
The potential role for metformin in the prevention and treatment of tuberculosis
Tuberculosis remains an ongoing health threat worldwide (1). The ability of this intracellular bacterial pathogen to evade the host’s innate and adaptive immune responses allows it to establish persistent intracellular infections (2). Spread through respiratory droplets, the disease primarily causes lung infection; however, pulmonary tuberculosis can disseminate throughout the body, leading to devastating health consequences (3). According to the World Health Organization, nearly 2 billion people worldwide are infected with Mycobacterium tuberculosis (M. tuberculosis); this is equivalent to about a quarter of the world’s population (1). While not all individuals infected with M. tuberculosis develop disease, those with HIV, diabetes, and other risk factors, such as smoking, alcohol consumption, and malnutrition, have a higher probability of presenting with active infection (1). In 2020, an estimated 9.9 million people throughout the world developed tuberculosis, and approximately 1.3 million died with this disease (1). The countries with the highest burden of incident cases included Southeast Asia (43%), Africa (25%), the Western Pacific (18%), Eastern Mediterranean (8.3%), the Americas (3.0%), and Europe (2.3%) (1).
Drug resistance is a well-known problem in the treatment of M. tuberculosis infections. Drug-resistant tuberculous bacilli are resistant to either isoniazid or rifampicin, two first-line anti-tuberculous medications, and multidrug-resistant bacilli are resistant to both medications (1). Throughout the world, the frequency of drug-resistant and multidrug-resistant tuberculosis has remained relatively stable for over 10 years (1). In patients with an initial diagnosis of tuberculosis, 3–4% have drug resistance (1). In patients previously treated for tuberculosis, the percentage of drug-resistant cases can reach 18–21% (1). This situation indicates the need for new drug development, different treatment regimens with current medications, and/or the use of adjunctive therapy with current medications.
Diabetes mellitus (DM) triples the risk of developing tuberculous infection and leads to increased disease severity and mortality (4). The poor clinical outcomes observed in patients with diabetes and tuberculosis can be explained in part by the suppressive effects of hyperglycemia on innate and adaptive immune responses (5). As reported by the World Health Organization in 2014, diabetes affects an estimated 422 million people globally; this number continues to increase as does the number of overweight and obese individuals who are at risk to develop diabetes (6). Patients with type 2 DM have several metabolic abnormalities, including hyperglycemia, elevated pro-inflammatory cytokine levels, and increased oxidative stress (7). These effects could adversely affect host defense responses to tuberculosis, and their reversal might explain the possible benefits of some diabetic medications. For example, metformin has several host-directed therapeutic effects, including increased host-mediated autophagy responses, reduced excess inflammation, and enhanced immune cell function (7,8). Modulation of host cell responses in addition to direct targeting of the pathogen by conventional anti-tuberculous medication could lead to better treatment outcomes in patients with tuberculosis and decrease the development of drug resistance (9).
Metformin can prevent the establishment of tuberculous infections
Several studies have suggested that metformin can reduce the establishment of tuberculous infections in diabetic patients. A retrospective case-control study by Marupuru et al. (10) analyzed 451 hospitalized patients comparing diabetics on metformin, diabetics not on metformin, and healthy subjects. Infection with tuberculous bacilli occurred more frequently in diabetics with higher HbA1c levels (>8%). Metformin dosages of 500 and 1,000 mg daily both significantly reduced the risk of tuberculous infection and had significant protective effects against tuberculosis (10).
A cross-sectional study using the National Health and Nutrition Examination Survey data from the United States found that combined metformin and statin use in diabetic patients was associated with less than half the prevalence of latent tuberculous infection (LTBI) identified by positive tuberculin skin tests, suggesting that combination therapy of metformin and other medications without antimicrobial effects might reduce the risk of latent infection in some individuals (11). In this study, metformin alone did not appear to have a protective effect, but metformin with 2 or more other diabetic medications did. Singhal et al. (12) prospectively studied 220 patients with diabetes, including 62 patients with latent tuberculosis infection. In this cohort, metformin treatment was associated with reduced T-SPOT®. TB test (Oxford Immunotec, Oxfordshire, UK) reactivity when compared to patients treated with other diabetic medications (25.6% vs. 42.4%). In addition, diabetics treated with metformin had a larger number of interferon-γ secreting T cells specific for the mycobacterial culture filtrate protein-10 (CFP-10) antigen. Since the prevention of LTBI is essential to prevent tuberculosis, metformin could be considered a medication that lowers the incidence of both LTBI and tuberculous disease in diabetic patients and might be especially useful in patients with diabetes who live in regions with high case rates of tuberculosis.
