
The transformation of atrial fibroblasts into myofibroblasts is promoted by trimethylamine N-oxide via the Wnt3a/β-catenin signaling pathway
Introduction
Atrial fibrosis is defined as the excessive deposition of collagen and extracellular matrix (ECM) protein (1). Meanwhile, atrial fibrosis is also an important pathophysiological mechanism in the development and maintenance of atrial fibrillation. A healthy heart has relatively less resident cardiac fibroblasts compared with the diseased heart, but when the cardiac fibroblast is activated by profibrotic stimulation, phenotypic transformation occurs whereby myofibroblasts are proliferated and differentiated by fibroblasts. Myofibroblasts actively produce collagen and other ECM proteins and act as the main drivers of fibrogenesis (2). Fibrosis can be induced by the excessive deposition of ECM protein, which leads to tissue dysfunction; thus, controlling the number and activity of myofibroblasts is important in inhibiting or reversing fibrosis (3). The expression of α-smooth muscle actin (α-SMA) is a marker of myofibroblast phenotype.
There is a close association among intestinal flora, diet, and cardiovascular health (4). Intestinal microorganisms convert dietary choline, betaine, phosphatidylcholine, and L-carnitine (e.g., from meat, eggs, and dairy products) into trimethylamine (TMA), which is absorbed by the intestine and delivered to the liver via portal circulation, and oxidized to trimethylamine N-oxide (TMAO) by flavin-containing liver enzyme 3 in the liver (5). TMAO is an intestinal bacteria-dependent metabolite and one of the most extensively studied microbial metabolites involved in promoting cardiac fibrosis (5-7). Previous study has shown that TMAO promotes phenotypic transformation, proliferation, and migration and increases collagen secretion in cardiac fibroblasts (8-10). The signaling pathways involved in mediating TMAO-induced pro-fibrosis include the transforming growth factor-β type I receptor (TGF-βRI/Smad2) signaling pathway (8), the TGF-β/Smad3 signaling pathway, and the activation of NOD-like receptor (NLR) pyrin domain containing 3 (NLRP3) inflammasomes (9).
The Wnt protein family is a secreted lipid-modified glycoprotein that regulates many intracellular signal transduction cascades. β-catenin is a key factor in signal transduction that mediates the canonical Wnt/β-catenin pathway (11). In the absence of Wnt ligands, β-catenin is phosphorylated in the cytoplasm and then β-catenin was ubiquitinated and degraded by proteasome, and Wnt signal transduction was inhibited (12). In the presence of Wnt ligands, Wnt ligands bind to receptors on the cell membrane, resulting in β-catenin is not degraded by phosphorylation, β-catenin accumulates in the cytoplasm and is translocated into the nucleus, where it mediates the transcription of target genes (12). The canonical Wnt pathway is usually highly conserved, which activates the β-catenin pathway by binding extracellular Wnt ligands to membrane receptors via an autocrine/paracrine approach, which is ultimately involved in promoting cell proliferation, differentiation, and migration (12).
The Wnt/β-catenin pathway plays a key role in cardiac fibrosis and cardiac fibroblasts activation (13). To date, 19 Wnt proteins have been identified in humans, among which Wnt3a and Wnt1 act as ligands that activate the Wnt/β-catenin signaling pathway after the translation and modification (12,14,15). Previous study has shown that targeting the Wnt3a/β-catenin cell pathway regulates atrial tissue fibrosis in a rat model of atrial fibrillation (16). Additionally, in vitro, the Wnt3a/β-catenin pathway is involved in promoting phenotypic transformation (i.e., the transformation of fibroblasts to myofibroblasts), collagen secretion, and the migration of fibroblasts (15,17,18).
To date, no research has been conducted on the Wnt/β-catenin pathway in study examining the pro-fibrosis of cardiac fibroblast phenotypic transformation induced by TMAO. Thus, this study sought to explore the role of the Wnt3a/β-catenin pathway in the phenotypic transformation of atrial fibroblasts promoted by TMAO. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-475/rc).
