Bronchodilator response in adults with bronchiectasis: correlation with clinical parameters and prognostic implications
Introduction
Bronchiectasis is a chronic debilitating airway disease characterized by chronic cough and sputum production associated with aberrant dilatation of bronchi (1-5), which predisposes to recurrent airway infections and mucus hypersecretion. These collectively contribute to airflow obstruction (4,6) associated with ventilation dyshomogeneity (4,7) which is common in bronchiectasis. Understanding the nature of these phenomena may offer new insights into developing future therapies, since β2-agonists have been associated with ameliorated ventilation heterogeneity and reduced airway impedance in asthma (8) and chronic obstructive pulmonary disease (COPD) (9,10).
Previous studies in COPD suggested that airway reversibility varied with repeated testing in the same individual, had poor prognostic implications and could not predict long-term response to maintenance bronchodilator treatments (11). However, COPD patients with greater airway reversibility had better long-term outcomes (12). Furthermore, reports on the association between bronchodilator response (BDR) and clinical characteristics and prognosis of bronchiectasis are lacking. The clinical utility of BDR in bronchiectasis needs to be systematically explored, which might better guide clinicians assessment of the prognosis and assist in individualised treatment.
Our objectives were two-fold: (I) characterize BDR and the association with clinical parameters in clinically stable bronchiectasis; (II) determine the utility of BDR to predict lung function decline and future risks of bronchiectasis exacerbations (BEs).
Methods
Subjects
Consecutive patients with symptoms of chronic cough, sputum production and/or hemoptysis, aged 18-75 years, remaining clinically stable for 4 weeks, were recruited from respiratory out-patient clinics between September 2012 and July 2013. Bronchiectasis was confirmed by study investigators via chest high-resolution computed tomography (HRCT) review. Those with malignancy, upper respiratory tract infection or antibiotic use within 4 weeks were excluded. To minimize the potential bias to BDR, we also excluded patients with physician-diagnosed asthma and COPD.
This study was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University. All patients signed informed consent.
Study design
At baseline, patients with clinically stable bronchiectasis (symptoms not exceeding normal day-to-day variations for 4 weeks) underwent baseline assessment consisting of history taking, sputum culture, measurement of sputum inflammatory markers and matrix metalloproteinases (MMPs), and spirometry.
Patients were prospectively followed up for 1 year. Patients underwent hospital visits (or telephone calls, if unavailable) at 3-month intervals. At 6 and 12 months, patients underwent spirometry and had sputum cultures collected, and BEs were diagnosed based on careful medical chart review. Data collection was not blinded to the investigators. Medications [mucolytics, theophylline, and (or) low-dose macrolides] remained unchanged throughout follow-up period but could be adjusted based upon the clinical decision made by their attending physicians.
Bronchiectasis exacerbations (BE)
BEs were defined as having 3 of the following items that persisted for at least 24 h: significantly increased sputum purulence/volume; dyspnea; considerably increased cough frequency; T >37.5 °C; hemoptysis; exercise intolerance or fatigue; increased crackles; increased pulmonary infiltrations (2,4,13-17). The magnitude of crackles was determined among patients who attended hospital visits only and was not a prerequisite to determine the presence of BEs. The diagnosis of BEs was made by the investigators (WJ Guan, YH Gao, G Xu), with any discrepancy resolved following group discussion.
Sputum processing
Following elimination of oral debris by thorough rinsing, patients expectorated sputum into a sterile plastic container, between 9:00 a.m. and 12:00 a.m. at hospital visits. Sputum induction was performed with hypertonic saline in case of insufficient sputum (18). Sputa with >25 leukocytes and <10 epithelial cells under microscopy (×100) were required for processing. Two random aliquots of sputum plugs were pipetted within 2 h for culture and ultracentrifugation (50,000 g) for 90 min, without pre-treatment with albumin or dithiothreitol, to harvest the sputum sol phase which was subsequently stored in −80 °C freezers.
Sputa at baseline visits were analyzed for baseline, whilst sputa were collected at subsequent visits were used to determine Pseudomonas aeruginosa infection, denoted as sputum culture positive to Pseudomonas aeruginosa for at least 2 occasions, with an interval of greater than 3 months, within 1 year. The presence of Pseudomonas aeruginosa infection could not indicate BEs, which was ascertained according to the diagnostic criteria outlined above (See online supplement for further details, also including Sputum MMPs measurement and inflammatory mediators and Spirometry).