Metformin may prevent the development of tuberculous disease in patients who have latent tuberculosis
In addition to reducing the frequency of LTBI, some studies have suggested that metformin can reduce the development of active disease. A retrospective cohort study by Lee et al. (13) found that diabetic patients with close contact to active cases not using metformin had significantly more incident tuberculosis than those using metformin and those without diabetes. Among 5,846 close contacts, 755 per 100,000 metformin users, 1,117 per 100,000 metformin non-users, and 526 per 100,000 healthy contacts developed active tuberculosis within 2 years following contact, indicating a protective effect of metformin against disease (13). A retrospective cohort study by Lin et al. (14) also reported a significantly lower incidence of active disease in type 2 DM patients using metformin than in type 2 DM patients not using metformin. In metformin users, a higher cumulative defined daily dose appeared to have a larger protective effect (14). Lee et al. (15) reported that patients with high-dose metformin use (>150 cumulative defined daily doses) had a significantly lower risk of incident tuberculosis than patients with low-dose metformin use (90–150 cumulative defined daily doses), but both dose ranges had protective effects against active disease. Therefore, these two studies suggest that metformin has a dose-dependent effect in the prevention of tuberculous disease in diabetic patients (14,15).
Another retrospective cohort study compared the effect of metformin and sulfonylureas, both considered first-line treatment options for type 2 DM, in reducing the risk of tuberculosis in 40,179 patients with type 2 DM (16). Two years of metformin therapy, but not sulfonylurea therapy, significantly reduced the development of tuberculosis in diabetic patients (16). Another retrospective cohort study supported these results and reported that metformin use in elderly patients (≥60 years) with DM lowered the risk for tuberculosis compared to sulfonylurea use (17). Since metformin and sulfonylureas both have similar effects on glycemic control, these studies suggest that metformin reduces the risk of tuberculosis by some mechanism other than its blood glucose lowering effect (16). Collectively, these results indicate that metformin may protect some DM patients from developing active disease and therefore might be considered a preventive measure against tuberculosis in diabetic patients. For example, it is possible that the combination of metformin and isoniazid would reduce the risk of active disease more than isoniazid alone in patients with LTBI. Testing this possibility would require a relatively simple study design but would require a large number of patients with a multiple year follow-up.
Metformin can change the disease course in active tuberculosis
Metformin also appears to modulate the course of pulmonary disease in patients with active tuberculosis, leading to enhanced immune responses and improved treatment outcomes. A retrospective cohort study by Ma et al. (18) evaluated the clinical outcomes of 58 diabetic patients either on metformin or not on metformin during standard anti-tuberculous treatment. A higher treatment success rate [93.8% vs. 71.4%, probability (P)—not significant], a higher proportion of culture conversions by the end of 2 months (87.5% vs. 71.4%, P—not significant), and lower relapse rates within 3 years (6.3% vs. 35.7%, P=0.045) occurred in patients on metformin in combination with an anti-tuberculous medication. The beneficial effect of metformin on the disease course is also supported by an observational study that examined two groups of type 2 DM patients with tuberculosis; one group received 1,000–1,500 mg metformin with insulin and anti-tuberculous medications and the other received only insulin and anti-tuberculous medications (19). One hundred percent of patients in the metformin group had negative acid-fast bacillus smears after 2 months of treatment, but only 75% of the non-metformin group had negative smears (P=0.046) (19). In addition, patients on metformin had increased autophagy based on higher serum levels of Microtubule Associated Protein 1 Light Chain 3 Beta, increased super oxide dismutase levels, and increased interferon-γ levels. Lee et al. (20) reported patients with DM and cavitary pulmonary tuberculosis taking metformin in combination with anti-tuberculous medications had significantly higher sputum culture conversion rates after 2 months of treatment compared to patients on no metformin. In these studies, metformin increased the bactericidal effects of anti-tuberculous medication and improved outcomes in diabetic patients.
