Outcomes of total cavopulmonary connection for single ventricle palliation
Introduction
Fontan procedures are widely used as definitive palliation for complex cardiac anomalies with a functional single ventricle. Some modifications to the procedure have been introduced during the past decades. De Leval (1) and Marcelletti (2) et al. independently developed the concept of a lateral tunnel and extracardiac conduit total cavopulmonary connection (TCPC). Bridges and Castaneda (3) described the concept of baffle fenestration. By the 1990s, staged TCPC by a previous bidirectional Glenn shunt (BDG) was firmly established and further decreased morbidity and mortality.
In the current era, the perioperative mortality of TCPC has declined steadily to <5% (4,5). Freedom from all-cause death or transplantation in perioperative survivors was reported to be 90% at 10 years, 83% at 20 years, and 70% at 25 years (6,7). However, the incidence of postoperative pleural effusions remains high and is a key determinant of postoperative length of hospital stay (4). Studies have shown that prolonged pleural effusions correlate with late protein-losing enteropathy and decreased survival (5).
We performed a two-stage TCPC operation in high risk patients, whereas a one-stage TCPC procedure was also undertook when patients were considered to be low risk candidates. The present study aimed to review the outcomes of all patients who underwent a TCPC procedure at a single institution and to evaluate contemporary risk factors for prolonged pleural effusions.
Materials and methods
Data collection
With the approval of the institutional review board at our medical center, a retrospective analysis of data was completed in a cohort of patients who had undergone the TCPC operation from January 2008 to December 2013 at our hospital. The preoperative, operative, and postoperative clinical and hemodynamic data were obtained from in-hospital and outpatient records.
Preoperative data, such as dominant ventricular morphology, anatomic subtypes, McGoon ratio, Nakata index, and aortopulmonary collaterals (APCs) were noted from echocardiography and enhanced computed tomography records. Preoperative atrioventricular (AV) valve regurgitation was also recorded from echocardiographic information. The AV valve regurgitation was graded as absent, mild, moderate, or severe based on pulse wave tracing and color Doppler mapping. Data from cardiac catheterizations, performed before the TCPC procedure, were reviewed. Systemic oxygen saturations on room air were recorded from arterial blood gas analysis. Mean pulmonary artery pressure (mPAP), and pulmonary vascular resistance (PVR) were noted from catheterization records.
Surgical details noted included previous palliation procedures [such as pulmonary artery banding (PAB), modified Blalock-Taussig (B-T) shunt, the BDG shunt or hemi-Fontan], the mPAP under general anesthesia, Fontan types (such as extracardiac conduit or lateral tunnel), concomitant procedures done at the time of the TCPC [such as pulmonary artery angioplasty, AV valvuloplasty, and fenestration], and aortic cross-clamp times and cardiopulmonary bypass (CPB) times.
Postoperative records were reviewed for duration of ventilation time, intensive care unit (ICU) stay, hospital stay, duration of pleural effusion, oxygen saturation at hospital discharge, and perioperative morbidity and mortality. Perioperative mortality was defined as mortality before hospital discharge or death occurring within 30 days after surgery. Duration of pleural drainage was defined as total days from surgery to removal of final chest tube, performed during initial admission or during a readmission within 30 days of surgery (4). Prolonged pleural effusions were defined as effusions requiring drainage for more than two weeks via systemic treatment (such as use of targeted drugs for pulmonary hypertension, glucocorticoid or maintained the stability of colloid osmotic pressure by adding albumin). All survivors were followed-up at regular intervals by a pediatric cardiologist in our outpatient department.
Statistical analysis
All data were expressed as median and range (or 1st Q−3rd Q), mean ± standard deviation, or both. Statistical analysis was performed with SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). The Fisher’s exact test or chi-square test was used to compare categorical variables. Kaplan-Meier survival analysis was used to assess survival with the log-rank test after the TCPC procedure. Associations between all potential covariates of interest and dichotomous outcomes were evaluated in univariate testing with logistic regression. A stepwise logistic regression analysis was performed to identify independent factors for the morbidity of prolonged pleural effusions. Statistical significance was defined as a P value less than 0.05.