Bronchial dilation test
Spirometers (QUARK PFT, COSMED Inc., Italy) were employed for assessment. Quality control met international guidelines for standardization (19). Predicted values were based on the model recommended by Zheng et al. (20). Salbutamol was withdrawn for at least 6 h, and salmeterol or formoterol for 24 h, prior to spirometry.
Bronchial dilation test was performed at the initial visit only, in patients with FEV1 predicted less than 80%. Salbutamol (GlaxoSmithKline Inc., UK) 400 mg was administered via spacer (Volumatic & Handbury’s, UK). This entailed spirometry reassessment at 15 min post-bronchodilation. BDR was defined as FEV1 improvement from pre-dose value by >12% and >200 mL (21). Patients with baseline FEV1 >80% predicted were not included in follow-up analyses.
Diffusing capacity
Diffusing capacity was tested using gas analyzers (QUARK PFT, COSMED Inc., Italy) with single-breath carbon monoxide washout technique. The interval between repetitive measurements was 4 min. Mean DLCO from two repeatable maneuvers was reported. Reduced diffusing capacity was referred to as DLCO being less than 80% predicted.
High-resolution computed tomography (HRCT) scores
Chest HRCT at 2 mm collimation within 12 months was evaluated. HRCT was assessed by an experienced radiologist (>10-year experience) who was blinded to patient’s allocations. Lingular lobe was scored as a separate lobe. Bronchiectasis was scored (0 for no, 1 for tubular, 2 for varicose and 3 for cystic) for individual lobes (22). For lung lobes with mixed types of bronchiectasis, the highest score was recorded for the most significant bronchiectatic segment. The maximal total score for 6 lobes was 18. Other imaging characteristics, including dyshomogeneity, atelectasis and infiltration, were also determined (See online supplement for further details).
Disease severity
Disease severity was assessed by the bronchiectasis severity index (BSI), which included age, body-mass index (BMI), prior exacerbations and hospitalization, Medical Research Council dyspnea score, FEV1 predicted%, colonization with Pseudomonas aeruginosa or other potentially pathogenic micro-organisms (i.e., Haemophilus influenzae, Klebsiella pneumoniae), and bronchiectatic lobes (23). The BSI of ≤4, 5–8, and ≥9 denoted mild, moderate and severe bronchiectasis, respectively.
Statistical analysis
We presented the mean ± standard deviation or median (interquartile range), as appropriate, for numerical data. Independent t-test or Mann-Whitney test was applied for two-group comparisons. Three-group comparisons were performed with analysis of variance or Kruskal-Wallis test. Time to BEs was analyzed with the log-rank test and presented in Kaplan-Meier survival plots. The last-observation-carry-forward algorithm was applied for missing lung function data during follow-up. Statistical analysis was performed using SPSS 16.0 (SPSS Inc., Ill, USA) and Graphpad Prism 5.0 (Graphpad Inc., USA). P<0.05 was deemed statistically significant.
Results
Subject enrollment
Of 160 subjects who underwent screening, 151 met eligibility criteria and 129 were included for baseline assessments and longitudinal follow-up visits. Twenty eight patients were lost to follow-up and were not included for follow-up survival analyses (Figure 1).
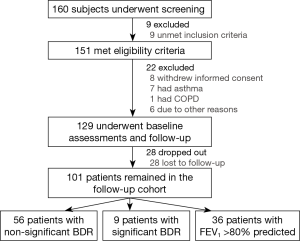
Characteristics of bronchiectasis patients
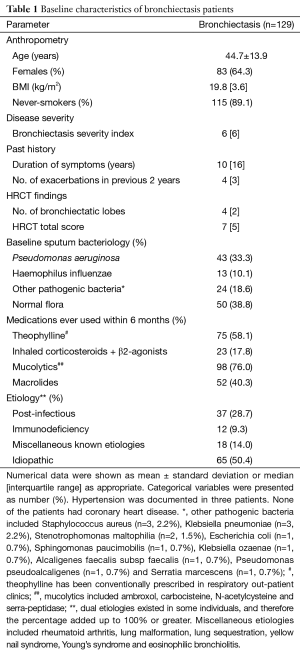
Baseline characteristics of bronchiectasis patients are displayed in Table 1. Bronchiectasis patients had a median of 4 bronchiectatic lobes, with median HRCT score of 7.0. In terms of sputum bacteriology, normal floras (including Neisseria, Streptococcus hemolyticus) (38.8%) were the most common findings, followed by Pseudomonas aeruginosa (33.3%). Mucolytics (76.0%) and theophylline (58.1%) constituted the medications most commonly used within 6 months. The most common etiology was idiopathic (50.4%), followed by post-infectious (28.7%). Patients had never used domiciliary mannitol or hypertonic saline, nor were on oral corticosteroids upon enrollment. Hypertension was documented in 3 patients. None of the patients had coronary heart disease.
