Update on anti-angiogenic therapy in non-small cell lung cancer: Are we making progress?
Department of Medical Oncology, Mater Misericordiae University Hospital, Eccles Street, Dublin 7, Ireland
|
Review Article
Update on anti-angiogenic therapy in non-small cell lung cancer: Are we making progress?
Department of Medical Oncology, Mater Misericordiae University Hospital, Eccles Street, Dublin 7, Ireland
|
|
Abstract
Non-small cell lung cancer (NSCLC) remains a leading cause of death worldwide among patients diagnosed with malignancy. Despite new chemotherapy regimens and new cytotoxic combinations investigated in multiple clinical trials in recent years, no significant improvement in the prognosis of patients with lung cancer was achieved. The five-year survival rate for all patients diagnosed with NSCLC is about 15%, only 5% better than that of more than 40 years ago. New therapeutic approaches that target various different aspects of tumor progression and metastasis are of particular interest in to NSCLC patients. Drugs that block tumor vascularization (angiogenesis) or interfere with the activity of growth factor receptors and molecular pathways that are triggered by activation of these receptors are already used in clinical practice. In this review we will briefly discuss briefly the basic mechanisms of lung cancer angiogenesis, rationale for using drugs that block this process and present the most current recent data on their clinical efficacy.
Key words
lung cancer; VEGF; angiogenesis; antiangiogenesis
J Thorac Dis 2011;3:19-29. DOI: 10.3978/j.issn.2072-1439.2010.11.11
|
|
Introduction
Over one million patients worldwide are diagnosed with non-small cell lung cancer (NSCLC) each year and nearly all of them will die from their malignancy (1). Although in most cases this is a preventable malignancy, lung cancer is the number one cause of cancer-related mortality both in women and men. The five-year survival rate for patients with stage I-IV according to TNM classification system is about 15%, which in the past 40 years improved only by about 5% (2). There are many factors that may be responsible for such a poor outcome, including no effective screening method, late diagnosis when disease is already clinically advanced and surgery with curative intent is not possible.
The adverse biology of lung cancer progression and metastasis is also a recognized factor that contributes to the grim prognosis since only about 65% patients were with stage IA disease according to the 7-th edition of TNM classification survive 5 years after curative surgery (3). Significant progress has been made in the recent years in understanding the molecular mechanisms of lung cancer. Multiple pathways those are active in NSCLC progression and metastasis are identified (4). The only two molecular pathways so far targeted therapeutically in clinical practice are epidermal growth factor (EGF) and the vascular endothelial growth factor (VEGF) pathways. In this review we will focus predominantly therapeutic implications of inhibiting VEGF and VEGF-related pathways in NSCLC patients.
|
|
Tumor angiogenesis
In 1971, Dr. Judah Folkman put forward the theory that malignant tumors cannot grow beyond a certain size without recruiting their own blood vessels (tumor angiogenesis) through a process that involved production of a soluble growth factor that was secreted by the tumor itself (5). He also proposed that the local tumor growth and formation of distant metastases can be prevented by inhibiting the tumor angiogenesis. Although controversial at that time, Judah Folkman’s hypothesis ignited extensive research into the molecular mechanisms of tumor angiogenesis and the ways of blocking it with the therapeutic intent in patients with cancer.
Although the list of growth factors that induce tumor angiogenesis is very long, the most important molecular player of tumor angiogenesis is vascular endothelial growth factor A (VEGF-A). VEGF was discovered in 1983 by Harold Dvorak and Donald Senger (6). In 1989, both the structure and the genetic sequence of VEGF was deciphered by Napoleone Ferrara’s and Daniel Connolly groups, respectively (7,8).
VEGF is the primary survival factor of vascular endothelial cells (ECs), stimulates proliferation and migration, inhibits apoptosis and modulates their permeability. VEGF belongs to a family of growth factors that includes VEGF-B, -C, -D, -E and placental growth factor (PlGF) (9). The biological functions of VEGF are mediated upon binding to receptor tyrosine kinases: vascular endothelial growth factor receptors-1, -2 and -3 (VEGFR1, 2, 3) (10,11). VEGFR2 is the key mediator of VEGF-driven angiogenesis. VEGFR1 and VEGFR3 are involved in embryonic vessel development (vasculogenesis) and formation of the lymphatic vascular network, respectively (Fig 1) (12,13).