Abinaya and coauthors randomized 100 nondiabetic patients with pulmonary tuberculosis into two groups (21). The control group received conventional anti-tuberculous treatment; the experimental group received the same treatment and metformin at 250 mg twice daily. The average time to sputum smear conversion was 3.4 weeks in the metformin group and 4.7 weeks in the control group (P=0.012). At the end of 2 months, the drug resistance studies indicated that 1 patient in the metformin group had resistance to rifampicin and 4 patients in the control group had resistance to anti-tuberculous medications (3 patients to rifampicin and 1 patient to isoniazid). Four patients in the control group and 6 patients in the metformin group had adverse events, mostly gastrointestinal related symptoms, such as nausea and vomiting. No significant differences in routine laboratory tests, including complete blood counts, renal function tests, and liver enzymes, occurred between the two groups. This study indicates that the use of metformin in patients on conventional therapy accelerates sputum conversion and possibly limits the development of drug resistance (21). In addition to improving clinical treatment outcomes, a prospective study of diabetic patients with tuberculosis reported that 500 mg of metformin twice daily improved the patients’ perceptions of quality of life in several domains, including symptom resolution, mental status and interest in work/activities, and exercise (22).
The attached table summarizes the clinical studies reviewed in this editorial (Table 1).
Table 1
| Author, year, country/region | Study design | Time frame | Met vs. no Met | Outcome |
|---|---|---|---|---|
| Retrospective studies | ||||
| Lee, 2018, Taiwan (15) | Retrospective: analysis of region wide data base, new diagnosis of DM; propensity matched | Incident TB, 2003–2006 | 88,866 DM cases with Met use, 88,866 DM cases with no Met use | 127 cases per 100,000 on Met, 140 cases per 100,000 no Met, HR =0.84 |
| Lee, 2019, Taiwan (13) | Retrospective: analysis of region wide database, close contacts of active case with DM and normal renal function; propensity matched | Incident TB, within 2 years of contact | Met >90 daily doses during year prior to contact date, 5,846 users | 526 cases per 100,000 in healthy controls, 755 cases per 100,000 in DM on Met, 1,117 cases per 100,000 in DM not on Met |
| Lin, 2018, Taiwan (14) | Retrospective: analysis of region wide database with new diagnosis of DM; propensity matched | Incident TB, 1998–2010 | 5,026 Met users, 5,026 non-users | Adjusted HR =2.01 (1.80–2.25) for TB in DM cases, adjusted RR =0.24 for TB with Met use |
| Marupuru, 2017, India (10) | Retrospective: case control DM with TB; DM without TB | 2015–2016 hospitalized patients | 152 cases, 299 controls | On Met, OR for TB =0.256; HbA1c <7, OR for TB =0.52 |
| Ma, 2018, China (18) | Retrospective: TB treatment centers | Treatment outcome, 3-year follow-up | 58 patients with DM, 16 on Met, 42 on no Met | Treatment success: 93.8% vs. 71.4%; culture conversion: 87.5% vs. 71.4%; relapse: 6.3% vs. 35.7% (P=0.045) |
| Lee, 2018, South Korea (20) | Retrospective: TB + DM; multivariate analysis | Treatment outcome, 1-year follow-up | 62 Met, 43 no Met | Sputum conversion higher in cavitary TB on Met, OR =10.8 |
| Pan, 2018, Taiwan (16) | Retrospective: multivariate analysis | TB incidence, 6.1 years follow-up | 3,161 on Met, 3,161 on sulfonylureas | TB incidence, adjusted HR =0.337, dose dependent reduced risk with Met |
| Park, 2019, South Korea (17) | Retrospective: patients >60 years old; propensity matching | TB incidence, 11 years follow up | 12,582 on Met, 12,582 on sulfonylurea | TB incidence 280.2 per 100,000 on Met, 394.5 per 100,000 on sulfonylurea, HR =0.74 |
| Singhal, 2014, Singapore (12) | Retrospective: TB with DM; multivariate analysis | Treatment outcome | 109 Met, 164 no Met | Met patients fewer cavities on X-ray (P=0.041), mortality—Met 3%, no Met 10% (P=0.013) |
| Prospective studies | ||||
| Novita, 2019, Indonesia (19) | Prospective study: RCT; TB + DM | Treatment outcome, 6 months TB treatment | 22 Insulin+ Met, 20 Insulin + no Met | Sputum reversion at 2 months, 22 Met vs. 15 no Met (P=0.046) |
| Abinaya, 2020, India (21) | Prospective: RCT; no DM | Treatment outcome, 6 months TB treatment | 50 Met, 50 no Met | Sputum conversion: 3.4 weeks on Met vs. 4.7 weeks on no Met (P=0.012) |
| Singhal, 2014, Singapore (12) | Prospective: DM; multivariate analysis | LTBI in cohort | 62 DM + LTBI | On Met T-spot reactivity 25.6%, on no Met T-spot reactivity 42.4%, P<0.05 |
| Mishra, 2021, India (22) | Prospective | 2018–2019, treatment outcome, 6 months treatment | 48 Met, 48 no Met | HRQoL score improved at end of intensive treatment (P<0.001) and at end of treatment (P=0.001) |
| Cross sectional studies | ||||
| Magee, 2019, USA (11) | Cross sectional study: NHANES | 2011–2012, 4,958 DM + QuantiFERON result | LTBI cohort | LTBI lower in DM plus Met + ≥2 DM drugs; LTBI lower in DM on pravastatin |
Met, metformin; TB, tuberculosis; DM, diabetes mellitus; HR, hazard ratio; RR, risk ratio; OR, odds ratio; RCT, randomized controlled trial; LTBI, latent tuberculous infection; HRQoL, Health-Related Quality of Life; NHANES, National Health and Nutrition Examination Survey.
