TNF-α were added in combination, the expression of vimentin, and α-SMA was increased significantly (P<0.05). The results above show that pirfenidone inhibits the degree of fibrosis in RLE-6TN cells by inhibiting TNF-α. WB experiments were used to detect the expression of STAT3, p-STAT3, and KL-6 in the cells in each group, and the results demonstrated that the expression levels of STAT3, p-STAT3, and KL-6 in the cells increased significantly after bleomycin induction (P<0.05). When pirfenidone was added, the expression levels of STAT3, p-STAT3, and KL-6 decreased (P<0.05). When pirfenidone and TNF-α were added in combination, the expression levels of STAT3, p-STAT3, and KL-6 increased significantly (P<0.05, Figure 2C-2G).
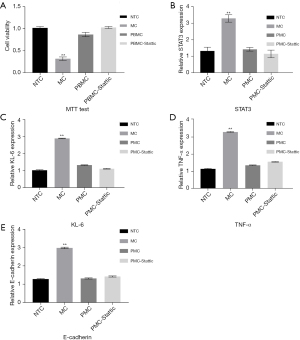
Pirfenidone inhibits bleomycin-induced damage to RLE-6TN cells through the STAT3 signaling pathway
After processing the cells in each group, the results of the MTT assays demonstrated that cell viability was significantly reduced after bleomycin induction, and the cell viability was significantly restored after the addition of pirfenidone. At the same time, cell viability was further increased after the STAT3 inhibitor Stattic was added (P<0.05, Figure 3A). RT-PCR detection of STAT3 and KL-6 gene expression in the cells in each group demonstrated that the expression of STAT3 and KL-6 increased significantly after bleomycin induction. When pirfenidone was added, the expression of STAT3 and KL-6 was significantly reduced. After the addition of Stattic, the expression of STAT3 and KL-6 in the cells was further reduced (P<0.05, Figure 3B,3C). ELISAs were used to detect the expression of TNF-α in the cells in each group, and the results demonstrated that the expression of TNF-α in the cells was significantly increased after bleomycin induction. When pirfenidone was added, the expression of TNF-α was significantly reduced (P<0.05); however, when Stattic was added, the expression of TNF-α in the cells did not change significantly (P>0.05). ELISAs were also used to detect the expression of E-cadherin in the cells in each group, and they demonstrated that the expression of E-cadherin was significantly increased after bleomycin induction; when pirfenidone was added, the expression of E-cadherin was significantly reduced (P<0.05). After the addition of Stattic, the expression of E-cadherin in the cells showed no significant difference compared to peripheral blood mononuclear cells (PBMCs) group (P>0.05, Figure 3D,3E).
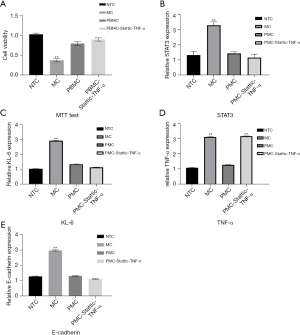
Pirfenidone inhibits bleomycin-induced damage to RLE-6TN cells through the TNF-α/STAT3/KL-6 signaling pathway
After processing each group of cells, the MTT assay results demonstrated that cell viability was significantly reduced after bleomycin induction, and cell viability was significantly restored after the addition of pirfenidone. At the same time, cell viability was significantly increased after the addition of the TNF-α/Stattic combination (P<0.05, Figure 4A). RT-PCR detection of STAT3 and KL-6 gene expression in the cells in each group demonstrated that the expression of STAT3 and KL-6 in the cells was significantly increased after induction with bleomycin. When pirfenidone was added, the expression of STAT3 and KL-6 was significantly reduced. After the addition of the TNF-α/Stattic combination, the expression of STAT3 and KL-6 in the cells was further reduced (P<0.05, Figure 4B,4C). ELISAs were used to detect the expression of TNF-α in the cells in each group, and the results demonstrated that TNF-α expression was significantly increased after bleomycin induction. When pirfenidone was added, the expression of TNF-α was significantly reduced (P<0.05); meanwhile, when the TNF-α/Stattic combination was added, the expression of TNF-α increased significantly (P>0.05, Figure 4D). ELISAs were used to detect the expression of E-cadherin in the cells of each group, and they demonstrated that the expression of E-cadherin in the cells was significantly increased after bleomycin induction. When pirfenidone was added, the expression of E-cadherin was significantly reduced (P<0.05); after the addition of the TNF-α/Stattic combination, the expression of E-cadherin was further reduced (P<0.05, Figure 4E).