Full table
Bronchodilator responses (BDR)
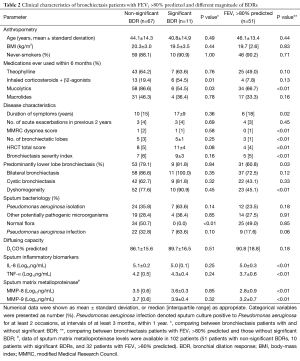
BDRs varied considerably among bronchiectasis patients (Table 2). Eleven patients were deemed to have significant BDR (mean baseline FEV1: 42.5%), with the median percentages of blood and sputum eosinophils of 2.7% and 1.0%. Ten patients had FEV1 change greater than 12% but less than 200 mL (mean baseline FEV1: 34.6%), 4 patients had FEV1 change less than 12% but greater than 200 mL (mean baseline FEV1: 67.4%), and the majority of patients (n=53) had FEV1 change less than 12% and 200 mL (mean baseline FEV1: 60.5%).
Full table
Inhaled corticosteroids and mucolytics were the most common and least common concomitant medications, respectively, in patients with significant BDR as compared with their counterparts (Table 2). However, the use of theophylline and macrolides did not differ statistically among patients with significant and non-significant BDR and those with FEV1 >80% predicted.
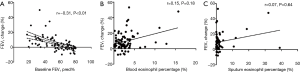
There was a significantly negative correlation between baseline FEV1% predicted and change in post-bronchodilator FEV1 (%) (Figure 2A). However, changes in post-bronchodilator FEV1 did not correlate with blood or sputum eosinophil percentage (Figure 2B,C). Patients with FEV1 change less than 12% but greater than 200 ml had the highest sputum eosinophil percentage (median: 2.3%), whereas differences in blood eosinophils did not reach significance (data not shown). There were only 3 patients with post-bronchodilator FEV1 increase being greater than 400 mL, therefore we did not perform further analyses.
Clinical characteristics among bronchiectasis patients with FEV1 >80% predicted and different bronchodilator responses (BDRs)
Next, we stratified bronchiectasis based on the magnitude of BDR. Patients with non-significant BDR did not differ statistically from those with significant BDR in anthropometry, the proportion of never-smokers, past history, HRCT characteristics, diffusing capacity, sputum inflammatory mediators (including interleukin-8, tumor necrosis factor-α) and matrix metalloproteinase levels (matrix metalloproteinase-8, matrix metalloproteinase-9) (all P>0.05). Patients with significant BDR had a significantly lower proportion of patients with normal flora isolated from sputum (Table 2, P<0.01), and a trend towards higher BSI and greater proportion of patients with Pseudomonas aeruginosa isolation and infection.
Patients with FEV1 >80% predicted were associated with a shorter duration of symptom onset, lower modified Medical Research Council (MMRC) dyspnea score, milder disease severity, minor HRCT abnormalities, and lower levels of sputum inflammation and matrix metalloproteinases, as compared with the other two groups (Table 2).
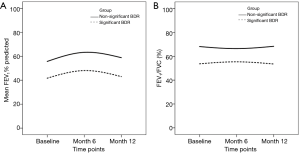
Trajectories of FEV1 and FEV1/FVC according to the magnitude of bronchodilator responses (BDRs)
FEV1 and FEV1/FVC ratios according to BDRs during 1-year follow-up, at 6-month intervals, are shown in Figure 3. Patients with significant BDR yielded consistently lower FEV1 percentage predicted and FEV1/FVC ratio throughout follow-up, compared with those who had non-significant BDR. Significant BDR was not associated with more rapid lung function decline during follow-up.
