
Safety of hybrid bronchial stents in transplant airway complications: a single center experience
Introduction
Lung transplant airway complications, including bronchial stenosis, anastomotic dehiscence, excess granulation tissue and bronchomalacia, are common with a reported incidence of 15–20% and an associated attributable mortality of 2–4% (1-4). The diagnosis of an airway complication has also been associated with an overall reduced survival measured at 30 days, 1 year and 5 years (5). For patients who do survive, airway complications can lead to a significant reduction in quality of life due to increased need for medications, procedures and hospital admissions which contribute to temporal and financial constraints. Lung transplant airway complications have a wide spectrum of severity and variable response to intervention, over a third of patients will suffer a complication recurrence after intervention and nearly 70% of those will have a third (2). Bronchial stenosis is the most common post-transplant airway complication and management requires a stepwise approach with potential interventions including dilation of the stenosis, ablation of excess tissue and stent placement (6).
Management of airway stenosis with tracheobronchial stents has increased over the last three decades. Stenting is a potential intervention for recurrent stenosis after other techniques like balloon dilation have failed (7). Initial stents were comprised of silicone and required rigid bronchoscopies for placement. However, the advent of self-expandable metallic stents (SEMS) allowed stents to be placed with flexible bronchoscopy, assisted by guidewires and fluoroscopy (8,9). Unfortunately, providers noted more complications over time with metallic stents including restenosis, bacterial colonization, granulation tissue formation, stent fracture and epithelialization into the airway wall (10,11). These issues led the United States Food and Drug Administration (FDA) to issue a black box warning in 2005 against the use of SEMS in benign tracheal disease. This warning was specific to benign disease processes because of prolonged survival in this group, increasing time of implantation as well as risk of complication compared to malignant airway disease (12).
Since the 2005 FDA warning, metallic stents have evolved. The third and newest generation was designed specifically to address issues related to removal; these stents are self-expandable or balloon expandable and are fully covered (Table 1). There is limited data evaluating the safety and efficacy of third generation stents in benign airway disease, with even less data specific to transplant airway complications (13-15). Our institution has multiple years of experience utilizing covered metallic stents for post-transplant airway complications. This is the largest analysis to date of covered metallic stents in transplant airway complications; we hypothesize that covered metallic stents placed by flexible bronchoscopy have an acceptable safety profile with a low risk of major morbidity and mortality. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-2003/rc).
Table 1
| Stents | iCAST | Bonastent | AERO |
|---|---|---|---|
| Description | Stainless steel frame encapsulated in expanded PTFE | Woven Nitinol stent frame lined with a silicone cover | Nitinol stent with a polyurethane coating |
| Originally a vascular stent but has a license for bronchial stent | Anti-migration struts | ||
| Sizes diameter × length, mm | 10×30.8; 6.9×15; 6.9×20.1 | 10, 12, 14, 16, 18, 20 mm diameter; variety of lengths | 8, 10, 12, 14 mm diameter; variety of lengths down as short as 10 mm |
| Deployment | Balloon mounted can be deployed through the 2.8 mm working channel of the bronchoscope or over a guide wire | Delivery catheter with distal deployment | Gun type deployment system |
| Ability to re-capture stent prior >70% deployment | |||
| 10 mm diameter stents can be deployed through the working channel 8 Fr delivery catheter | |||
| Properties | Easy to deploy | Easy to deploy especially through the bronchoscope | 2 deployment methods over the scope or via guidewire |
| Flared ends anchor the stents well but do create granulation tissue | |||
| Easy to deploy | Conforms to airway | ||
| Fracture risk with excessive pressure | |||
| Advantages | Helpful for smaller airways | Easy to place and remove | – |
| Customizable in the airway | Conforms to airway shape |
PTFE, polytetrafluoroethylene.