Results
Clinical and hemodynamic characteristic
The preoperative clinical characteristic and hemodynamic data are summarized in Table 1. There were 82 consecutive TCPC operations with 55 (67.1%) procedures performed in male patients. The median age at surgery was 3.0 years (range, 1.3−14 years; 1st−3rd quartile, 3.0−5.0 years) and weight was 14.5 kg (range, 8.9−49.5 kg; 1st−3rd quartile, 12.6−17.9 kg). Ten patients underwent the PAB procedure, and three underwent modified B-T shunts in the neonatal period as stage I palliation. Twenty-two patients had a one-stage TCPC procedure without any type of stage II palliation. Sixty patients underwent stage II palliation at a median age of 12 months (range, 3 months−12 years), including BDG shunt in 43 (52.4%), bilateral BDG in 14 (17.1%), Kawashima procedure in one (1.2%) and hemi-Fontan in two (2.4%) patients. Preoperative cardiac functional status of all candidates was New York Heart Association (NYHA) class I or II. Most patients (n=80, 97.6%) were in sinus rhythm before the Fontan procedure.
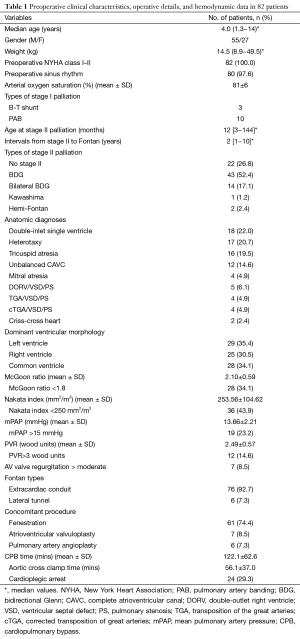
Full table
The main single ventricle diagnoses included 18 cases of a double-inlet single ventricle, 17 cases of heterotaxy (14 cases of right atrial isomerism/asplenia and 3 cases of left atrial isomerism/polysplenia), 16 cases of tricuspid atresia, 4 cases of mitral atresia, 12 cases of unbalanced CAVC, 5 cases of double-outlet right ventricle (DORV) combined with ventricular septal defect (VSD) and pulmonary stenosis (PS), 4 cases of transposition of the great arteries (TGA) combined with VSD and PS, 4 cases of corrected transposition of great arteries (cTGA) combined VSD and PS, and 2 cases of criss-cross heart. Dominant ventricular morphology was categorized as left dominant ventricle (n=29, 35.4%), right dominant ventricle including mitral atresia (n=25, 30.5%), or common ventricle (n=28, 34.1%). Accompanying malformations included the common AV valve (n=16, 19.5%), obstructive TAPVC (n=12, 14.6%), anomalous systemic venous return (n=18, 21.9%), and APCs (n=12, 14.6%).
Anomalous systemic venous return was categorized as bilateral superior vena cava in 14, interrupted inferior vena cava in one, and hepatic vein connected to right atrium independently in three patients.
Preoperative mean McGoon ratio was 2.10±0.59, and in 34.1% (n=28) of patients was less than 1.8. Mean Nakata index was 253.56±104.62 mm2/m2, and in 43.9% (n=36) of patients was lower than 250 mm2/m2. Pre-operative mPAP was 13.66±2.21 mmHg with 23.2% (n=19) higher than 15 mmHg. Seventy-five patients (91.5%) accepted cardiac catheterization, and the total PVR was 2.49±0.57 Wood units, with 14.6% (n=12) higher than 3 Wood units. A ratio of AV valve regurgitation more than moderate was present in 8.5% (n=7) of patients.
Surgical details
A summary of operative details is shown in Table 1. At the time of the BDG shunt, the azygos vein was ligated and antegrade pulmonary blood flow was preserved. The median interval between stage II palliation and TCPC procedure was 2 years (range, 1−10 years). Seventy-six (92.7%) candidates underwent extracardiac conduit Fontan procedure, whereas six patients underwent lateral tunnel. For extracardiac conduit Fontan, a polytetrafluoroethylene (PTFE) tube (Gore-Tex®; W.L. Gore & Associates, Inc., Flagstaff, Arizona, USA), with the diameter of 18−22 mm, was used to connect the inferior vena cava to the pulmonary artery. For the lateral tunnel procedure, a PTFE (Gore-Tex®) patch material was used to baffle the inferior vena cava to the Glenn cavopulmonary connection. Most of the TCPC procedure was performed on a beating heart under mild hypothermia or moderate hypothermic CPB. Cardioplegic arrest was used in 24 patients because associated intracardiac procedures were required. Mean CPB times and aortic cross clamp times were 122.1±62.6 minutes and 56.1±37.0 minutes, respectively.