The effect of metformin on the pathogenetic factors in tuberculosis infections
Matrix metalloproteases (MMPs), a family of enzymes with proteolytic and cell recruiting activity, contribute to lung tissue destruction and disease severity in tuberculosis (23). MMPs may contribute to pulmonary cavitation in tuberculosis, which is usually associated with a higher bacillary burden, delayed sputum culture conversion, and the development of drug resistance (23). Metformin therapy lowers systemic MMP-1, -2, -3, -7, -9 and -12 levels in diabetic patients with tuberculosis and could possibly decrease MMP-mediated tissue destruction in tuberculous patients without DM (24). Furthermore, patients with diabetes and tuberculosis have higher levels of systemic monocyte activation markers, including soluble cluster of differentiation 14 (sCD14), soluble CD163 (sCD163), soluble tissue factor (sTF), and C-reactive protein (CRP), indicating increased inflammatory responses of monocytes and macrophages contributing to tissue damage and progression of pulmonary disease (25). Specifically, Kumar et al. (25) showed that sCD14 and sCD163 levels were higher in diabetic patients with bilateral versus unilateral disease and cavitary versus non-cavitary disease and thus are associated with increased disease severity and bacterial burden. In diabetic patients on metformin therapy, the levels of sCD14, sCD163, and CRP were significantly decreased compared to those not on metformin. By modulating monocyte activation, metformin has an anti-inflammatory effect that could help decrease disease severity in these patients (25).
Several in vitro studies with peripheral blood mononuclear cells (PBMCs) have provided evidence of improved immune responses associated with metformin treatment that could provide the basis for improved clinical outcomes (12,26). Lachmandas et al. (26) studied PBMC function in blood samples from 11 healthy patients on 1,000 mg metformin twice daily. Metformin decreased the release of inflammatory markers, such as tumor necrosis factor-α, interleukin (IL)-1β, IL-6, interferon-γ, and IL-17, from PBMCs and also increased macrophages’ phagocytic capacity and reactive oxygen species (ROS) production, indicating enhanced microbicidal ability (26). The increased phagocytic and oxidative abilities of PBMCs due to metformin could reduce the ability of the mycobacterial bacilli to escape from phagolysosomes and resist oxidative bursts; this would help control disease progression in patients both with and without diabetes. In another in vitro study Singhal et al. (12) examined the human monocytic cell line THP-1 (a spontaneously immortalized monocyte-like cell line) and human monocyte-derived macrophages and found that metformin treatment inhibited intracellular growth of tuberculous bacilli by inducing mitochondrial ROS production. Both these in vitro studies suggest that increased mitochondrial ROS production associated with metformin treatment helps control tuberculous infections (12,26). Figure 1 provides an overview of the effects of metformin on host defenses relevant to tuberculous infections (12,23-26).