Methods
This is a single center retrospective cohort study using patient data collected between April 2016 and April 2021 at Temple University Hospital in Philadelphia, Pennsylvania. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Research Ethics Board at Temple University Hospital, protocol 28636, and individual consent for this retrospective analysis was wavied. We reviewed the electronic medical record, specifically the bronchoscopy procedure notes, of all lung transplant patients who had covered metal stents placed for bronchial stenosis or anastomotic dehiscence. All stents were placed and removed with general anesthesia using flexible bronchoscopy by two proceduralists. Most of these patients (88%, n=44) were also treated with balloon dilation and some form of tissue ablation. Data specific to these ablative interventions is not included in this analysis. Follow up bronchoscopy was scheduled on a case-by-case basis or performed as needed. All patients were encouraged to perform regular airway clearance maneuvers and prescribed twice daily inhaled mucolytics. The specific covered metallic stents included in this analysis are the Bonastent® (EndoChoice Inc., Alpharetta, GA, USA), the AERO® (Merit Medical Systems, South Jordan, UT, USA), and the Atrium iCASTTM (Atrium Medical, Hudson, NH, USA). Of note, the Atrium iCASTTM is not self-expandable like the Bonastent® or the AERO®; but it is a fully covered metallic stent or hybrid metallic stent. See Table 1 for specific details about each stent type.
Information obtained from the electronic medical record include patient gender, patient age at transplantation, patient BMI at transplantation, date of lung transplant, lung transplant laterality (right, left, double), underlying diagnosis leading to lung transplant, bronchoscopy intervention date, bronchoscopy procedure description, date of stent placement, date of stent removal, type of stent, stent size, stent location, stent removal reason and any potential procedural complications. The potential etiologies for stent removal and stent complications included secretions, granulation tissue, stent migration or malposition, stent fracture or stent hemorrhage. Complications were defined as minor if they were able to managed with bronchoscopy and did not require further intervention. Complications were defined as major if they required intervention beyond bronchoscopy for management or led to direct morbidity or mortality.
Statistical analysis
The primary objective of this study is to assess the safety profile of covered hybrid metallic stents in the management of transplant airway disease. This was done by determining the incidence of each procedural complication and etiology for stent removal, the incidence of major stent related complication and by determining the total and average number of stent days each patient tolerated. More than one etiology for stent removal was allowed. Descriptive statistics including mean, median, standard deviation and range were utilized to determine the incidence of stent related complications and the number of stent days tolerated. Chi square analysis was used to determine the statistical significance of complications by stent.
Results
We identified a total of 50 patients with transplant airway disease who underwent stent placement with a covered metal stent between April 2016 and April 2021. Baseline characteristics are shown in Table 2. During this time 645 transplants were performed at our center. The mean age at time of transplantation was 63 years old. The majority of patients were male (n=35, 70%) and the most common indication for lung transplantation was interstitial lung disease (n=29, 58%). More than half of the patients (n=26, 52%) were deceased at the time of analysis. The most frequent indication for intervention was bronchial stenosis (n=44, 88%) rather than anastomotic dehiscence (n=6, 12%).
Table 2
| Patient characteristics | Values |
|---|---|
| Total patients | 50 |
| Age at transplantation, years | 63.44±7.90 |
| Male | 35 (70%) |
| Female | 15 (30%) |
| BMI at transplantation, kg/m2 | 26.91±4.88 |
| Primary diagnosis | |
| Chronic obstructive pulmonary disease | 10 (20%) |
| Interstitial lung disease | 29 (58%) |
| Pulmonary hypertension | 1 (2%) |
| Combined pulmonary fibrosis and emphysema | 10 (20%) |
| Deceased at time of analysis | 26 (52%) |
| Alive at time of analysis | 24 (48%) |
| Right lung transplant | 16 (32%) |
| Left lung transplant | 11 (22%) |
| Double lung transplant | 23 (46%) |
| Redo lung transplant | 4 (8%) |
| Indication for intervention | |
| Bronchial stenosis | 44 (88%) |
| Anastomotic dehiscence | 6 (12%) |
Values presented as mean ± standard deviation or n (%).