A total of 61 (74.4%) patients underwent a fenestration with the diameter of 3−4 mm. Concomitant intracardiac procedures included AV valvuloplasty in 7 (8.5%) and pulmonary artery angioplasty in 6 (7.3%) patients.
Early mortality and morbidity
The perioperative outcomes of TCPC are presented in Table 2. The overall perioperative mortality was 4.9%. There were both two perioperative deaths in the one- and two-stage TCPC group. Overall the early mortality (within 30 days) in one-stage group was 9.1% compared to 3.3% in two-stage group, for a P value of 0.284. Of the two early deaths in the two-stage TCPC group, both were diagnosed with heterotaxy, right atrium isomerism and asplenia syndrome, obstructed totally anomalous pulmonary venous connection (TAPVC) and complete atrioventricular canal (CAVC). Of the two perioperative deaths in the one-stage TCPC group, one case was a 14 years old girl and died due to undetected bleeding of ovarian cyst rupture during surgery. Another death case was a 2 years and 3 months old boy, diagnosed with heterotaxy, right atrium isomerism and asplenia syndrome, and abnormal hepatic veins return, died because of postoperative low cardiac output.
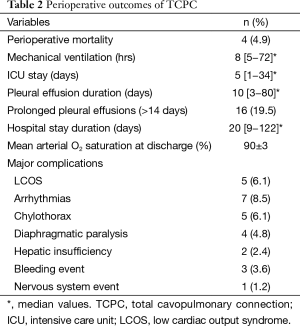
Full table
Other perioperative variables are shown in Table 2. The length of mechanical ventilation time was 8 hours (range, 5−72 hours) and ICU stay was 5 days (range, 1−34 days). The median duration of pleural effusion was 10 days (range, 3−80 days), and prolonged pleural effusions occurred in 16 (19.5%) patients. Transient supraventricular tachycardia (SVT) occurred in seven (8.5%) patients, and all converted to sinus rhythm within two weeks. No patient required implantation of a pacemaker. The duration of hospital stay was 20 days (range, 9−122 days), and all survivors were discharged with a mean arterial oxygen saturation of 90%±3%.
Risk factors associated with prolonged pleural effusion
Significant risk factors associated with prolonged pleural effusion longer than 14 days are described in Table 3. Risk factors for prolonged pleural effusion (>14 days) based on univariable analysis included obstructed TAPVC, APCs, and Mpap >15 mmHg. Multivariable analysis revealed that only mPAP >15 mmHg was independently associated with prolonged pleural effusion. Creation of a fenestration was associated with decreased odds of prolonged pleural effusion on both univariate and multivariable analysis.
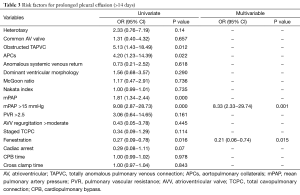
Full table
Follow up
There were two late deaths during a median follow-up of 36 months (range, 12−60 months). They both underwent a one-stage TCPC procedure. Of the two late deaths, one patient was diagnosed with heterotaxy, left atrium isomerism and polysplenia syndrome, associated with interrupted inferior vena cava. The death occurred in the first year after operation due to protein-losing enteropathy. The other death case occurred in the second year after operation with arrhythmias.
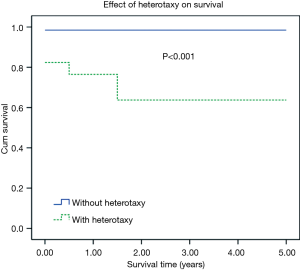
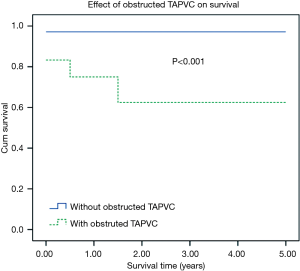
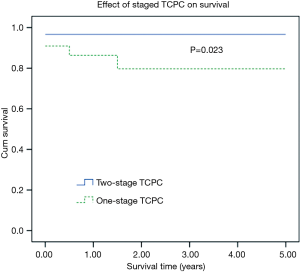
One and 5-year Kaplan-Meier survivals in the one-stage TCPC group were 86.4% and 79.7%. Respective survivals for two-stage TCPC group were 96.7% and 96.7%. There was significant difference between two groups (Figure 1, Log-rank, P=0.023). Besides, Kaplan-Meier survival analysis showed that patients with heterotaxy or obstructed TAPVC had significantly worse mid-term survival. Five-year Kaplan-Meier survivals in patients with heterotaxy was significant lower than that of in the patients without heterotaxy (98.5% vs. 63.7%, P<0.001). Five-year Kaplan-Meier survivals in patients with obstructed TAPVC was significant lower than that of in the patients without obstructed TAPVC (97.1% vs. 62.5%, P<0.001). The effects of heterotaxy or obstructed TAPVC on Kaplan-Meier survival curve of patients are presented in Figures 2,3.