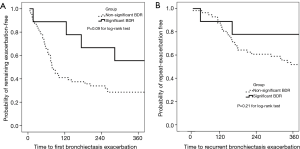
Association between bronchiectasis exacerbations (BEs) and bronchodilator responses (BDRs)
The incidence of and time to BEs during 1-year follow-up are displayed in Figure 4. A total of 99 exacerbation events were documented among the 65 patients who were included in survival analysis, and the median incidence was 1.5/patient/year. Of these, 73 events were analyzed because we only calculated the time to first (n=44) exacerbations. Overall median time to the first exacerbations was 65.0 days and 149.5 days in patients with non-significant BDR and those with significant BDR, respectively. Survival analysis showed that patients with significant BDR demonstrated non-significantly lower risks of having the first BEs compared to those without (P=0.09 for log-rank test).
Discussion
Principal findings
We found that patients with non-significant BDR did not differ statistically from those with significant BDR regarding the majority of clinical variables, including demographics, the duration of symptoms and radiologic manifestations. Patients with significant BDR tended to have a higher BSI and a greater proportion of Pseudomonas aeruginosa isolation and infection. Significant BDR was associated with poorer lung function at baseline, but not more rapid lung function decline during the 1-year follow-up. Patients with significant BDR showed a non-significant trend towards lower risks of experiencing BEs than those without.
Interpretation
Could bronchodilator response (BDR) be influenced by the blood or sputum eosinophil count in bronchiectasis?
BDR has been a feature of asthma and a considerable proportion of patients with COPD. One might speculate that significant BDR could still be anticipated in bronchiectasis. To this end, we have first excluded patients with asthma and COPD, to reduce potential bias. Next, we analyzed blood and sputum eosinophil count in patients with different magnitude of BDRs. Patients with significant BDR had lower sputum eosinophil percentage than those with change in post-bronchodilator FEV1 being less than 12% but greater than 0.2 L. Furthermore, no remarkable differences in blood eosinophil count were found among different magnitude of BDRs. Therefore, BDR was unlikely to have been biased by the blood or sputum eosinophil count.
Clinical characteristics of bronchiectasis patients with or without significant bronchodilator response (BDR)
A greater magnitude of BDR might be an indicator of prescribing bronchodilators as one of the maintenance therapeutic regimens in a subgroup of patients, therefore it would be helpful to unravel the characteristics of BDR in bronchiectasis patients. In this study, we found that greater magnitude of BDR was indeed associated with poorer baseline FEV1 percentage predicted, but not significantly higher blood or sputum eosinophil count. This might have pointed to the greater room for lung function improvement and alleviation of dyspnea in some patients who had more significant lung function impairment at baseline. However, patients with significant BDR had a higher baseline FEV1 percentage predicted than those with FEV1 change less than 12% but greater than 200 mL, hence there might be other factors (differences in sputum bacteriology, the ease of removal of mucus plugging because of greater contact for bronchodilators with the airway smooth muscles) that could help interpret the greater magnitude of BDR in this cohort.
Could airway inflammation or remodeling be the effect modifier of BDR? Unfortunately, we did not find the statistically different levels of sputum inflammatory markers (including interleukin-8 and tumor necrosis factor-α) or matrix metalloproteinases (matrix metalloproteinase-8 and matrix metalloproteinase-9), indicating that airway inflammation and remodeling might have played a less prominent role in explaining the differences in the magnitude of BDR in bronchiectasis.
Could there be a role for certain bacteria that could have mediated the magnitude of BDR in bronchiectasis? We speculated that Pseudomonas aeruginosa infection-driven Th2 airway inflammation, which has been associated with Th2-skewed responses in cystic fibrosis (24), might be partially responsible for the differential BDR. Indeed, the trend towards a numerically higher proportion of Pseudomonas aeruginosa isolation and infection might support to the above hypothesis. We have also noted a trend towards the numerically higher BSI and HRCT total score which were related to the significant BDR. During database review, we found that this might be interpreted by the greater number of bronchiectatic lobes, and again, Pseudomonas aeruginosa isolation and infection in patients with significant BDR. As we did not evaluate the extent of mucus plugging and airway wall thickness in individual lung lobes, we could not comment on whether these differences would contribute substantially to the differences in BDR. Since this was not a mechanistic study, data were not available to fully elucidate the relationship between BDR and baseline lung function or the vicious cycle (airway infection-inflammation-destruction) in bronchiectasis.