A total of 376 stents were placed or manipulated in 50 patients for a total of 15,711 stent days (Table 3) over the course of 774 bronchoscopies. The average patient had a median total of 176 stent days (IQR, 45.5–351.25). The median duration per stent was 22.5 days (IQR, 13–29). The most frequent reasons for stent removal were secretions (n=193), granulation tissue (n=131) and migration or malposition (n=51) as described in Figure 1. There were two major complications related to hemorrhage with one case of stent related mortality from major hemorrhage after removal; in that case there was concern that the tissue in the bronchus intermedius had thinned and there was direct bleeding from the pulmonary artery after the stent was removed. The second case involved major bleeding after a left mainstem stent was removed and balloon dilation was done. That patient went into hemorrhagic shock and ultimately required a pneumonectomy.
Table 3
| Stent characteristics | Values |
|---|---|
| Total stents | 376 |
| Total bronchoscopies involving stents | 774 |
| Bonastent® | 219 (58.24%) |
| Bonastent® length, mm | 30+10 |
| #Diameter 10 | 69 |
| #Diameter 12 | 125 |
| #Diameter 14 | 25 |
| Atrium iCASTTM | 130 (34.57%) |
| Atrium iCASTTM length, mm | 16+22 |
| #Diameter 6 | 4 |
| #Diameter 7 | 84 |
| #Diameter 10 | 42 |
| AERO® | 27 (7.18%) |
| AERO® length, mm | 15+15 |
| #Diameter 8 | 4 |
| #Diameter 10 | 4 |
| #Diameter 12 | 16 |
| #Diameter 14 | 3 |
| Days per stent | 22.5+22.9 |
| Stents per patient | 4+8.75 |
| Stent location | |
| RMSB | 147 (39.10%) |
| RUL | 33 (8.78%) |
| BI | 77 (20.48%) |
| RLL | 14 (3.72%) |
| RML | 8 (2.13%) |
| LMSB | 82 (21.81%) |
| LUL | 7 (1.86%) |
| LLL | 8 (2.13%) |
Values presented as median + IQR or n (%). #, number of stents. RMSB, right mainstem bronchus; RUL, right upper lobe; BI, bronchus intermedius; RLL, right lower lobe; RML, right middle lobe; LMSB, left mainstem bronchus; LUL, left upper lobe; LLL, left lower lobe.
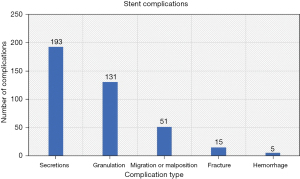
The Bonastent® was the most frequent utilized covered metal stent in our analysis (n=219, 58.24%), the least utilized was the AERO® (n=27, 7.18%). Stents were placed most frequently in the right mainstem bronchus (n=147, 39.10%) followed by the left mainstem bronchus (n=82, 21.81%) and the bronchus intermedius (n=77, 20.48%). See Table 3 for further details. Analysis of complication by stent demonstrated that each stent’s individual complication rate was similar to the overall complication rate with the exception of stent fracture which only occurred more frequently in the Bonastent® subgroup (P=0.04). See Table 4 for further details.
Table 4
| Complication | Stent | N (%) | P value |
|---|---|---|---|
| Secretions (n=193) | Bonastent® | 112 (51.14) | 0.462 |
| Atrium iCASTTM | 70 (53.85) | ||
| AERO® | 11 (40.74) | ||
| Granulation (n=131) | Bonastent® | 72 (32.88) | 0.235 |
| Atrium iCASTTM | 46 (35.38) | ||
| AERO® | 13 (48.15) | ||
| Migration (n=51) | Bonastent® | 29 (13.24) | 0.869 |
| Atrium iCASTTM | 19 (14.62) | ||
| AERO® | 3 (11.11) | ||
| Fracture (n=15) | Bonastent® | 15 (6.52) | 0.04 |
| Atrium iCASTTM | 0 | ||
| AERO® | 0 |
More than one complication was allowed for some stents. The percentage is the incidence of the complication for each stent type. Chi square analysis was used to determine statistical significance.