Discussion
This study confirmed that pirfenidone can effectively control CTD-ILD progression in patients and restore the patient’s pulmonary ventilation function. Cellular experiments were conducted to establish cell fibrosis injury models, and TNF-α expression and STAT3 expression were inhibited.
Interstitial pneumonia is a common complication of connective tissue disease, and its main clinical manifestations are restrictive pulmonary ventilation dysfunction, decreased diffusion function, and hypoxemia (12). Epidemiological survey results show that CTD-ILD patients have a high fatality rate and poor prognosis, which seriously threaten the life and health of patients (13). The current routine clinical treatment for CTD-ILD is glucocorticoids combined with immunosuppressive therapy (14). However, the clinical effects of the above treatments are limited. As an antifibrotic drug, pirfenidone has benefits in the treatment of IPF, renal fibrosis, scar contracture, and other diseases (15-17). Therefore, this study proposes that pirfenidone can effectively treat clinical CTD-ILD patients. To verify the effect of pirfenidone in patients with CTD-ILD, a randomized controlled clinical study was conducted. To ensure the reliability of the results, the sample size estimation in the early stage of the research design was approximately 50 according to PASS 15.0. For CTD-ILD patients, the combined application of pirfenidone with basic conventional treatment (glucocorticoids combined with immunosuppressive agents) resulted in improved lung function compared with patients who only received conventional treatment. Serological examination demonstrated that the expression levels of serum TNF-α, STAT3, and KL-6 in the study group were noticeably higher than those found in the control group. The aforementioned clinical research results show that pirfenidone can effectively treat CTD-ILD and simultaneously reduce serum TNF-α, STAT3 and KL-6 levels in patients. The main reason for lung function improvements was considered that pirfenidone can reduce the accumulation of cellular inflammation, prevent the spread of fibrosis and inhibit the decrease of vital capacity (18).
A previous medical study has shown that in the course of ILD, the serum level of TNF-α, a key cytokine that causes lung tissue fibrosis, is elevated (19). TNF-α is an inflammatory factor that can further promote the proliferation and differentiation of fibroblasts by mediating inflammatory damage in alveolar epithelial cells, thus ultimately promoting the development of lung tissue fibrosis. Previous studies have shown that pirfenidone can effectively reduce the serum TNF-α level in patients with pulmonary fibrosis, interstitial pneumonia, and silicosis and the degree of pulmonary fibrosis (20-22). At the same time, a study has shown that pirfenidone can be used to inhibit the synthesis and secretion of TNF-α in cells, thereby demonstrating its therapeutic effect (23). On this basis, in our study, the RLE-6TN cell line was cultured in vitro, and cell fibrosis damage was stimulated by bleomycin administration. After the addition of pirfenidone, cell activity was restored, and the expression of fibrosis-related proteins was significantly reduced. The expression of TNF-α, STAT3 and KL-6 was significantly reduced. Thus, the addition of TNF-α inhibited cell activity, increased the expression of STAT3 and KL-6, and increased the expression of fibrotic proteins. The results above indicate that pirfenidone can effectively treat pulmonary fibrosis by inhibiting the expression of TNF-α in cells.
Previous studies have shown that the STAT3 protein family is closely related to the inflammatory response and immune response. As a transcription factor, STAT3 can participate in the regulation of the expression of inflammation-related genes (24,25). At the same time, a study has shown that STAT3 is closely related to the degree of tissue fibrosis. Fibroblasts associated with liver cancer can activate STAT3 by secreting IL-6, further promoting the degree of fibrosis in liver tissue (26). Moreover, a previous study has demonstrated that the activation of STAT3 in the microenvironment can mediate the Smad/TGF-β signaling pathway, thereby further promoting the occurrence of tissue fibrosis. After blocking the STAT3 signaling pathway, the degree of fibrosis was significantly reduced (27). The above results suggest that STAT3 plays an important regulatory role in the process of fibrosis (28). The results of our clinical study demonstrated that the expression of STAT3 in the serum of CTD-ILD patients was significantly reduced after pirfenidone treatment, and lung function was significantly improved. Cell experiments demonstrated that by inhibiting the expression of STAT3, cell viability was significantly increased, while the degree of fibrosis was reduced. Activating TGF-α while inhibiting STAT3 expression increased cell activity and reduced the degree of fibrosis, but the degree of TGF-α inhibition was not obvious. Therefore, this study proposes that pirfenidone can regulate the process of pulmonary fibrosis through the TNF-α/STAT3 signaling axis, achieving an effective treatment for CTD-ILD. At the same time, the cell experiment results indicated that TNF-α and STAT3 can regulate the expression of KL-6.