Methods
Isolation and culture of atrial fibroblasts from primary newborn rabbits
Newborn New Zealand rabbits (1–3 days old) were anesthetized and sacrificed. The atria from the heart was taken, quickly finely minced, and digested with 2.5 g/L of trypsin (Gibco, Invitrogen, California, USA) and 2 g/L of Collagenase Type II (Gibco). The subsequent supernatant was collected and centrifuged at 1,000 rpm for 5 min and cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Gibco) at 37 ℃. The subsequent supernatant were left for 90 min to attach differentially, and the atrial fibroblasts were first attached and then cultured in new culture flasks. After 2–3 generations, atrial fibroblasts were used for experiments. To assess the effect of TMAO (Sigma-Aldrich CAS1184-78-7, Missouri, USA) on cultured cardiac fibroblasts, the atrial fibroblasts were treated with different concentrations (100 or 200 µM) of TMAO. The control group was treated with PBS only. This study was approved by the Animal Ethics Committee of Chinese PLA General Hospital (No. 2020-X16-100). All the procedures in this study were performed in accordance with the institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Cell proliferation assays
Cell viability was measured to detect cell proliferation by the cell counting Kit-8 (CCK8; Biyuntian Biological Company, China). The cells were seeded in 96-well plates at 5,000 cells/well (200 µL). The cells were treated with different concentrations (100 or 200 µM) of TMAO for 24 and 48 h, respectively. Next, CCK8 (20 µL) was immediately added to each well, and the cells were incubated for 2 h at 37 ℃. The absorbance was measured at 450 nm (OD450) by a microplate reader.
Cell migration assays
The migration assays were performed by a Transwell chamber (24-well, 8.0-µm, Cornin Company, China). The cells (1.5×104) were seeded in the upper chamber in serum-free DMEM medium. The complete medium contained different TMAO concentrations (100 or 200 µM), which was added to the bottom wells. After incubation at 37 ℃ for 24 h, the cells were removed from the upper chamber, and the cells were stained by adding 0.1% crystal violet. The cells were counted under a microscope, and 5 random images were taken of each chamber.
Western blot
The collected atrial fibroblasts were lysed by radioimmunoprecipitation protein extraction reagent containing protease inhibitors (Beyotime, Shanghai, China). Next, equal amounts of proteins were added to sodium dodecyl sulfate polyacrylamide gel for electrophoresis, and the proteins were transferred to polyvinylidene fluoride membranes. Subsequently, the membranes were blocked with 5% non-fat dry milk in tris-buffered saline with Tween (TBST) and incubated with primary antibodies overnight at 4 ℃. The next day, the corresponding secondary antibodies were added, and the membranes were washed. Enhanced chemiluminescence imaging was then performed, and the optical density was quantified. The main primary antibodies included Collagen I (1:2,000, Invitrogen, California, USA), TGF-β1 (1:1,000, Invitrogen, California, USA), α-SMA (1:1,000, abcam, Cambridge, UK), Wnt3a (1:1,000, Aviva Systems Biology, San Diego, USA) and β-catenin (1:1,000, Aviva systems biology, San Diego, USA), while glyceraldehyde 3-phosphate dehydrogenase (1:2,500; Solarbio, Beijing, China) was set as a control.
Immunocytochemistry
The atrial fibroblasts were fixed in 4% paraformaldehyde and treated with 0.5% Txiton X-100 for 20 min (at room temperature). Next, they were sealed with 10% TBST and goat serum. Subsequently, the cells were incubated with a α-SMA antibody (1:200, Abcam), Col-I (1:2,000, Invitrogen), and β-catenin (1:1,000, AVIVA SYSTEMS BIOLOGY) overnight in the dark, respectively. The cells were then washed twice, and incubated with Goat Anti-Mouse IgG H&L (heavy chain and light chain) (Alexa Fluor® 488) secondary antibody pre-adsorbed (1:200, ab150117, abcam, Cambridge, UK) or Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) secondary antibody pre-adsorbed (1:200, ab150081) for 2 h at room temperature in the dark in accordance the manufacturer’s instructions. The nucleus staining was conducted by 4',6-diamidino-2-phenylindole. Images were obtained using a confocal laser scanning microscope (Leica, Germany). Mean fluorescence Intensity (MFI) was measured by ImageJ software (National Institutes of Health, Bethesda, MD, USA). The MFI was calculated by the estimated total fluorescence per field, and a total of 5 different fields were calculated.