Discussion
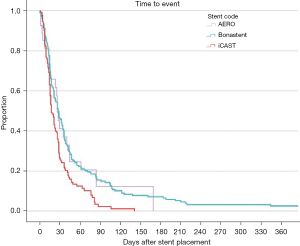
The United States FDA warned against the safety of SEMS in benign airway primarily due to concerns about safe removal after prolonged implantation (12). There were only two major complications out of the 376 stents that were placed over a total of 15,711 stent days. The average patient had a median total of 176 stent days (IQR, 45.5–351.25). The median duration per stent was 22.5 days (IQR, 13–29). See Figure 2 for more details. Mucus plugging around the stents was the most frequent reason why stents were removed in our patients (n=193). Despite differences in stent diameter (see Table 3), there was no difference in incidence of stents removed due to secretions among the different stent types (see Table 4). Although patients are instructed to perform airway clearance techniques after stent placement, mucus plugging of stents is an inevitable minor complication that can easily be treated with repeat bronchoscopy. Granulation tissue was the second most common indication for stent removal, the decision to treat granulation tissue or exchange a stent was at the discretion of the proceduralist. Subgroup analysis of complications by stent type demonstrated that individual complication rates for each stent were consistent with the overall complication rate. The exception was stent fracture which only occurred in the Bonastent® subgroup (n=15, P=0.04). However, this occurred infrequently and was not associated with major morbidity or mortality. An increased incidence of stent fracture with Bonastent® has previously been described in the management of gastric outlet obstructions (16).
In this retrospective study, we provide the largest analysis to date demonstrating the safety of hybrid covered metallic stents in transplant airway disease. Unlike prior studies reviewing metallic stents in benign airway disease, which primarily used rigid bronchoscopy, we utilized flexible bronchoscopy for placement and removal of all stents. We also have a significantly larger number of stents in our analysis, 376, as compared to 20, 40 and 47 in the papers by Dooms et al., Fortin et al. and Ma et al. respectively (13-15). One major difference in our study is that we have a significantly shorter average duration of stent placement (median: 22.5 + IQR: 29) as compared to Fortin et al. (median: 77.0±96.6 days) and Ma et al. (median: 258 + IQR: 308). We attribute this difference to multiple factors. Our entire cohort of 50 patients consists of lung transplant patients unlike Fortin et al. (14) who had only 8 post-transplant patients. Transplant patients may be more prone to mucus plugging in the immediate post operative period due to sloughing of ischemic mucosa and transplant specific medications may interfere mucociliary clearance. Secondly, stent variation between studies may account for some differences. All the stents in our cohort were covered metal stents unlike Ma et al. (15), who primarily utilized silicone stents. We would have anticipated less mucus plugging with the covered metal stent versus the silicone stents and cannot explain the contrary. The average stent duration for covered metal stents in Ma et al. (15) was shorter (median: 108 + IQR: 107) than for silicone stents (median: 296 + IQR: 317) demonstrating that stent duration is not equivalent for metallic and silicone stents. Additionally, Ma et al. study only evaluated stenting for anastamotic pathology and the majority of stents placed in Fortin et al. were placed in proximal airways (14,15). Nearly half of our stents were placed in distal airways (Table 3), the smaller lumen size may account for worse mucus plugging and may have contributed to the intensity of surveillance and revision. We believe that based on the number of hybrid metallic stents included in our cohort and use of flexible bronchoscopy, our data adds valuable evidence that this intervention can be safely utilized for lung transplant airway complications.
Our study does have several limitations. This is a retrospective cohort analysis with data that is reliant on interpretation of procedural documentation. Ideally, this would have been performed as a prospective analysis with predefined complication outcomes as well as a defined protocol for intervention and follow up bronchoscopy. Decisions regarding stent placement and other complementary procedures were at the discretion of the performing proceduralists. Furthermore, this is a single center experience and the practice patterns of our institution and the performing bronchoscopists likely impacted the results, specifically our stent duration which is shorter than what has previously been reported. It is hard to grade the severity of complications retrospectively. Additionally, our analysis does not account for variations in patient’s baseline comorbidities or for synchronous interventions that were performed to treat airway complications which certainly could have impacted the safety profile of the covered metal stents. Complementary interventions for bronchial stenosis including dilation and tissue ablation procedures like argon plasma coagulation (APC) are generally regarded as safe but can cause airway perforation and hemorrhage in rare circumstances (17).