Notably, FEV1 >80% predicted should not be viewed as a predictor of the clinical characteristics of bronchiectasis, since our goal was to demonstrate that patients with and without significant BDR indeed had poorer clinical conditions than those with FEV1 >80% predicted, which reflected that the latter subgroup had a milder forms degree of bronchiectasis.
Association between BDR and the outcomes of bronchiectasis during 1-year follow-up
Throughout follow-up, patients with significant BDR presented with consistently poorer lung function. However, BDR was not an indicator of lung function decline, suggesting that the latter might be a pulmonary aging process which was independent of the airway smooth muscle tones. Nonetheless, this did not contradict with the findings in COPD, since airflow obstruction predicted more rapid lung function decline in females with COPD (25,26), as did cigarette smoking, obesity (25) and exertional desaturation (27) in male COPD patients. In light of our relatively short (1 year) follow-up period and small sample size (n=65), follow-up studies on a long-term basis are warranted to confirm these findings.
Significant BDR was, compared with non-significant BDR, associated with lower risks of experiencing the first BEs. This was surprising since we have shown that significant BDR was linked to poorer baseline lung function, which might predispose to more frequent exacerbations. So what is the most likely explanation for this phenomenon? One might postulate that the greater prescription rates of inhaled corticosteroids (46.2% vs. 23.9%) may be responsible for the differential susceptibility of experiencing BEs. This was because physicians were prone to prescribe inhaled corticosteroids and (or) β2-agonists in patients with significant BDR, which would help reduce the rate of BEs (2,27). However, the prescription rates of other medications (i.e., macrolides, theophylline) were similar or even lower in patients with significant BDR. Alternatively, significant BDR has been associated with an improved deposition of inhaled medication particles in the small airways and the ease of expectoration, which alleviates mucus plugging of smaller airways. This might help interpret the association between BDR and the reduced risks of BEs. However, whether more active prescription of inhaled corticosteroids and β2-agonists in patients with non-significant BDR would lead to reduced future risks and longer time to having BEs remains unclear.
Our study did not fully rule out the predictive roles of BDR with regards to the future risks of BEs. Inhaled corticosteroids and (or) β2-agonists might be the optimal long-term regimen for patients with significant BDR. Since our study did not investigate the association between BDR and the efficacy of medications, we were unable to comment further on the utility of BDR to indicate the therapeutic outcomes in terms of symptom and quality of life improvement and alleviation of BEs.
Limitations
Our sample size has limited the generalizability of our findings.
- A larger sample size is needed to clarify the clinical value of BDR to long-term prognosis of bronchiectasis. Due to the limited sample sizes, our findings should be interpreted with caution.
- Ipratropium was not administered following salbutamol inhalation, thus the magnitude and prevalence of BDR might have been underestimated.
- Underreporting of BEs has been fairly common and we were unable to assess the potential impacts on our data analyses without validated tools (i.e., mobile phones installed with EXACTpro questionnaire).
- The use of concomitant medications was not well balanced among the three groups: patients with FEV1 >80% predicted, patients with significant BDR, and those with non-significant BDR. However, mucolytics have no known effects on BDR; whereas patients with significant BDR had greater, not less, use of inhaled corticosteroids than their counterparts, suggesting that significant BDR was a bona fide clinical manifestation and has not been influenced by the imbalance of medication usage.
Conclusions
Significant BDR exists in a subgroup of bronchiectasis patients and is associated with poorer lung function at baseline but reduced risks of BEs. Further studies that elucidate the mechanisms responsible for BDR in bronchiectasis are of merit.
Acknowledgements
We wholeheartedly thank Dr. Yu-Hua Li (Conghua Central Hospital, Guangzhou, China) for recruiting patients.
Footnote
Conflicts of Interest: Profs. NS Zhong and RC Chen declared that they had received Changjiang Scholars and Innovative Research Team in University ITR0961, The National Key Technology R&D Program of the 12th National Five-year Development Plan 2012BAI05B01 and National Key Scientific & Technology Support Program: Collaborative innovation of Clinical Research for chronic obstructive pulmonary disease and lung cancer No. 2013BAI09B09. Dr. Guan declared that he has received National Natural Science Foundation No. 81400010 and 2014 Scientific Research Projects for Medical Doctors and Researchers from Overseas, Guangzhou Medical University No. 2014C21. None of the funding sources had any role on this study. All other authors declared no potential conflicts of interest.