Statistical analysis
All the data are expressed as the mean ± standard deviation. The statistical analysis of the data was performed using GraphPad Prism 8 software (La Jolla, CA). Significant differences between the 2 groups were determined using the unpaired Student’s t-test test for the treatment groups. Comparison among groups were conducted by a one-way analysis of variance, followed by the Tukey test. A P value <0.05 was considered statistically significant.
Results
TMAO promotes the proliferation and migration of atrial fibroblasts
The effects of different concentrations of TMAO on the proliferation of atrial fibroblasts was detected using the CCK-8 method. The results are shown in Figure 1A. Regardless of whether TMAO was co-cultured with atrial fibroblasts for 24 or 48 h, the absorbance of the TMAO cells was observed to be significantly increased compared to that of the control group, which indicates that TMAO promotes the proliferation of the atrial fibroblasts. Additionally, Transwell assays were conducted to detect the response of the atrial fibroblasts to different doses of TMAO. The results are shown in Figure 1B. Compared to the control group, the number of cells migrating from the Transwell chamber to the lower chamber was significantly increased in the 100 and 200 µM treatment groups. Thus, the results indicated that TMAO enhances the migration ability of atrial fibroblasts.
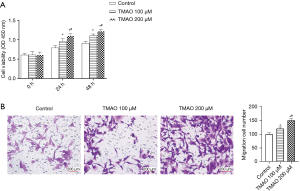
TMAO promotes the phenotypic transformation and collagen deposition of atrial fibroblasts
The phenotypic transformation of atrial fibroblasts, which increases collagen secretion, and ECM imbalance are important pathophysiological mechanisms of atrial fibrosis. The α-SMA is a fibroblast marker of myofibroblast transformation, and Collagen I is an important component of the ECM. The Western blot results showed that compared to the control group, TMAO promoted a significant increase in the expression of α-SMA and Collagen I. Additionally, the increase was more significant at a concentration of 200 µM (see Figure 2A-2C). The immunofluorescence results revealed that TMAO promoted the expression of α-SMA (see Figure 2D,2E). Thus, TMAO appears to promote the phenotypic transformation and collagen deposition of atrial fibroblasts.
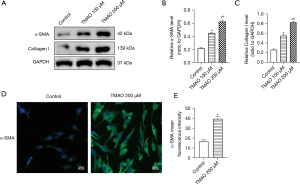
In conclusion, the proliferation, migration, phenotypic transformation, and collagen secretion of atrial fibroblasts is promoted by TMAO, which indicates that TMAO promotes cardiac fibrosis by regulating the function of atrial fibroblasts.
According to the above CCK-8 and Transwell assay results, the most significant effect on the proliferation and migration of atrial fibroblasts occurred at a concentration of 200 µM of TMAO, which significantly promoted the expression of α-SMA and Collagen I. Thus, 200 µM of TMAO was selected as the concentration for the subsequent experiments.
TMAO activates the β-catenin pathway and promotes the phenotypic transformation, migration, and collagen secretion of atrial fibroblasts
TMAO promotes the transformation of cardiac fibroblasts into myofibroblasts (i.e., phenotypic transformation), and the Wnt3a/β-catenin signaling pathway is involved in the regulation of atrial fibrosis; however, the role of the Wnt3a/β-catenin signaling pathway in the profibrotic process of TMAO is unclear. The protein expression of the atrial fibroblasts was examined after 48 h of co-culturing with TMAO. The Western blot results revealed that compared to the control group, the expression of Wnt3a and β-catenin in atrial fibroblasts was significantly increased in the TMAO group (see Figure 3A-3C). The expression of α-SMA, Collagen I, and TGF-β1 was also significantly increased (see Figure 3A,3D-3F). Additionally, the immunofluorescence results suggested that nuclear translocation occurred due to increased β-catenin expression and nuclear accumulation in the atrial fibroblasts after the TMAO intervention (see Figure 3G), which suggested that the Wnt3a/β-catenin signaling pathway was activated. The expression of α-SMA and Collagen I was also significantly increased in the atrial fibroblasts of the TMAO group (see Figure 3H-3I). These results suggest that TMAO promotes the phenotypic transformation and collagen secretion of atrial fibroblasts by promoting Wnt3a/β-catenin pathway activation.