While our study contributes significantly to the safety profile of stents, it does not include a distinct measure of clinical efficacy. Prior studies have utilized changes in spirometry, frequency of bronchoscopy and lack of follow-up after removal as markers of clinical success (13-15). Unfortunately, this data can easily be confounded by other acute complications in lung transplant patients. Given that stent placement for bronchial stenosis is largely a palliative intervention, future prospective studies should include a validated dyspnea or quality of life questionnaire to assess the true benefit of each intervention.
In conclusion, we provide the largest analysis to date of the safety of covered metal stents. Prior warnings against SEMS should be updated to reflect the enhanced safety of hybrid covered metallic bronchial stents for transplant airway complications. Future studies should account for patient specific comorbidities and the impact of other interventions, while also objectively measuring the benefit of each intervention for patients with airway complications after lung transplantation.
Acknowledgments
We acknowledge the Temple Lung Center for their support in this work.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-2003/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-2003/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-2003/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-2003/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Research Ethics Board of Temple University Hospital, protocol 28636, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;6:79-93. [Crossref] [PubMed]
- Murthy SC, Blackstone EH, Gildea TR, et al. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg 2007;84:401-9, 409.e1-4.
- Date H, Trulock EP, Arcidi JM, et al. Improved airway healing after lung transplantation. An analysis of 348 bronchial anastomoses. J Thorac Cardiovasc Surg 1995;110:1424-32; discussion 1432-3. [Crossref] [PubMed]
- Shennib H, Massard G. Airway complications in lung transplantation. Ann Thorac Surg 1994;57:506-11. [Crossref] [PubMed]
- Awori Hayanga JW, Aboagye JK, Shigemura N, et al. Airway complications after lung transplantation: Contemporary survival and outcomes. J Heart Lung Transplant 2016;35:1206-11. [Crossref] [PubMed]
- Frye L, Machuzak M. Airway Complications After Lung Transplantation. Clin Chest Med 2017;38:693-706. [Crossref] [PubMed]
- Chhajed PN, Malouf MA, Tamm M, et al. Interventional bronchoscopy for the management of airway complications following lung transplantation. Chest 2001;120:1894-9. [Crossref] [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [Crossref] [PubMed]
- Dasgupta A, Dolmatch BL, Abi-Saleh WJ, et al. Self-expandable metallic airway stent insertion employing flexible bronchoscopy: preliminary results. Chest 1998;114:106-9. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Gottlieb J, Fuehner T, Dierich M, et al. Are metallic stents really safe? A long-term analysis in lung transplant recipients. Eur Respir J 2009;34:1417-22. [Crossref] [PubMed]
- FDA. FDA public health notification: complications from metallic tracheal stents in patients with benign airway disorders, 2005.
- Dooms C, De Keukeleire T, Janssens A, et al. Performance of fully covered self-expanding metallic stents in benign airway strictures. Respiration 2009;77:420-6. [Crossref] [PubMed]
- Fortin M, Lacasse Y, Elharrar X, et al. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017;93:430-5. [Crossref] [PubMed]
- Ma KC, Li M, Haas AR, et al. Efficacy and safety of airway stenting to treat anastomotic complications after lung transplant: a cohort study. J Thorac Dis 2020;12:3539-48. [Crossref] [PubMed]
- Ye BW, Lee KC, Hsieh YC, et al. Self-Expandable Metallic Stent Placement in Malignant Gastric Outlet Obstruction: A Comparison Between 2 Brands of Stents. Medicine (Baltimore) 2015;94:e1208. [Crossref] [PubMed]
- Seaman JC, Musani AI. Endobronchial ablative therapies. Clin Chest Med 2013;34:417-25. [Crossref] [PubMed]