Supplementary
Methods
Assessment of chest HRCT scores and disease severity assessment
Chest HRCT at collimation of 2 mm or less within 12 months was captured. Bronchiectasis was diagnosed if the internal diameter of bronchi was greater than accompanying pulmonary artery. Miscellaneous signs of bronchiectasis included the lack of normal bronchial tapering along travel on sequential slices and (or) visible bronchi within 10 mm to the pleura. The HRCT score was assessed on a lobar basis, with lingular lobe being regarded as a separate lobe. For an individual lung lobe, the radiological severity of bronchiectasis were scaled by using modified Reiff score (22). The maximal total score was 18 for a total of six lobes. Miscellaneous characteristics of HRCT, including the predominant bronchiectatic lobes, dyshomogeneity, atelectasis and infiltration, were also determined.
Bronchiectasis Severity Index (BSI) (23), a novel tool previously validated, was applied to determine the severity of bronchiectasis. The BSI was a composite of clinical parameters, including the age, body-mass index, prior exacerbations and prior hospitalization in the preceding year, Medical Research Council dyspnea score, FEV1 predicted%, P. aeruginosa infection, colonization with other PPMs and the number of bronchiectatic lobes. The cutoff values of BSI were ≤4, 5-8 and ≥9 for mild, moderate and severe bronchiectasis, respectively.
Spirometry
Spirometry was conducted by using spirometers (QUARK PFT, COSMED Co. Ltd., Milan, Italy) according to American Thoracic Society/European Respiratory Society guidelines (19). Variation between the best two maneuvers was <5% or 200 mL in FVC and FEV1, with maximal values being reported. Predicted values were derived from the model by Zheng et al. (20).
For follow-up visits, we only compared the pre-bronchodilator FEV1 and FEV1/FVC ratio.
Sputum sampling and processing
Sputum was sampled during hospital visits, between 9:00 a.m. and 12:00 a.m. Following removal of contents in oral cavity through rinsing, patients expectorated sputum in 60 mL sterile plastic container. Hypertonic saline was employed for induction if sputum yield was insufficient (18). Sputum samples with 25 leukocytes or greater and 10 epithelial cells or fewer under microscopy (×100) were deemed eligible. Two random aliquots were pipetted within 2 h for bacterial culture and ultracentrifugation (50,000 g) at 4 °C for 90 min to yield sputum sol that was subsequently stored in −80 °C freezers.
Sputum bacterial culture
Blood agar and chocolate agar plates (Biomeurix Inc., France) were applied for culture. Fresh sputum was homogenized with SPUTASOL (Oxoid SR089A, UK) and serially diluted with natural saline (10−4, 10−5 and 10−6). This was followed by addition of 10µl respective diluent to the plates with micropipette tubes and inoculation using 10 µL standardized rings. Plates were positioned in thermostatic box containing 5% carbon dioxide at 37 °C for overnight incubation.
Potentially pathogenic microorganisms (PPMs) were categorized as Pseudomonas aeruginosa (P. aeruginosa), Haemophilus influenzae, Haemophilus parainfluenzae (18) and miscellaneous clinically significant bacteria. Non-PPMs (commensals) comprised Neisseria, α-Streptococcus hemolyticus, Bacilli diphtheria and coagulase-negative staphylococcus. Prolonged culture was done for negative plates. Bacterial load, measured as colony forming unit per milliliter (CFU/mL), was reported for PPMs only. Plates with 30 to 300 CFU were counted for bacterial load.
Sputum MMPs and inflammatory mediators
Sputum sol was defrozen for at least 0.5 h before measurements.
Sputum sol MMP-8, MMP-9, IL-6, IL-8 and TNF-α were measured by using Luminex assay system (Biorad Inc., USA), which enables simultaneous measurements of multiple biomarkers. Briefly, samples were pipetted to 96-well plate coated with monoclonal antibodies for incubation for 1 h. Following plate rinsing with buffer solution, the antibody solution containing magnetic microbeads was added for further incubation. This was followed by the addition of streptavidin-phycoerythrin for 15 min. Stop solution was added to terminate reactions. Concentrations of MMP and inflammatory mediators were assessed using Bio-Plex reader (Biorad Inc., USA) and recorded as ng/mL.
