Differing histopathology and prognosis in pulmonary adenocarcinoma at central and peripheral locations
Introduction
Lung cancer is the leading cause of cancer death globally (1), and adenocarcinoma is the most common histologic type (2). Often situated at the periphery of the lung, pulmonary adenocarcinoma commonly displays a mix of microscopic features (3). A newly proposed classification of adenocarcinoma subtypes has been formulated jointly by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) (3). According to revised criteria, five major histopathologic patterns (acinar, papillary, micropapillary, solid and lepidic) are recognized and occur in varying ratios as tumor components. Furthermore, each of these elements impacts prognosis differently (4,5).
Using these microscopic parameters, the impact of morphology on prognosis in adenocarcinoma is currently under investigation (6-9). However, no study as yet has linked microscopic features and prognosis with tumor location (i.e., central vs. peripheral). Among non-small cell lung cancers, squamous cell carcinoma (SqCC) is classifiable by primary location as central (encompassing most of these cancers) or peripheral type. One particular study has found that SqCC differs by location, noting a better prognosis in peripheral tumors, and that peripheral SqCC is on the rise (10). Other sources have also elaborated upon clinicopathologic and biologic variability in central and peripheral types of SqCC (11,12), although more work is clearly needed. Central and peripheral adenocarcinoma is subject to such variability as well.
This study was conducted to compare clinicopathologic characteristics of central and peripheral adenocarcinoma, exploring pathologic and biologic differences primarily through micromorphologic analysis. In addition, location-related differences in degree of tumor differentiation and patient prognosis were investigated.
Patients and methods
Patients
Between August, 2010 and December, 2013, a total of 486 patients diagnosed with non-small cell lung cancer (NSCLC) underwent complete curative resection at Seoul St. Mary’s Hospital in Korea. Of these, 321 patients with adenocarcinoma qualified for retrospective chart review. After excluding 13 patients who were given induction chemotherapy prior to surgery (possibly altering tumor characteristics), 308 patients were ultimately included in the study. TNM staging was based on the 7th American Joint Committee on Cancer (AJCC) guidelines (13). Operative procedures included wedge resection, segmentectomy, lobectomy, bilobectomy, and pneumonectomy. Systemic lymph node dissection (en bloc) or sampling (partial node resection) was carried out in most instances, encompassing more than three mediastinal lymph node stations. Tumor recurrence anywhere within ipsilateral hemithorax was considered locoregional equating distant recurrence with extrathoracic involvement. Any pulmonary nodule harboring a lepidic growth pattern was viewed as metachronous (rather than recurrenct) lung cancer. This study was approved by the institutional Review Board of Seoul St. Mary’s Hospital (The Catholic University of Korea).
Histologic evaluation
Central lung lesions were defined as a tumor location limited to the trachea, bronchi, or segmental bronchi; and peripheral lesions as a tumor location limited more to the periphery than the subsegmental bronchi (10,11,14,15). Pathology reports of all specimens were rendered by certified pathologists, and adenocarcinoma subtyping adhered to the 2011 revised classification (IASLC/ATS/ERS). In particular, five major proliferative patterns (acinar, papillary, micropapillary, solid, and lepidic) of tumors were quantified microscopically in 5% increments (3).
Statistical analysis
Clinicopathologic characteristics of central and peripheral adenocarcinoma at all stages were compared, conducting the same comparison for selectively for stage-I adenocarcinoma. Student’s t-test was used for continuous variables, and χ2 test was applied for categorical variables. Follow-up data for the interval between surgical resection and last follow-up visit were analyzed, using confirmed recurrences/deaths to calculate recurrence-free survival (RFS) via Kaplan-Meier method. Survival of each group was compared by log-rank test, and the Cox proportional hazards model of multivariate analysis was engaged to determine risk of recurrence in stage I pulmonary adenocarcinoma. A value of P<0.05 was considered statistically significant.