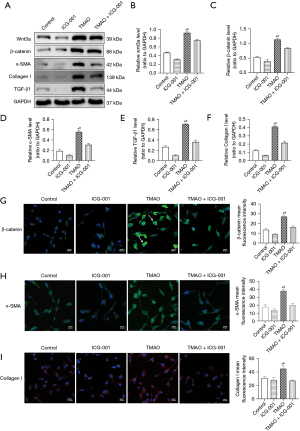
Blocking the β-catenin pathway inhibits the migration, phenotypic transformation, and collagen secretion of atrial fibroblasts induced by TMAO
ICG-001 (10 µM, Selleckchem, Houston, TX, USA) is a small molecule inhibitor that specifically inhibits β-catenin–mediated gene transcription (19). Changes in the effects of TMAO on atrial fibroblasts were observed by inhibiting the β-catenin pathway by adding ICG-001. The Western blot results revealed that compared to the TMAO group, the atrial fibroblasts in the TMAO + ICG-001 group not only decreased the protein expression of Wnt3a and β-catenin, but also significantly inhibited the expression of α-SMA and Collage I (see Figure 3A-3D,3F). Additionally, the immunofluorescence revealed that β-catenin expression and its nuclear translocation were inhibited in atrial fibroblasts in the TMAO + ICG-001 group compared to the TMAO group (see Figure 3G), while the expression of α-SMA and Collagen I was also significantly inhibited (see Figure 3H-3I). The increased migration of atrial fibroblasts is a feature of myofibroblasts, and we found that TMAO enhanced the migration capacity of the atrial fibroblasts. The inhibition effect of the β-catenin pathway on atrial fibroblast migration was further assessed. Compared to the TMAO treatment alone, the number of cells migrating from the Transwell to the lower chamber was significantly reduced after the co-treatment of TMAO and ICG-001 (P<0.05; see Figure 4), which suggests that the migration ability of atrial fibroblasts promoted by TMAO is attenuated by the inhibition of the β-catenin pathway. In conclusion, these results suggest that blocking the β-catenin pathway inhibits TMAO-induced migration, phenotypic transformation, and collagen secretion in atrial fibroblasts.

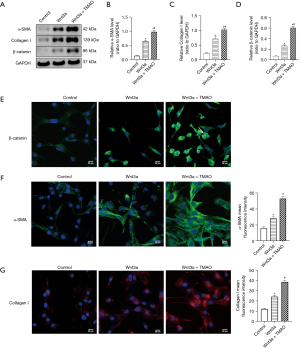
Exogenous Wnt3a and TMAO synergistically promote the activation of the β-catenin pathway and phenotypic transformation in atrial fibroblasts
To examine whether Wnt3a and TMAO had a synergistic effect on the atrial fibroblasts, atrial fibroblasts with exogenous Wnt3a were treated for 24 h. The Western blot results showed that the expression of β-catenin was more upregulated in the exogenous Wnt3a group than the control group (P<0.05). Further, the Wnt3a and TMAO co-treatment significantly increased the expression of β-catenin compared to the Wnt3a treatment alone (P<0.05). Additionally, exogenous Wnt3a upregulated the expression of α-SMA and Collagen I compared to the control group (P<0.05). The Wnt3a and TMAO co-treatment significantly increased the protein expression of α-SMA and Collagen I compared to the Wnt3a treatment alone (P<0.05; see Figure 5A-5D). When β-catenin expression was assessed by immunofluorescence, we found that β-catenin expression and translocation to the nucleus in atrial fibroblasts were promoted by Wnt3a, compared to the controls. Additionally, the nuclear translocation was further strengthened by the co-treatment of Wnt3a with TMAO (see Figure 5E). The immunofluorescence experiments showed that the co-treatment of Wnt3a with TMAO significantly increased the protein expression of α-SMA and Collagen I, compared to the Wnt3a treatment alone (see Figure 5F-5G). In conclusion, these results suggest that exogenous Wnt3a and TMAO synergistically promote the activation of the β-catenin pathway and phenotypic transformation in atrial fibroblasts.