Expression of VEGF within tumors is regulated by multiple factors including the level of oxygen within the tumor, growth factors and cytokines produced by the tumor, and mechanisms involving oncogene activation/tumor suppressor inactivation (14). Hypoxia in the tumor microenvironment is however one of the most important factors driving VEGF production during tumor growth and progression (15,16).
|
|
Mechanisms of action of anti-angiogenic agents
There is no single mechanism which explains how anti-angiogenic agents actually work in terms of combating cancer (17). According to Judah Folkman’s hypothesis, blocking vessel growth impairs substantially or abolishes completely formation of blood vessels, which slows tumor growth and causes the tumor to regress to a clinically undetectable “state of dormancy” (5).
While this hypothesis may explain most of the preclinical data, in clinical setting when tumor is discovered, it has already established its own vascular network. However, not all the tumor vessels are created equal. Only a fraction of tumor blood vessels are associated intimately with pericytes which makes them more functional and stable when compared with vessels lacking that support (18). Pericyte-covered tumor vessels are usually located at the periphery of the tumor and do not depend on VEGF as a survival and growth factor. In contrast, a majority of tumor blood vessels are not supported by pericyte coverage and in result present as tortuous, leaky, and immature. These are dependent on continuous stimulation by growth factors including VEGF to survive and grow. Upon VEGF deprivation, these vessels regress, while stable, pericyte-covered vascular network remains mostly unaffected. Therefore, after anti-VEGF therapy tumor vasculature that remains consists of a higher percentage of mature, pericyte-covered blood vessels that provide more efficient perfusion of the tumor. This process known as “normalization of tumor vasculature” was proposed by Rakesh Jain who hypothesizes that anti-VEGF therapy may transiently improve blood flow within the tumor and enhancing the delivery of chemotherapy (19,20). This is not only observed in the animal and pre-clinical models but also in clinical setting where efficacy of radiation therapy was increased the due to transient improvement in tumor oxygenation as a result of anti-angiogenic treatment (17).
|
|
Clinical applications of anti-angiogenic therapy
Upon binding to its receptors, VEGF triggers a cascade of molecular events that drives tumor angiogenesis (Fig 1). Generally, there are two major concepts with respect to interfering with the VEGF pathway – blocking the activation of extracellular part of VEGF receptor by neutralizing/blocking of VEGF molecule or blocking the activation of tyrosine kinase within the intracellular part of VEGF receptor by tyrosine kinase inhibitors (TKIs) (21).
Bevacizumab is a humanized, monoclonal antibody that binds to VEGF. It is the first anti-angiogenic agent that demonstrates survival benefit in patients with metastatic colorectal cancer, when added to standard chemotherapy (22).
Aflibercept is a recombinant fusion protein (VEGF-trap) that works similarly to bevacizumab and binds with high affinity to VEGF and PlGF. It is not an antibody, but a “molecular construct” that consists of the extracellular domains of VEGFR1 and VEGFR2 fused to the Fc region of human IgG (23).
TKIs, unlike monoclonal antibodies or fusion proteins, are small molecules that interfere directly with tyrosine kinase activity (Fig 2). The intracellular domain of the receptor targeted by TKIs is structurally similar in many tyrosine kinase receptors, thus a single TKI interferes with the activity of multiple receptors (24).
|
|
Efficacy of anti-VEGF therapy in lung cancer
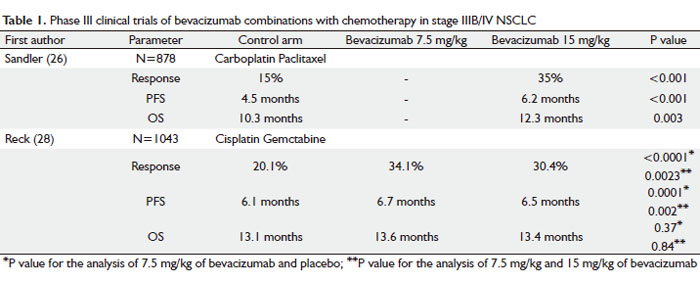
In 2004, a phase II clinical trial investigated the use of bevacizumab in newly diagnosed stage IIIB/IV or recurrent NSCLC patients (25). Patients were randomized to receive bevacizumab at 7.5 or 15 mg/kg with carboplatin (area under the curve; AUC = 6) and paclitaxel 200 mg/m2 chemotherapy every 3 weeks or chemotherapy alone. The primary end-points were the median time to progression (TTP) and tumor response rate (RR). Patients who received higher dose of bevacizumab combined with chemotherapy showed improved TTP (7.4 months) when compared with those underwent either chemotherapy alone (4.2 months) or the lower dose of bevacizumab (4.3 months). RR was also higher in patients who received higher dose of bevacizumab (31.5%) when compared with chemotherapy alone arm (18.8%). There was no statistically significant difference in overall survival (OS) in all three arms, however the trial was not powered to address the question of survival benefit. Bevacizumab was overall well tolerated, with hypertension, proteinuria, thrombotic events and grade 3/4 leukopenia most commonly reported in patients who received bevacizumab. The most notable side-effect of bevacizumab in this phase II study was the increased frequency of bleeding events. The bleeding in all patients had two distinct patterns: mucocutaneous that was minor and did not require changes in treatment protocol, or major hemoptysis/pulmonary hemorrhage that was fatal in four out of six symptomatic patients. Further analysis showed that the majority of patients (67%) who had serious pulmonary bleeding had tumors with squamous-cell histology and the tumors were centrally-located in close vicinity to major blood vessels. In future clinical trials with bevacizumab, patients with squamous histology were excluded from participation based on this data.