Results
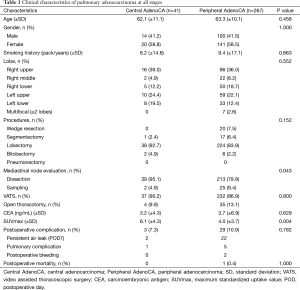
Among 308 patients with pulmonary adenocarcinoma at all stages, 41 (13.3%) lesions were central, and 267 (86.7%) were peripheral. Clinical characteristics of study population are summarized in Table 1, stratified by central and peripheral tumor locations. These population subsets were similar in age, male-to-female ratio, and smoking history. Right upper lobe was most often affected in both groups (39.0% and 36.0%, respectively), and lobectomy was primarily performed (92.7% and 83.9%, respectively). Although some resections were limited [central adenocarcinoma: segmentectomy, 1 (2.4%); peripheral adenocarcinoma: wedge resection, 20 and segmentectomy, 17 (13.9%)], the groups did not differ significantly by extent of surgery (P=0.152). Mediastinal node evaluation was done routinely (100% vs. 89.2%; P=0.043), and video-assisted thoracoscopic surgery (VATS) usually took place (90.2% and 86.9%, respectively). Mean maximum standardized uptake value (SUVmax) of fluorodeoxyglucose on positron emission tomography (PET) was higher in central (vs. peripheral) lesions (6.1 vs. 4.0; P=0.004).
Full table
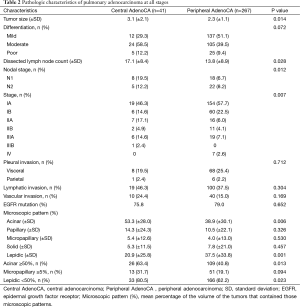
In comparing pathologic characteristics of pulmonary adenocarcinoma (Table 2), central lesions surpassed peripheral lesions in terms of mean size (3.1 vs. 2.3 cm; P=0.014), nodal metastasis rate (P=0.012), and incidence of advanced disease (stages II and III; P=0.007). However degrees of tumor differentiation (P=0.072) and local invasion (pleural; P=0.072, lymphatic; P=0.304; vascular; P=0.169) were similar. With respect to patterns of tumor proliferation, central lesions differed significantly from peripheral lesions, diverging in mean percentages of acinar (53.3% vs. 38.9%; P=0.006) or lepidic (20.9% vs. 37.5%; P=0.001) growth by tumor volume.
Full table
To better assess tumor subsets, clinicopathologic characteristics of patients with stage-I pulmonary adenocarcinoma [central, 25 (10.5%); peripheral, 214 (89.5%)] were compared (Table 3). Age, gender, smoking history, tumor site (lobe), extent of surgery, mediastinal node evaluation, CEA, and SUVmax did not differ by group. Although mean size of central tumors tended to be larger (2.4 vs. 2.0 cm), statistical significance was not reached (P=0.091), and degrees of differentiation were similar for these subsets. Mean dissected lymph node count for central adenocarcinoma also exceeded that of peripheral adenocarcinoma (16.8 vs. 12.8; P=0.035), but extent of invasion (pleural and lymphatic/vascular) and the frequency of EGFR mutation were comparable, as were observed percentages of microscopic growth patterns.
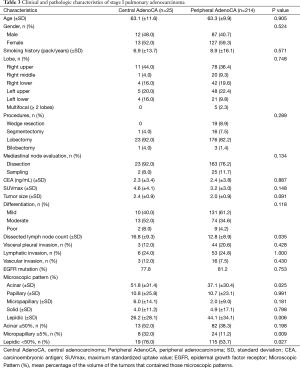
Full table
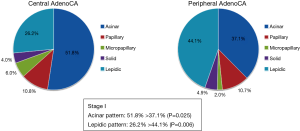
In stage I, central tumors typically displayed more acinar (51.8% vs. 37.1%; P=0.025) and less lepidic (26.2% vs. 44.1%; P=0.006) growth (Figure 1). Micropapillary growth ≥5% and lepidic areas <50% bode poorly in adenocarcinoma as prognostic indices (4,7) and were documented more often in central (vs. peripheral) tumors (micropapillary ≥5%: 32% vs. 11.2%, P=0.009; lepidic <50%: 76% vs. 53.8%, P=0.027).