Discussion
We first investigated the effects of TMAO on atrial fibroblasts in this study, and found that TMAO promotes the proliferation and migration of atrial fibroblasts, and the transformation of fibroblasts into myofibroblasts (i.e., phenotypic transformation), and increases collagen secretion. In response to the above phenomena and to explore the mechanism by which this occurs, we conducted further experiments and found that TMAO activates the β-catenin pathway and promotes phenotypic transformation and collagen secretion in atrial fibroblasts. However, these changes could be reversed by inhibiting the β-catenin pathway. Additionally, we found that TMAO promotes the increase of Wnt3a protein expression in atrial fibroblasts, and exogenous Wnt3a and TMAO synergistically promote the activation of the β-catenin pathway and phenotypic transformation in atrial fibroblasts. Thus, Wnt3a/β-catenin signaling plays an important role in mediating atrial fibroblast activation and matrix production induced by TMAO. To the best of our knowledge, this is the first time to reveal that TMAO can pass Wnt3a/β-catenin signaling pathway promotes the phenotypic transformation of cardiac fibroblasts. This is the first study to reveal the mechanism by which the Wnt/β-catenin pathway mediates promotes the phenotypic transformation of cardiac fibroblasts by TMAO. Our results can be used as a basis for further research to elucidate the potential effects of TMAO on atrial fibrosis.
Clinical studies have shown that circulating TMAO levels are closely associated with various cardiovascular diseases (such as coronary heart disease, heart failure, and hypertension) (20-22), and indicate a poor prognosis for cardiovascular disease patients (23). Fibroblast proliferation, migration, and phenotypic transformation constitute the major hallmarks of tissue fibrosis. First, we investigated the effects of TMAO on the proliferation and migration of atrial fibroblasts. These effects play an important role in the process of atrial fibrosis. Preclinical study has shown that TMAO promotes cardiac hypertrophy and fibrosis through the TGF-β1/smad3 signaling pathway (24). TMAO was shown to exacerbate bad left ventricular remodeling in mice with myocardial infarction, and in-vitro experiments suggest that the transformation of cardiac fibroblasts into myofibroblasts is induced by the activation of TGF-βRI/Smad2 (8). Additionally, TMAO aggravates doxorubicin-induced cardiac fibrosis and relies on TGF-β/Smad3 signaling and the activation of NLRP3 inflammatory bodies to proliferate, migrate, and secrete collagen in cardiac fibroblasts (9). Our study also confirmed that TMAO promotes the transformation, proliferation, and migration of atrial fibroblasts into myofibroblasts in vitro, and that the activation of the Wnt3a/β-catenin pathway also plays an important role.
TGF-β1 is a known pro-fibrogenic factor. TMAO promotes TGF-β1 expression upregulation in atrial fibroblasts, and the β-catenin pathway inhibition downregulates TGF-β1 expression, which suggest that TGF-β1 may be regulated by the Wnt/β-catenin pathway. Our findings are consistent with previous study that have found an interaction between Wnt/β-catenin and TGF-β/smad signaling in controlling gene transcription and cell phenotype (15,25). Notably, TGF-β1 has also been shown to reverse regulate Wnt/β-catenin to promote the phenotypic transformation of fibroblasts (14,26). However, regardless of the upstream and downstream relationship between TGF-β/smad and Wnt/β-catenin signaling regulation, we found that TMAO promotes the protein expression of Wnt3a, β-catenin, and β-catenin pathway activation (nuclear translocation). Moreover, the inhibition of β-catenin pathway significantly affects the profibrotic effect of TMAO on atrial fibroblasts, such that the expression of α-SMA, Collagen I, and migration ability are inhibited. These findings suggest that the Wnt3a/β-catenin signaling pathway plays an important role in mediating the phenotypic transformation process of atrial fibroblasts induced by TMAO. Our important findings also complement mechanistic research on cardiac fibrosis mediated by TMAO. In conclusion, TMAO appears to promote the activation of cardiac fibroblasts and cause cardiac fibrosis via the activation of multiple profibrotic signaling pathways.
Previous study has found conflicting results as to whether exogenous Wnt3a directly contributes to the phenotypic transformation of cardiac fibroblasts (15). The cardiac fibroblasts of Wnt3a-treated mice showed transformation to myofibroblasts (i.e., phenotypic transformation) (15,27). However, two studies have shown that exogenous Wnt3a does not lead to the transformation of cardiac fibroblasts to myofibroblasts (17,26). In the present study, we found that exogenous Wnt3a promotes the phenotypic transformation of atrial fibroblasts and increases the secretion of Collagen I. However, the differences in the conclusions reached by various studies may be due to the different activation states of the fibroblasts under different culture conditions, the isolation procedures or cell sources, or the different treatment concentrations of Wnt3a. Consistently, when Wnt3a was co-treated with other stimulating factors (e.g., TGF-β1), it was shown to synergistically increase the phenotypic transformation of fibroblasts (17).