Following the encouraging results of the phase II study, Eastern Cooperative Oncology Group conducted a phase III clinical trial (E4599) in 878 patients with stage IIIB/IV NSCLC using higher dose of bevacizumab (15 mg/kg) combined with chemotherapy as investigational arm vs chemotherapy alone (Table 1) (26). Patients were randomized to receive chemotherapy alone (control arm), carboplatin (AUC = 6) and paclitaxel (200 mg/m2) or chemotherapy with bevacizumab at 15 mg/kg once every three weeks. After completion of 6 cycles of treatment, patients receiving bevacizumab with chemotherapy continued on bevacizumab as a single agent until disease progression or intolerable toxicities occurred. OS was the primary end point in this study. Based on the safety issues that were raised in the phase II study, patients with squamous histology, brain metastases, clinically significant hemoptysis or receiving anticoagulation therapy were excluded from the study.
The study met its primary end-point and the combination of bevacizumab and chemotherapy resulted in the significant improvement in median survival by 2 months when compared with chemotherapy alone group, 12.3 vs 10.3 months respectively. The median PFS and the RR were also statistically significantly better in combination group vs chemotherapy alone group, 6.2 vs 4.5 months, and 35% vs 15%, respectively. Even in this highly selected group of patients (non-squamous histology, no hemoptysis, no anticoagulation treatment), patients who received bevacizumab combined with chemotherapy experienced higher rates of clinically significant bleeding when compared with patients who received chemotherapy alone, 4.4% vs 0.7%, respectively. There were 15 treatment-related deaths in the chemotherapy-plus-bevacizumab group, and 10 out of 15 patients died of hemorrhagic or thromboembolic complications in this group (5 from pulmonary hemorrhage, 2 from gastrointestinal bleeding, 2 from a stroke and 1 of pulmonary embolus). In the control arm there were two deaths related to toxic effects of therapy.
Retrospective analysis of the outcome in patients over 70 years old who received bevacizumab showed significantly higher rates of side effects and no PFS or OS benefit when compared with younger patients (27).
The AVAiL trial investigated similar approach as ECOG 4599 study did in 1043 stage IIIB/IV NSCLC patients, comparing cisplatin/gemcitabine chemotherapy alone vs chemotherapy combined with bevacizumab (Table 1) (28). Highly selected patients with non-squamous tumors, with no history of hemoptysis, brain metastases, tumor invading major vessels, uncontrolled hypertension, spinal cord compression, thrombotic or hemorrhagic conditions within the past 6 months from randomization were randomized to receive cisplatin 80 mg/m2 and gemcitabine 1250 mg/m2 for up to six cycles plus low-dose bevacizumab (7.5 mg/kg), high-dose bevacizumab (15 mg/kg), or placebo every 3 weeks until disease progression. Although the study was powered for OS, the primary end point was changed from OS to PFS during accrual. Median PFS improved upon adding bevacizumab to chemotherapy both in 7.5 mg/kg (6.7 months) and 15 mg/kg dose group (6.5 months) when compared with chemotherapy alone (6.1 months). The most surprising and puzzling finding in the AVAiL study was no survival benefit associated with adding bevacizumab to standard chemotherapy as was shown in ECOG 4599 trial. No clear explanation for this disappointing result is available. Multiple possible reasons were proposed including insufficient statistical power of the study or the type of platinum doublet combined with bevacizumab. Generally, patients in the AVAiL study had more favourable prognostic features when compared with patients in the E4599 trial: they were younger (median age 57–59 vs 63 years), 8% of patients had dry stage IIIb disease (E4599 trial enrolled only wet stage IIIb) and a high proportion had adenocarcinoma histology (82%–85%) and were never smokers (22%–26%). The above factors may have contributed to 3.2 months longer median survival for patients in the chemotherapy- only group when compared with E4599 trial and may also explain the less significant benefit from adding bevacizumab to chemotherapy in this highly selected group of patients.