In recent years, studies have found that KL-6 can be used as a CTD-ILD serum marker molecule because it is mainly expressed in bronchial epithelial cells and type II alveolar epithelial cells but not in type I alveolar cell epithelial cells. When alveoli are damaged, type I alveolar epithelial cells die, and the replacement of type II alveolar cells in the alveolar basement membrane can lead to an increase in the expression of KL-6. Due to damage to the capillary endothelium and lung tissue, KL-6 can be released into alveolar lavage fluid as well as into the serum. The higher the KL-6 level is, the more severe the alveolar damage, and the worse the prognosis (29,30). In this study, after treatment of patients in the study group, lung function indicators were significantly better than those in the control group, while the KL-6 level was noticeably lower than that in the control group. Pirfenidone is a new type of small-molecule drug used to treat pulmonary fibrosis, and it has antioxidant, anti-inflammatory, and antifibrotic activity (31). Initially, pirfenidone was recommended by many experts and multiple guidelines worldwide for the treatment of IPF. In recent years, it has been used in the treatment of CTD-ILD, and a large number of clinical studies have been conducted (32). TNF-α is a cytokine with proinflammatory effects that can trigger and aggravate inflammation, causing damage to tissues and cells that leads to apoptosis and tissue necrosis and thereby triggers tissue self-repair and subsequent fibrosis processes (33). Meanwhile, STAT3 is a transcription factor belonging to the STAT protein family. After phosphorylation, STAT3 is activated, forms homodimers or heterodimers and translocates to the nucleus. It acts as an activated transcription factor to mediate the expression of a variety of genes and is involved in many cellular processes. A study has shown that the TNF-α/STAT3 pathway plays an important role in tumor metastasis, autophagy, epithelial-mesenchymal transition (EMT), apoptosis, and regeneration after ischemia-reperfusion injury (34). Clinical research results indicate that pirfenidone can significantly reduce serum TNF-α and STAT3 levels, improve lung function indicators, and reduce KL-6 levels compared with conventional treatments, indicating that pirfenidone can effectively alleviate the CTD-ILD process; such results are consistent with those of previous studies. Furthermore, inhibiting the inflammatory response caused by the TNF-α/STAT3 pathway reduces the damage to type I alveolar epithelial cells and the compensatory replacement of type II alveolar epithelial cells, thereby restoring patient lung function (35). The clinical results of this study demonstrated that the expression level of KL-6 in the serum of CTD-ILD patients treated with pirfenidone was significantly reduced compared with conventional treatment. Increasing pirfenidone can effectively reduce the expression of KL-6 in patient serum. In cell experiments, the expression level of KL-6 in RLE-6TN cells was significantly reduced after bleomycin induction, and the expression level of KL-6 was significantly reduced after the addition of pirfenidone. On this basis, after overexpression of TNF-α, the expression of KL-6 was also upregulated, indicating that pirfenidone regulates the expression of KL-6 through TNF-α; after inhibition of STAT3 expression, the expression of KL-6 was further suppressed, indicating that pirfenidone regulates the expression of KL-6 through STAT3. Hence, after overexpression of TNF-α combined with STAT3 inhibition, the expression of KL-6 was further reduced. These results suggest that pirfenidone regulates the expression of KL-6 through TNF-α/STAT3. And the specific mechanism was shown in Figure 5.