It is currently believed that there is heterogeneity in cardiac fibroblasts, and fibroblasts in different anatomical locations are phenotypically and functionally different (28), which may be related to the different microenvironments in which the cells are located. A direct comparison of ventricular fibroblasts and atrial fibroblasts revealed differences between these 2 cells, including different growth characteristics, secretory, and proliferative functions, different responses to growth factors, and differences in the gene expression (29). In vitro, compared to the ventricular fibroblasts, the atrial fibroblasts proliferated more significantly in response to growth factors, such as platelet derived growth factor (PDGF) and TGF-β1, a higher expression α-SMA was observed, and the atrial fibroblasts appeared to show a more significant fibrotic and proliferative response to injury (29). Additionally, the significant differences in marked atrial fibrosis and ventricular fibrosis were observed in animal models with characteristic over-expressions of the pro-fibrotic factor TGF-β1 (30,31).
In previous studies (8,9) on the effects of TMAO on cardiac fibroblasts, ventricle-derived fibroblasts were selected, and it was obviously inappropriate to extrapolate the effect on ventricular fibroblasts to atrial fibroblasts. The major difference between this study and previous study may be that atrial fibroblasts rather than ventricular fibroblasts were selected as the study subjects. In this study, atrial fibroblasts were selected as the study subjects to reflect the potential association between TMAO and atrial fibrosis better; however, our findings should be further verified in animal models. As a member of the Wnt family, Wnt3a acts on cells in an autocrine manner (27), which is consistent with the findings of this study. It could be that the expression of Wnt3a in atrial fibroblasts cultured in vitro increases under TMAO induction and decreases when β-catenin is blocked, which would indicate that the expression of Wnt3a in atrial fibroblasts is regulated by β-catenin and acts on fibroblasts in an autocrine-like manner.
In conclusion, our study demonstrated that TMAO promotes the proliferation and migration of atrial fibroblasts, promotes the phenotypic transformation of atrial fibroblasts and increases collagen secretion by activating the Wnt3a/β-catenin signaling pathway. These results suggest that there may be a link between TMAO and atrial fibrosis, which is critical in the pathophysiological mechanism of atrial fibrillation or atrial cardiomyopathy. Further research needs to be conducted with animal models to validate these findings.
Acknowledgments
Funding: The study was supported by Beijing Natural Science Foundation (No. Z141100002114050).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-475/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-475/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-475/coif). All authors report that the study was supported by Beijing Natural Science Foundation (No. Z141100002114050). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. This study was approved by the Animal Ethics Committee of Chinese PLA General Hospital (No. 2020-X16-100). All the procedures in this study were performed in accordance with the institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kurose H. Cardiac Fibrosis and Fibroblasts. Cells 2021;10:1716. [Crossref] [PubMed]
- Reese-Petersen AL, Olesen MS, Karsdal MA, et al. Atrial fibrillation and cardiac fibrosis: A review on the potential of extracellular matrix proteins as biomarkers. Matrix Biol 2020;91-92:188-203. [Crossref] [PubMed]
- Gourdie RG, Dimmeler S, Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov 2016;15:620-38. [Crossref] [PubMed]
- Thomas MS, Fernandez ML. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr Atheroscler Rep 2021;23:12. [Crossref] [PubMed]
- Zhang Y, Wang Y, Ke B, et al. TMAO: how gut microbiota contributes to heart failure. Transl Res 2021;228:109-25. [Crossref] [PubMed]