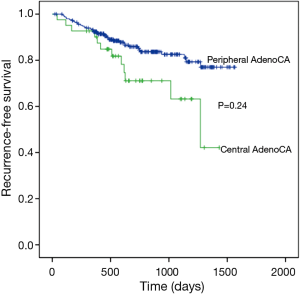
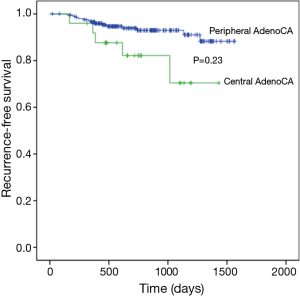
Median follow up time for all patients was 727 days (range, 12–1564 days) and recurrences were recorded in 50 patients. Three-year RFS rates in central and peripheral adenocarcinoma differed significantly (63.2% vs. 82.5%; P=0.024) (Figure 2), although advanced disease (stages II and III) was more frequent in central adenocarcinoma, so a valid comparison could not be made. Instead, 3-year RFS rates were compared at stage I only. During follow-up monitoring, 19 (7.9%) of 239 patients suffered recurrences [central adenocarcinoma, 5 (2.1%) (locoregional, 2; distant, 2; both, 1); peripheral adenocarcinoma, 14 (5.8%) (locoregional, 7; distant, 4; both, 3)]. Three-year RFS rates of central and peripheral adenocarcinoma differed significantly (70.4% vs. 91.0%; P=0.023) (Figure 3), with better outcomes in the peripheral adenocarcinoma subset.
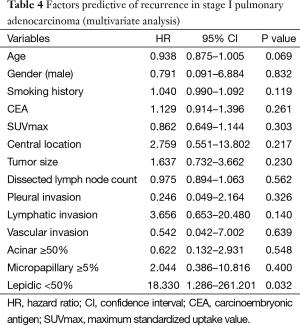
Multivariate analysis, using the Cox proportional hazards model to determine factors associated with recurrence of stage-I pulmonary adenocarcinoma (Table 4), indicated that lepidic growth <50% posed a statistically significant risk (HR=18.330, 95% CI: 1.286–261.210; P=0.032). The between-group difference recorded in RFS is thus explainable. Lepidic growth fell below the 50% threshold significantly more often in central adenocarcinoma. Although central location did not impact RFS to a statistically significant extent, differing lepidic growth in central and peripheral tumors may explain the disparate prognoses observed by location in early-stage disease.
Full table
Discussion
Pulmonary adenocarcinoma generally occurs in peripheral lung tissue, although centrally located primary tumors are not uncommon. In this study, central lesions accounted for 13.3% of all patients undergoing surgical resection. Despite the fact that central and peripheral adenocarcinoma are believed to differ fundamentally, there has been no formal documentation to date confirming this precept, and at least some of our data seem to refute this. For example, one may assume a link between central adenocarcinoma and smoking (16), but our patient subsets were similar in terms of smoking history. In comparing all stages of central and peripheral adenocarcinoma, central adenocarcinoma was often more advanced (i.e., larger primary tumor) and more likely to involve nodal metastasis. On the basis of patient outcomes, however, it could not be said that central adenocarcinoma behaves more aggressively, but it is likely to present at a more advanced stage. In terms of the major microscopic patterns used for classification purposes, only acinar and lepidic growth differed significantly by tumor location. Lepidic elements are apt to appear in early-stage pulmonary adenocarcinoma, which explains their relative scarcity in more advanced tumors that are centrally located.
For better insight, central and peripheral tumors at stage I only were compared. Again, clinicopathologic characteristics generally did not differ, with the exception of a significant disparity in microscopic features. As in pulmonary adenocarcinoma overall, stage I central tumors displayed more acinar growth patterns (likely to invade) and fewer lepidic areas (unlikely to invade). In other words, higher malignant potential may be ascribed to central adenocarcinoma, given its more invasive attributes (4). Furthermore, micropapillary growth ≥5% and lepidic content <50%, both imparting a poor prognosis (4,7,17-20), were more frequently seen in central (vs. peripheral) adenocarcinoma. Thus, microscopic features of central adenocarcinoma signaled a comparatively worse prognosis for stage I disease (and therefore heightened malignant potential), even though other clinicopathologic characteristics of central and peripheral lesions were similar.