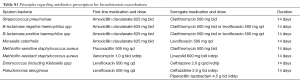
Principles regarding antibiotics prescription
See Table S1 (3). Patients with resistance to oral antibiotics, or had severe exacerbations, received intravenous antibiotics.
Full table
Results
Changes in pre-bronchodilator FEV1% predicted and FEV1/FVC after 1-year follow-up in patients with different BDRs
Changes in pre-bronchodilator FEV1% predicted and FEV1/FVC after 1-year follow-up in patients with different BDRs are compared in Table S1.
Patients with significant BDR yielded consistently lower FEV1% predicted and FEV1/FVC ratio throughout follow-up, compared with those who had non-significant BDR. Significant BDR was not associated with greater lung function decline.
References
- Barker AF. Bronchiectasis. N Engl J Med 2002;346:1383-93. [PubMed]
- Tsang KW, Ho PL, Lam WK, et al. Inhaled fluticasone reduces sputum inflammatory indices in severe bronchiectasis. Am J Respir Crit Care Med 1998;158:723-7. [PubMed]
- Pasteur MC, Bilton D, Hill AT, et al. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65 Suppl 1:i1-58. [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Characterization of lung function impairment in adults with bronchiectasis. PLoS One 2014;9:e113373. [PubMed]
- Zheng L, Lam WK, Tipoe GL, et al. Overexpression of matrix metalloproteinase-8 and -9 in bronchiectatic airways in vivo. Eur Respir J 2002;20:170-6. [PubMed]
- Roberts HR, Wells AU, Milne DG, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax 2000;55:198-204. [PubMed]
- Rowan SA, Bradley JM, Bradbury I, et al. Lung clearance index is a repeatable and sensitive indicator of radiological changes in bronchiectasis. Am J Respir Crit Care Med 2014;189:586-92. [PubMed]
- Goo HW, Yu J. Redistributed regional ventilation after the administration of a bronchodilator demonstrated on xenon-inhaled dual-energy CT in a patient with asthma. Korean J Radiol 2011;12:386-9. [PubMed]
- da Costa GM, Faria AC, Di Mango AM, et al. Respiratory impedance and response to salbutamol in healthy individuals and patients with COPD. Respiration 2014;88:101-11. [PubMed]
- Timmins SC, Diba C, Schoeffel RE, et al. Changes in oscillatory impedance and nitrogen washout with combination fluticasone/salmeterol therapy in COPD. Respir Med 2014;108:344-50. [PubMed]
- Hanania NA, Celli BR, Donohue JF, et al. Bronchodilator reversibility in COPD. Chest 2011;140:1055-63. [PubMed]
- Marín JM, Ciudad M, Moya V, et al. Airflow reversibility and long-term outcomes in patients with COPD without comorbidities. Respir Med 2014;108:1180-8. [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Effect of airway Pseudomonas aeruginosa isolation and infection on steady-state bronchiectasis in Guangzhou, China. J Thorac Dis 2015;7:625-36. [PubMed]
- Tsang KW, Tan KC, Ho PL, et al. Inhaled fluticasone in bronchiectasis: a 12 month study. Thorax 2005;60:239-43. [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Impulse oscillometry in adults with bronchiectasis. Ann Am Thorac Soc 2015;12:657-65. [PubMed]
- Gao YH, Guan WJ, Xu G, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: a prospective study. Chest 2015;147:1635-43. [PubMed]
- Guan WJ, Gao YH, Xu G, et al. Sputum bacteriology in steady-state bronchiectasis in Guangzhou, China. Int J Tuberc Lung Dis 2015;19:610-9. [PubMed]
- Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med 2013;187:1118-26. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Zheng J, Zhong N. Normative values of pulmonary function testing in Chinese adults. Chin Med J (Engl) 2002;115:50-4. [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [PubMed]
- Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000;162:1277-84. [PubMed]
- Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85. [PubMed]
- Hartl D, Griese M, Kappler M, et al. Pulmonary TH2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol 2006;117:204-11. [PubMed]
- Watson L, Vonk JM, Löfdahl CG, et al. Predictors of lung function and its decline in mild to moderate COPD in association with gender: results from the Euroscop study. Respir Med 2006;100:746-53. [PubMed]
- Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis 2012;7:95-9. [PubMed]
- Kim C, Seo JB, Lee SM, et al. Exertional desaturation as a predictor of rapid lung function decline in COPD. Respiration 2013;86:109-16. [PubMed]