- Huang R, Yan L, Lei Y. The Gut Microbial-Derived Metabolite Trimethylamine N-Oxide and Atrial Fibrillation: Relationships, Mechanisms, and Therapeutic Strategies. Clin Interv Aging 2021;16:1975-86. [Crossref] [PubMed]
- Witkowski M, Weeks TL, Hazen SL. Gut Microbiota and Cardiovascular Disease. Circ Res 2020;127:553-70. [Crossref] [PubMed]
- Yang W, Zhang S, Zhu J, et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J Mol Cell Cardiol 2019;134:119-30. [Crossref] [PubMed]
- Li X, Geng J, Zhao J, et al. Trimethylamine N-Oxide Exacerbates Cardiac Fibrosis via Activating the NLRP3 Inflammasome. Front Physiol 2019;10:866. [Crossref] [PubMed]
- Kapetanaki S, Kumawat AK, Persson K, et al. The Fibrotic Effects of TMAO on Human Renal Fibroblasts Is Mediated by NLRP3, Caspase-1 and the PERK/Akt/mTOR Pathway. Int J Mol Sci 2021;22:11864. [Crossref] [PubMed]
- Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017;169:985-99. [Crossref] [PubMed]
- Liu J, Xiao Q, Xiao J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 2022;7:3. [Crossref] [PubMed]
- Tao H, Yang JJ, Shi KH, et al. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolism 2016;65:30-40. [Crossref] [PubMed]
- Xiang FL, Fang M, Yutzey KE. Loss of β-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun 2017;8:712. [Crossref] [PubMed]
- Carthy JM, Garmaroudi FS, Luo Z, et al. Wnt3a induces myofibroblast differentiation by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent manner. PLoS One 2011;6:e19809. [Crossref] [PubMed]
- Lv X, Li J, Hu Y, et al. Overexpression of miR-27b-3p Targeting Wnt3a Regulates the Signaling Pathway of Wnt/β-Catenin and Attenuates Atrial Fibrosis in Rats with Atrial Fibrillation. Oxid Med Cell Longev 2019;2019:5703764. [Crossref] [PubMed]
- Działo E, Czepiel M, Tkacz K, et al. WNT/β-Catenin Signaling Promotes TGF-β-Mediated Activation of Human Cardiac Fibroblasts by Enhancing IL-11 Production. Int J Mol Sci 2021;22:10072. [Crossref] [PubMed]
- Działo E, Rudnik M, Koning RI, et al. WNT3a and WNT5a Transported by Exosomes Activate WNT Signaling Pathways in Human Cardiac Fibroblasts. Int J Mol Sci 2019;20:1436. [Crossref] [PubMed]
- Methatham T, Tomida S, Kimura N, et al. Inhibition of the canonical Wnt signaling pathway by a β-catenin/CBP inhibitor prevents heart failure by ameliorating cardiac hypertrophy and fibrosis. Sci Rep 2021;11:14886. [Crossref] [PubMed]
- Ge X, Zheng L, Zhuang R, et al. The Gut Microbial Metabolite Trimethylamine N-Oxide and Hypertension Risk: A Systematic Review and Dose-Response Meta-analysis. Adv Nutr 2020;11:66-76. [PubMed]
- Salzano A, Cassambai S, Yazaki Y, et al. The Gut Axis Involvement in Heart Failure: Focus on Trimethylamine N-oxide. Heart Fail Clin 2020;16:23-31. [Crossref] [PubMed]
- Heianza Y, Ma W, DiDonato JA, et al. Long-Term Changes in Gut Microbial Metabolite Trimethylamine N-Oxide and Coronary Heart Disease Risk. J Am Coll Cardiol 2020;75:763-72. [Crossref] [PubMed]
- Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948-56. [Crossref] [PubMed]
- Li Z, Wu Z, Yan J, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest 2019;99:346-57. [Crossref] [PubMed]
- Li T, Weng X, Cheng S, et al. Wnt3a upregulation is involved in TGFbeta1-induced cardiac hypertrophy. Cytokine 2021;138:155376. [Crossref] [PubMed]
- Blyszczuk P, Müller-Edenborn B, Valenta T, et al. Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur Heart J 2017;38:1413-25. [PubMed]
- Zhao Y, Wang C, Wang C, et al. An essential role for Wnt/β-catenin signaling in mediating hypertensive heart disease. Sci Rep 2018;8:8996. [Crossref] [PubMed]
- Soliman H, Rossi FMV. Cardiac fibroblast diversity in health and disease. Matrix Biol 2020;91-92:75-91. [Crossref] [PubMed]
- Burstein B, Libby E, Calderone A, et al. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation 2008;117:1630-41. [Crossref] [PubMed]
- Nakajima H, Nakajima HO, Salcher O, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res 2000;86:571-9. [Crossref] [PubMed]
- Rahmutula D, Marcus GM, Wilson EE, et al. Molecular basis of selective atrial fibrosis due to overexpression of transforming growth factor-β1. Cardiovasc Res 2013;99:769-79. [Crossref] [PubMed]