RFS rates were compared overall and at stage I. Although 3-year RFS of central adenocarcinoma was significantly lower than that of peripheral adenocarcinoma overall, the fact that central adenocarcinoma presented a generally higher stages than those of peripheral adenocarcinoma undermined our comparison. Indeed, central adenocarcinoma was detected as a more advanced cancer in many cases. However, 3-year RFS for stage I disease also proved significantly lower for central (vs. peripheral) adenocarcinoma, indicating a comparatively worse prognosis. In central tumors, lymphatic and vascular spread may be facilitated by topography, but at stage I, nodal metastasis is not an issue―thus, our focus on early disease. Furthermore, pathologic characteristics (size; tumor differentiation; invasion of pleura, lymphatics, and blood vessels) did not differ significantly by tumor subset at stage I disease. Consequently, prognostic differences in early-stage central and peripheral pulmonary adenocarcinoma were attributed to micromorphologic divergence.
Using the Cox proportional hazards model for multivariate analysis, risk factors for 3-year recurrence of pulmonary adenocarcinoma at stage I disease were assessed. Accordingly, a lepidic growth pattern <50% was identified as the sole statistically significant prognosticator for recurrence (HR=18.330; P=0.032). In central PA, fewer lepidic areas amounting to <50% of tumor were found by comparison. Although central location did not impact RFS to a statistically significant extent, differing lepidic growth in central and peripheral tumors may explain the disparate prognoses observed by location in early-stage disease. Thus it appears that central adenocarcinoma poses a greater risk of recurrence than does peripheral adenocarcinoma, based on microscopic attributes.
Disease-specific prognosis better reflects cancer-related RFS, as opposed to overall survival. Moreover, overall survival is a poor gauge of prognosis in stage I disease comparisons, because deaths are less likely a direct result of cancer (4). For this study, comparison of cancer-specific prognosis was our aim, examining RFS overall and separately at stage I. Given that our data was so recent, 3-year survival was the only option for analyzing prognosis. Our facility initiated VATS fairly recently, starting in August, 2010. Due to inconsistencies in technique, surgical data generated before that date was disqualified, thus limiting our resources. In addition, efforts made since then to ensure staging accuracy included mediastinal lymph node dissection or sampling in most patients. Likewise, standard histomorphologic assessments at our facility as of 2011 incorporated the new IASLC/ATS/ERS international multidisciplinary classification of lung adenocarcinoma (transitioning with some patients in late 2010). Thereafter, accuracy and consistency of recorded data was ensured. Comparing prognosis via 3-year RFS seemed reasonable, knowing that postoperative recurrences of non-small cell lung cancer typically occur within 2 years and that any cancer surfacing after 2 years has a good possibility of being metachronous (21). Of note, a related study has documented that 2-year recurrence reflects overall prognosis of surgically resected lung cancer, especially in early stage disease (22).
Peripherally situated adenocarcinoma enabled wedge resection of suitably small lesions, especially ground glass opacity nodules. Due to access issues, adenocarcinoma in central locations usually required lobectomy, regardless of tumor size or opacity. If more than lobectomy was needed, mediastinal lymph node evaluation (dissection or sampling) was routinely performed at the same time in most central tumor resections resulting in higher nodal counts for greater accuracy of staging. Hence, operative procedure had no bearing on the lower RFS rate we determined for central adenocarcinoma.
Some researchers also maintain that the clinicopathologic and prognostic profiles of central and peripheral SqCC in the lung are fundamentally different (10,11). However, studies comparing central and peripheral adenocarcinoma have been few, and none has addressed the inherent micromorphologic heterogeneity of these tumors. Staging of esophageal cancers (SqCC), which sometime differ in tumor progression and prognosis, reflects tumor location (13). Perhaps tumor location is a matter of importance in the staging of non-small cell lung cancers as well. Of course large-scale clinical studies and molecular-level biologic investigation would be needed for corroboration.