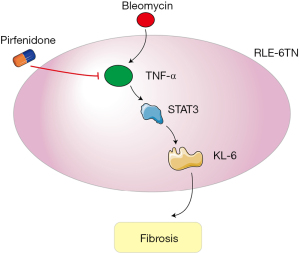
In summary, through clinical experiments and cell model studies, this study revealed that pirfenidone can reduce EMT and fibrosis in lung epithelial cells and lung tissues. Pirfenidone reduces EMT and fibrosis in lung epithelial cells by inhibiting the expression of the marker molecule KL-6 through the TNF-α/STAT3 pathway.
Limitation
The number of clinical samples in this study is small, and there may be some deviation in the results of clinical research. The basic research was mainly based on in vitro cytological tests, which may be different from in vivo research. Therefore, further in vivo experiments will be carried out to verify the results.
Acknowledgments
Funding: This study was funded by the Project of Cangzhou Science and Technology Bureau (162302163).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-41/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-41/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Cangzhou Central Hospital (No. 2016-098-01). Patient’s informed consent was waived in view of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antin-Ozerkis D, Rubinowitz A, Evans J, et al. Interstitial lung disease in the connective tissue diseases. Clin Chest Med 2012;33:123-49. [Crossref] [PubMed]
- Khanna D, Mittoo S, Aggarwal R, et al. Connective Tissue Disease-associated Interstitial Lung Diseases (CTD-ILD) - Report from OMERACT CTD-ILD Working Group. J Rheumatol 2015;42:2168-71. [Crossref] [PubMed]
- Solomon JJ, Fischer A. Connective Tissue Disease-Associated Interstitial Lung Disease: A Focused Review. J Intensive Care Med 2015;30:392-400. [Crossref] [PubMed]
- Bonella F, Long X, Ohshimo S, et al. MUC1 gene polymorphisms are associated with serum KL-6 levels and pulmonary dysfunction in pulmonary alveolar proteinosis. Orphanet J Rare Dis 2016;11:48. [Crossref] [PubMed]
- Fathi M, Barbasso HS, Lundberg IE. KL-6: a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis. J Intern Med 2012;271:589-97. [Crossref] [PubMed]
- Solomon JJ, Danoff SK, Goldberg HJ, et al. The Design and Rationale of the Trail1 Trial: A Randomized Double-Blind Phase 2 Clinical Trial of Pirfenidone in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Adv Ther 2019;36:3279-87. [Crossref] [PubMed]
- Iwata T, Yoshida S, Nagato K, et al. Experience with perioperative pirfenidone for lung cancer surgery in patients with idiopathic pulmonary fibrosis. Surg Today 2015;45:1263-70. [Crossref] [PubMed]
- Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann Rheum Dis 2018;77:175-87. [Crossref] [PubMed]
- Chung MP. Connective Tissue Disease-Associated Interstitial Lung Disease. J Intensive Care Med 2013;84:498.
- Jee AS, Adelstein S, Bleasel J, et al. Role of Autoantibodies in the Diagnosis of Connective-Tissue Disease ILD (CTD-ILD) and Interstitial Pneumonia with Autoimmune Features (IPAF). J Clin Med 2017;6:51. [Crossref] [PubMed]
- Koslow M, Maleki-Fischbach M, Keith RC. Diagnosis and Management of Interstitial Lung Disease in Patients with Connective Tissue Diseases. Case Rep Rheumatol 2021;2021:6677353. [Crossref] [PubMed]
- Shi S, Chen L, Gui X, et al. Association of Red Blood Cell Distribution Width Levels with Connective Tissue Disease-Associated Interstitial Lung Disease (CTD-ILD). Dis Markers 2021;2021:5536360. [Crossref] [PubMed]
- Erre GL, Sebastiani M, Manfredi A, et al. Antifibrotic drugs in connective tissue disease-related interstitial lung disease (CTD-ILD): from mechanistic insights to therapeutic applications. Drugs Context 2021;10:e2020-8-6.