During the follow-up period, no more than moderate arrhythmia, thrombosis, restenosis, or AV valve regurgitation occurred. More than 30% of fenestrations closed spontaneously with a follow-up of 1−2 years. All survivors showed preserved ventricular function and were in NYHA class I or II. All children continued to live normally with mean oxygen saturation of 91%±4%.
Discussion
In the current era, the Fontan procedure is widely used as the definitive palliation for complex cardiac anomalies with a single functional ventricle. The ultimate goal of the sequence of surgeries is to achieve optimal systemic oxygen delivery at as low a systemic venous pressure as possible. During the past decades, Choussat’s “Ten Commandments” have been constantly revised (8). In general terms, there are three essential ingredients to asuccessful Fontan: good ventricularfunction, good lungs (PVR <4 U·m2), and an unobstructed pulmonary vascular architecture (9).
In the early years after the Fontan procedure was initiated, a McGoon ratio greater than 1.8 and a Nakata index of more than 250 mm2/m2 were thought to be the minimum requirements for a successful Fontan operation. In recent decades, Fiore and colleagues (10) identified a McGoon ratio of <1.5 as a risk factor for the operation. A study by Ovroutski and colleagues also showed that a Nakata index below 150 mm2/m2 correlated with an unfavorable late result (11). Hosein et al. reviewed the late outcomes of 406 patients over a 16-year period and found that the only two significant risk factors for both early and late Fontan procedure were preoperative impaired ventricular function and mPAP >15 mmHg (12). Other studies demonstrated that a diagnosis of heterotaxy (13), significant AV valve regurgitation, and obstructed TAPVC (14), APCs (15), and longer aortic cross clamp times (5) were also associated with decreased survival.
One of the major advances of the last two decades has been the concept of staged TCPC by a previous BDG shunt. Many studies have revealed that the staged strategy can reduce morbidity and mortality, particularly for high risk Fontan candidates (16).
In the present study, we reviewed the early and mid-term results of all patients who underwent one- or two-stage TCPC operation concurrently over the last five years at a single institution, and found an overall perioperative mortality of 4.9%. The staged TCPC strategy was applied for most of the diseased children (73.2%) in this study, whose median age at the time of the stage II Glenn shunt procedure was 12 months, and significantly greater than the current mainstream age of 4−6 months. This was mainly because most of the diseased children were from remote and secluded mountain areas, and they were relatively older by the time they came in for their first hospital visits. In this study, the ages of the youngest and oldest diseased child who underwent the Glenn shunt procedure were 3 months old and 12 years old, respectively. This highlights the great age disparities of the study subjects. In fact, BDG shunt was performed in all the diseased children who had undergone neonatal palliation at our department at the age of 4−6 months. The percentage of diseased children who underwent one-stage TCPC was also relatively high. Owing to China’s natural topography, some of the diseased children from remote and secluded areas did not have regular hospital visits, and were usually over 2 years old at the time of their first hospital visits. Additionally, these families cannot afford the huge medical expenses that are associated with multiple surgeries. For these reasons, and unless combined with significant risk factors, we may discreetly choose one-stage TCPC and fenestration for these diseased children. Among the cases included in this study, the median age in one-stage TCPC group was 4.5 years (rang, 1.3−14 years; 1st−3rd quartile, 2.9−6.5 years), which seems to be older than the currently recommended age. The criteria for one-stage TCPC in our hospital included age >18 months, McGoon ratio >1.8, mPAP <15 mmHg, PVR <3 wood units, no more than moderate AV valve regurgitation, and good ventricular function. Actually, the youngest patient in the one-stage TCPC group was one year and four months old with a diagnosis of double-inlet single ventricle. Although the early mortality of one-stage TCPC was higher than that of two-stage group (9.1% vs. 3.3%), there was no significant difference, which may be associated with smaller sample size. However, five-year Kaplan-Meier survival of the one-stage TCPC group was much higher than that of two-stage group. This result maybe demonstrated the superiority of staged TCPC strategy. We also found that patients with heterotaxy or obstructed TAPVC had significantly worse mid-term survival, which was similar to many previous studies.