A number of study limitations are acknowledged, the first being that this was retrospective review conducted at a single center. The small patient sampling with fewer instances of central adenocarcinoma also may have introduced bias. Greater data accrual through multicenter studies may remedy this problem. As already explained, the short follow-up duration was also a weakness. Regardless of the importance assumed by pathologic features of pulmonary adenocarcinoma, RFS was limited to a 3-year period rather than the customary 5-year interval relied upon for prognostication. Still, most recurrences of non-small cell lung cancer are known to occur within a 2-year time frame (21), and early recurrence has been shown to mirror extended prognosis (22).
In conclusion, centrally located pulmonary adenocarcinoma is detected in an advanced stage for many patients. Even with standard resection and mediastinal lymph node dissection, central tumors overall and in stage I disease have a poorer early prognosis than peripheral lesions. This disparity is related to the microscopic features of these cancers, particularly a notable departure in extent of lepidic growth. With a pursuit of large-scale micromorphologic and molecular biologic studies, the impact of location on intrinsic properties of adenocarcinoma may be better understood.
Acknowledgements
This article has been edited by native English-speaking experts of BioMed Proofreading, LLC.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Parkin DM, Ferlay J, Curado MP, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer 2010;127:2918-27. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [PubMed]
- Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Oncol 2014;12:388. [PubMed]
- Ito M, Miyata Y, Kushitani K, et al. Prediction for prognosis of resected pT1a-1bN0M0 adenocarcinoma based on tumor size and histological status: relationship of TNM and IASLC/ATS/ERS classifications. Lung Cancer 2014;85:270-5. [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [PubMed]
- Sasada S, Nakayama H, Miyata Y, et al. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann Thorac Surg 2015;99:956-60. [PubMed]
- Zhang J, Wu J, Tan Q, et al. Why do pathological stage IA lung adenocarcinomas vary from prognosis?: a clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol 2013;8:1196-202. [PubMed]
- Kinoshita T, Ohtsuka T, Hato T, et al. Prognostic factors based on clinicopathological data among the patients with resected peripheral squamous cell carcinomas of the lung. J Thorac Oncol 2014;9:1779-87. [PubMed]
- Funai K, Yokose T, Ishii G, et al. Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung. Am J Surg Pathol 2003;27:978-84. [PubMed]
- Saijo T, Ishii G, Nagai K, et al. Differences in clinicopathological and biological features between central-type and peripheral-type squamous cell carcinoma of the lung. Lung Cancer 2006;52:37-45. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- Shimosato Y, Suzuki A, Hashimoto T, et al. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol 1980;4:365-73. [PubMed]
- Sakurai H, Asamura H, Watanabe S, et al. Clinicopathologic features of peripheral squamous cell carcinoma of the lung. Ann Thorac Surg 2004;78:222-7. [PubMed]
- Gonzalez M, Vignaud JM, Clement-Duchene C, et al. Smoking, occupational risk factors, and bronchial tumor location: a possible impact for lung cancer computed tomography scan screening. J Thorac Oncol 2012;7:128-36. [PubMed]
- Chao L, Yi-Sheng H, Yu C, et al. Relevance of EGFR mutation with micropapillary pattern according to the novel IASLC/ATS/ERS lung adenocarcinoma classification and correlation with prognosis in Chinese patients. Lung Cancer 2014;86:164-9. [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [PubMed]
- Sumiyoshi S, Yoshizawa A, Sonobe M, et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer 2013;81:53-9. [PubMed]
- Zhang J, Liang Z, Gao J, et al. Pulmonary adenocarcinoma with a micropapillary pattern: a clinicopathological, immunophenotypic and molecular analysis. Histopathology 2011;59:1204-14. [PubMed]
- Tremblay L, Deslauriers J. What is the most practical, optimal, and cost effective method for performing follow-up after lung cancer surgery, and by whom should it be done? Thorac Surg Clin 2013;23:429-36. [PubMed]
- Kiankhooy A, Taylor MD, LaPar DJ, et al. Predictors of early recurrence for node-negative t1 to t2b non-small cell lung cancer. Ann Thorac Surg 2014;98:1175-83. [PubMed]