- Wells AU. New insights into the treatment of CTD-ILD. Nat Rev Rheumatol 2021;17:79-80. [Crossref] [PubMed]
- Chen T, Li QH, Zhang Y, et al. The role of pirfenidone in the treatment of interstitial pneumonia with autoimmune features. Clin Exp Rheumatol 2022;40:560-7. [Crossref] [PubMed]
- Feng H, Zhao Y, Li Z, et al. Real-life experiences in a single center: efficacy of pirfenidone in idiopathic pulmonary fibrosis and fibrotic idiopathic non-specific interstitial pneumonia patients. Ther Adv Respir Dis 2020;14:1753466620963015. [Crossref] [PubMed]
- Sakamoto S, Shimizu H, Isshiki T, et al. Effectiveness of pirfenidone for idiopathic pulmonary fibrosis associated with pleuroparenchymal fibroelastosis-like lesions and nonspecific interstitial pneumonia. Clin Respir J 2021;15:272-9. [Crossref] [PubMed]
- Dellaripa PF. Interstitial lung disease in the connective tissue diseases; a paradigm shift in diagnosis and treatment. Clin Immunol 2018;186:71-3. [Crossref] [PubMed]
- Lim DH, Lee EJ, Lee HS, et al. Acetylated Diacylglycerol 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol in Autoimmune Arthritis and Interstitial Lung Disease in SKG Mice. Biomedicines 2021;9:1095. [Crossref] [PubMed]
- Yang H. Cytokine expression in patients with interstitial lung disease in primary Sjogren's syndrome and its clinical significance. Am J Transl Res 2021;13:8391-6. [PubMed]
- Li J, Chen X, Qu Y. Effects of cyclophosphamide combined with prednisone on TNF-α expression in treatment of patients with interstitial lung disease. Exp Ther Med 2019;18:4443-9. [Crossref] [PubMed]
- Picchianti Diamanti A, Markovic M, Argento G, et al. Therapeutic management of patients with rheumatoid arthritis and associated interstitial lung disease: case report and literature review. Ther Adv Respir Dis 2017;11:64-72. [Crossref] [PubMed]
- Tan J, Ni X. TNF-α antagonist may not be suitable for severe rituximab-induced interstitial lung disease. J Clin Pharm Ther 2015;40:249-50. [Crossref] [PubMed]
- Gothe F, Gehrig J, Rapp CK, et al. Early-onset, fatal interstitial lung disease in STAT3 gain-of-function patients. Pediatr Pulmonol 2021;56:3934-41. [Crossref] [PubMed]
- Silva-Carmona M, Vogel TP, Marchal S, et al. Successful Treatment of Interstitial Lung Disease in STAT3 Gain-of-Function Using JAK Inhibitors. Am J Respir Crit Care Med 2020;202:893-7. [Crossref] [PubMed]
- Chen JH, Bao YM, Li ZC, et al. Immunodeficiency diseases with interstitial lung disease as major clinical manifestations: report of six cases. Zhonghua Er Ke Za Zhi 2020;58:228-32. [PubMed]
- Zhang J, Wang D, Wang L, et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am J Physiol Lung Cell Mol Physiol 2019;316:L487-97. [Crossref] [PubMed]
- He Q, Tang Y, Huang J, et al. The value of KL-6 in the diagnosis and assessment of interstitial lung disease. Am J Transl Res 2021;13:9216-23. [PubMed]
- Singh S, Collins BF, Sharma BB, et al. Interstitial Lung Disease in India. Results of a Prospective Registry. Am J Respir Crit Care Med 2017;195:801-13. [Crossref] [PubMed]
- Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016;352:h6819. [Crossref] [PubMed]
- El-Beheidy R, Domouky AM, Zidan H, et al. Serum KL-6 as predictive and prognostic marker of interstitial lung disease in childhood connective tissue diseases: a pilot study. Reumatismo 2021;73. [Crossref] [PubMed]
- Pourgholamhossein F, Rasooli R, Pournamdari M, et al. Pirfenidone protects against paraquat-induced lung injury and fibrosis in mice by modulation of inflammation, oxidative stress, and gene expression. Food Chem Toxicol 2018;112:39-46. [Crossref] [PubMed]
- Fang C, Huang H, Guo J, et al. Real-world experiences: Efficacy and tolerability of pirfenidone in clinical practice. PLoS One 2020;15:e0228390. [Crossref] [PubMed]
- Li D, Ji H, Zhao B, et al. Therapeutic effect of ulinastatin on pulmonary fibrosis via downregulation of TGF-β1, TNF-α and NF-κB. Mol Med Rep 2018;17:1717-23. [PubMed]
- Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 2018;15:234-48. [Crossref] [PubMed]