The superiority of the lateral tunnel over the extracardiac conduit Fontan has been debated (17). We have used an extracardiac conduit in all patients since 2009 in order to avoid cross-clamping of the aorta, sometimes without the use of CPB. There is no consensus regarding the optimal diameter of the extracardiac conduit. It is generally felt that the conduit should be replaced if the diameter is too small. However, the energy loss will increase and hemodynamic advantages will be compromised if the diameter is too large. Therefore, we performed the extracardiac conduit Fontan using a Gore-Tex® graft with a diameter of 18−22 mm. The early postoperative results showed good hemodynamic performance and there was no incidence of either stenosis or thrombosis during follow-up.
The incidence of postoperative pleural effusions after Fontan remains high and is a key determinant of length of postoperative hospital stay (4). However, the pathogenesis of the prolonged pleural effusions after the Fontan procedure is not established. Reported risk factors associated with prolonged pleural effusions include elevated preoperative mPAP (18), APCs (19), and elevated levels of aldosterone, renin and antidiuretic hormones (20). In the present study, we identified mPAP >15 mmHg as significantly associated with prolonged pleural effusions on multivariable analysis (P=0.001), and found no correlation between prolonged pleural effusions and either APCs, obstructed TAPVC, or longer CPB times.
Bridges and Castaneda described the concept of fenestrated Fontan in 1990, and demonstrated that fenestration is associated with low mortality and significantly less pleural effusions among high-risk patients (21). Although some groups continue to debate whether a fenestration is helpful in the majority of patients undergoing the Fontan procedure (22), the majority of recent reports suggests that a fenestration is helpful in increasing cardiac output, maintaining early postoperative hemodynamic stability, reducing morbidity (particularly pleural effusions), shortening hospital stays, and possibly reducing mortality at the expense of decreased oxygen saturation. Bradley recommended that fenestration should be routinely performed during the Fontan procedure (23).
Salazar et al. developed a highly selective approach to Fontan fenestration, limiting its application to only very high-risk candidates, such as patients with elevated PVR or transpulmonary gradient, significant AV valve regurgitation, poor ventricular function, intracardiac anatomy not amenable to extracardiac conduit, significant concomitant procedures, or single-lung Fontan palliation (24). We accepted this strategy in the present study and performed fenestration selectively. The rate of fenestration was 74.4% and multivariable analysis revealed that fenestration was significantly associated with a lower incidence of prolonged pleural effusions (P=0.015). It is worth mentioning that all 22 children with one-stage TCPC underwent extracardiac conduit Fontan operation, and 95.5% (n=21) of them received fenestration. The incidence rate of refractory pleural effusion was 13.6% (3/22) in one-stage TCPC cases and 21.7% (13/60) in staged TCPC cases, showing a statistically insignificant difference (P>0.05), which was also confirmed by the multivariate analysis. In addition, five cases in the present study sustained postoperatively severe low cardiac output syndrome (LCOS), which were confirmed by pulse-indicated continuous cardiac output (PiCCO, Pulsion, Munich, Germany) device. They all required an emergency shunt between the extracardiac conduit and the right atrium with a 4−8 mm Gore-Tex® graft, of which two patients required another surgical ligation of the graft three days after the shunt due to severe hypoxemia. The exact reason was unclear, and we speculated that it was associated with a transient increase in PVR due to CPB. The five patients recovered well and were discharged with an oxygen saturation of approximately 90%. This result suggested that fenestration may have had a positive effect on postoperative outcomes. There is ongoing controversy as to whether the fenestration should be closed in the catheterization laboratory. In our cohort, more than 30% of fenestrations were found to close spontaneously over the first postoperative year, similar to a previous report (25).
Study limitations
Our study had several limitations including its small sample size and the absence of long term survival data. For this reason, we did not identify independent risk factors associated with early and late mortality. However, close follow-up on our patients will continue and we will revise our results once this data becomes available. In addition, the study was retrospective and the criteria by which the patients were divided into the different groups were not standardized.
Conclusions
The present study reviewed the early and mid-term outcomes of all patients who underwent a TCPC procedure at a single institution and showed favorable results. In this cohort, we found that staged TCPC improved the early and mid-term survival of patients with a single ventricle, and patients with heterotaxy or obstructed TAPVC had significantly worse mid-term survival. Our results also revealed that Mpap >15 mmHg is independently associated with prolonged pleural effusions and a fenestration significantly correlated with a lower odds of a prolonged pleural effusion.