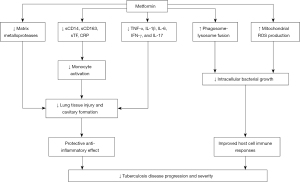
In summary, the studies used in this literature review suggest that metformin can reduce the establishment of LTBI after the exposure to active cases, reduce the progression of LTBI to active disease, modulate the course of active pulmonary disease by increasing sputum conversion rates and reducing tissue injury, and improve health-related quality of life in patients. Therefore, these studies support the use of metformin in patients, especially patients with diabetes, with tuberculous disease. Additional studies should be conducted to better evaluate metformin’s immunomodulatory effects in humans, especially when used as adjunctive therapy with first-line anti-tuberculous medication. Randomized control trials are essential to study the use of metformin in patients with both latent tuberculosis and active infection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-39/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Global tuberculosis report 2021. WHO 2021. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021
- Yew WW, Chang KC, Chan DP, et al. Metformin as a host-directed therapeutic in tuberculosis: Is there a promise? Tuberculosis (Edinb) 2019;115:76-80. [Crossref] [PubMed]
- Ryndak MB, Laal S. Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells. Front Cell Infect Microbiol 2019;9:299. [Crossref] [PubMed]
- Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008;5:e152. [Crossref] [PubMed]
- Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis (Edinb) 2013;93:S10-4. [Crossref] [PubMed]
- World Health Organization. Global report on diabetes. WHO 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf
- Restrepo BI. Diabetes and Tuberculosis. Microbiol Spectr 2016; [Crossref] [PubMed]
- Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015;15:255-63. [Crossref] [PubMed]
- Padmapriyadarsini C, Bhavani PK, Natrajan M, et al. Evaluation of metformin in combination with rifampicin containing antituberculosis therapy in patients with new, smear-positive pulmonary tuberculosis (METRIF): study protocol for a randomised clinical trial. BMJ Open 2019;9:e024363. [Crossref] [PubMed]
- Marupuru S, Senapati P, Pathadka S, et al. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Braz J Infect Dis 2017;21:312-6. [Crossref] [PubMed]
- Magee MJ, Salindri AD, Kornfeld H, et al. Reduced prevalence of latent tuberculosis infection in diabetes patients using metformin and statins. Eur Respir J 2019;53:1801695. [Crossref] [PubMed]
- Singhal A, Jie L, Kumar P, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med 2014;6:263ra159. [Crossref] [PubMed]
- Lee MC, Lee CH, Lee MR, et al. Impact of metformin use among tuberculosis close contacts with diabetes mellitus in a nationwide cohort study. BMC Infect Dis 2019;19:936. [Crossref] [PubMed]
- Lin SY, Tu HP, Lu PL, et al. Metformin is associated with a lower risk of active tuberculosis in patients with type 2 diabetes. Respirology 2018;23:1063-73. [Crossref] [PubMed]
- Lee MC, Chiang CY, Lee CH, et al. Metformin use is associated with a low risk of tuberculosis among newly diagnosed diabetes mellitus patients with normal renal function: A nationwide cohort study with validated diagnostic criteria. PLoS One 2018;13:e0205807. [Crossref] [PubMed]
- Pan SW, Yen YF, Kou YR, et al. The Risk of TB in Patients With Type 2 Diabetes Initiating Metformin vs Sulfonylurea Treatment. Chest 2018;153:1347-57. [Crossref] [PubMed]
- Park S, Yang BR, Song HJ, et al. Metformin and tuberculosis risk in elderly patients with diabetes mellitus. Int J Tuberc Lung Dis 2019;23:924-30. [Crossref] [PubMed]
- Ma Y, Pang Y, Shu W, et al. Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up. Eur J Clin Microbiol Infect Dis 2018;37:1259-63. [Crossref] [PubMed]
- Novita BD, Ali M, Pranoto A, et al. Metformin induced autophagy in diabetes mellitus - Tuberculosis co-infection patients: A case study. Indian J Tuberc 2019;66:64-9. [Crossref] [PubMed]
- Lee YJ, Han SK, Park JH, et al. The effect of metformin on culture conversion in tuberculosis patients with diabetes mellitus. Korean J Intern Med 2018;33:933-40. [Crossref] [PubMed]
- Abinaya E, Meenakshi N, Ruckmani A, et al. Clinical evaluation of efficacy and safety of metformin add-on therapy to standard ATT in newly diagnosed pulmonary tuberculosis patients. Biomed Pharmacol J 2020;13:299-309. [Crossref]
- Mishra R, Krishan S, Siddiqui AN, et al. Impact of metformin therapy on health-related quality of life outcomes in tuberculosis patients with diabetes mellitus in India: A prospective study. Int J Clin Pract 2021;75:e13864. [Crossref] [PubMed]
- Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med 2014;190:9-18. [Crossref] [PubMed]
- Kumar NP, Moideen K, Viswanathan V, et al. Elevated levels of matrix metalloproteinases reflect severity and extent of disease in tuberculosis-diabetes co-morbidity and are predominantly reversed following standard anti-tuberculosis or metformin treatment. BMC Infect Dis 2018;18:345. [Crossref] [PubMed]
- Kumar NP, Moideen K, Bhootra Y, et al. Elevated circulating levels of monocyte activation markers among tuberculosis patients with diabetes co-morbidity. Immunology 2019;156:249-58. [PubMed]
- Lachmandas E, Eckold C, Böhme J, et al. Metformin Alters Human Host Responses to Mycobacterium tuberculosis in Healthy Subjects. J Infect Dis 2019;220:139-50. [Crossref] [PubMed]