Acknowledgements
Funding: This work was supported by a grant from National Natural Science Foundation of China (No. 8110023).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- de Leval MR, Kilner P, Gewillig M, et al. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg 1988;96:682-95. [PubMed]
- Marcelletti C, Corno A, Giannico S, et al. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. J Thorac Cardiovasc Surg 1990;100:228-32. [PubMed]
- Bridges ND, Lock JE, Castaneda AR. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation 1990;82:1681-9. [PubMed]
- Rogers LS, Glatz AC, Ravishankar C, et al. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol 2012;60:1018-25. [PubMed]
- Hirsch JC, Goldberg C, Bove EL, et al. Fontan operation in the current era: a 15-year single institution experience. Ann Surg 2008;248:402-10. [PubMed]
- Mondésert B, Marcotte F, Mongeon FP, et al. Fontan circulation: success or failure? Can J Cardiol 2013;29:811-20. [PubMed]
- Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85-92. [PubMed]
- Stern HJ. Fontan "Ten Commandments" revisited and revised. Pediatr Cardiol 2010;31:1131-4. [PubMed]
- Petit CJ. Staged single-ventricle palliation in 2011: outcomes and expectations. Congenit Heart Dis 2011;6:406-16. [PubMed]
- Fiore AC, Turrentine M, Rodefeld M, et al. Fontan operation: a comparison of lateral tunnel with extracardiac conduit. Ann Thorac Surg 2007;83:622-9; discussion 629-30. [PubMed]
- Ovroutski S, Ewert P, Alexi-Meskishvili V, et al. Absence of pulmonary artery growth after fontan operation and its possible impact on late outcome. Ann Thorac Surg 2009;87:826-31. [PubMed]
- Hosein RB, Clarke AJ, McGuirk SP, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The 'Two Commandments'? Eur J Cardiothorac Surg 2007;31:344-52; discussion 353. [PubMed]
- Wolff D, van Melle JP, Ebels T, et al. Trends in mortality (1975-2011) after one- and two-stage Fontan surgery, including bidirectional Glenn through Fontan completion. Eur J Cardiothorac Surg 2014;45:602-9. [PubMed]
- Anagnostopoulos PV, Pearl JM, Octave C, et al. Improved current era outcomes in patients with heterotaxy syndromes. Eur J Cardiothorac Surg 2009;35:871-7; discussion 877-8. [PubMed]
- Grosse-Wortmann L, Drolet C, Dragulescu A, et al. Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: a multimodality study. J Thorac Cardiovasc Surg 2012;144:1329-36. [PubMed]
- Bridges ND, Jonas RA, Mayer JE, et al. Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation 1990;82:IV170-6. [PubMed]
- Kogon B. Is the extracardiac conduit the preferred Fontan approach for patients with univentricular hearts? The extracardiac conduit is the preferred Fontan approach for patients with univentricular hearts. Circulation 2012;126:2511-5; discussion 2515. [PubMed]
- Mascio CE, Wayment M, Colaizy TT, et al. The modified Fontan procedure and prolonged pleural effusions. Am Surg 2009;75:175-7. [PubMed]
- Glatz AC, Rome JJ, Small AJ, et al. Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging 2012;5:218-25. [PubMed]
- François K, Bové T, De Groote K, et al. Pleural effusions, water balance mediators and the influence of lisinopril after completion Fontan procedures. Eur J Cardiothorac Surg 2009;36:57-62. [PubMed]
- Bridges ND, Mayer JE Jr, Lock JE, et al. Effect of baffle fenestration on outcome of the modified Fontan operation. Circulation 1992;86:1762-9. [PubMed]
- Harada Y, Uchita S, Sakamoto T, et al. Do we need fenestration when performing two-staged total cavopulmonary connection using an extracardiac conduit? Interact Cardiovasc Thorac Surg 2009;9:50-4; discussion 54. [PubMed]
- Bradley SM. Use of a fenestration should be routine during the Fontan procedure: PRO. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2010;13:55-9. [PubMed]
- Salazar JD, Zafar F, Siddiqui K, et al. Fenestration during Fontan palliation: now the exception instead of the rule. J Thorac Cardiovasc Surg 2010;140:129-36. [PubMed]
- Atz AM, Travison TG, McCrindle BW, et al. Late status of Fontan patients with persistent surgical fenestration. J Am Coll Cardiol 2011;57:2437-43. [PubMed]