In the number of small phase II, clinical trials that examined efficacy of combining bevacizumab with various platinum-based chemotherapy doublets, overall response rate ranged from 30-74% depending on the chemotherapy doublet. However these trials were not designed to address the difference in PFS or OS (29-31). Post marketing, phase IV clinical trial (SAiL – Safety of Avastin in Lung) results published recently confirmed already known safety and toxicity profile of bevacizumab combined with platinum-based chemotherapy used as first-line treatment in non-squamous NSCLC (32).
Squamous NSCLC comprises about 25-40% of all histological subtypes. Based on the earlier phase II trial (25) that highlighted increased risks of hemorrhagic complications in patients with squamous histology, bevacizumab is not recommended for this substantial portion of NSCLC patients. The BRIDGE trial investigated the safety profile of delayed administration of bevacizumab in patients with squamous histology (33). Patients with recent arterial thromboembolic events, gastrointestinal perforation, hemoptysis, untreated brain metastases, intrathoracic lesion(s) with cavitation, or anticoagulation therapy were not eligible. Patients were treated with carboplatin/paclitaxel for the first two initial cycles and with carboplatin/paclitaxel/bevacizumab for cycles 3-6. No new safety signals were identified and the incidence of pulmonary hemorrhage was 3.2% (1 patient). However, it is too early to recommend routine use of bevacizumab in this group of patients outside the clinical trial setting and more studies are needed.
Similarly, patients with brain metastases are excluded from routine use of bevacizumab. PASSPORT study evaluated use of bevacizumab combined with chemotherapy in patients with treated brain metastases by means of surgery, whole brain radiation or stereotactic radiotherapy (34). No increase of brain hemorrhage in this study was observed. Currently there is ongoing study (NCT00800202) of bevacizumab in patients with non-squamous NSCLC with asymptomatic and untreated brain metastases which may shed more light specifically on the question of bevacizumab use is patients with CNS metastatic disease (www.clinicaltrials.gov).
Similarly to monoclonal antibodies that bind to VEGF, afliberept (VEGF trap), was investigated as single agent in phase II clinical trial in 98 patients with platinum- and erlotinib-resistant stage IIIB/IV lung adenocarcinoma (35). The primary end point of the study was response rate and additional endpoints included safety, duration of response, PFS and OS. Aflibercept was given intravenously every 2 weeks until progression of disease or intolerable toxicity. Aflibercept as single agent had a very limited activity in this heavily pretreated group of patients with the overall response rate of 2.0%, Median PFS was 2.7 months, and OS was 6.2 months. Common grade 3/4 toxicities included dyspnea (21%), hypertension (23%), and proteinuria (10%). There are ongoing further studies evaluating the use of aflibercept in combination with chemotherapy and other targeted therapies in patients with NSCLC (www.clinicaltrials.gov). The new antibodies that target molecules involved directly in tumor angiogenesis like VEGFR-2 and PDGFR are tested in early phase clinical trials as reported recently, however no randomized phase III data is available (36,37).
 |
|
Multi-targeted therapy in NSCLC
There is strong pre-clinical evidence regarding the close relationship of the EGF and VEGF pathways in cancer (38). By targeting both pathways with separate drugs one would expect that it would have an additive or synergistic inhibitory effect on tumor progression. The EGF pathway can be modulated by monoclonal antibodies that block EGFR (cetuximab, panitumumab) or by TKIs (erlotinib, gefitinib) that interfere with activation of EGFR. It is well established at present that clinical activity of TKIs is associated with specific mutations within the tyrosine kinase domain of EGFR (39). Particular group of patients (never-smokers, patients from Asia, patients with adenocarcinoma histology) have higher incidence of these mutations thus deriving the most clinical benefit from these drugs.
The concept of dual blockade in NSCLC patients was evaluated in a phase I/II trial combining bevacizumab and erlotinib in stage IIIB/IV non-squamous NSCLC (40). Majority of patients tumors were adenocarcinomas and majority of patients were current or past tobacco smokers. Out of 40 patients enrolled, eight achieved a partial response and 26 patients had stable disease. There were no pharmacokinetic interactions between bevacizumab and erlotinib and there were no severe adverse reactions noted.
The combination of bevacizumab and EGFR TKIs was also investigated in patients who progressed during or after platinum-based treatment (41). One hundred and twenty patients were randomized in the phase II trial to one of three arms: 1) bevacizumab combined with either chemotherapy (docetaxel or pemetrexed), or 2) bevacizumab combined with erlotinib and 3) chemotherapy alone. The median PFS was 3 months for chemotherapy alone arm and 4.8 and 4.4 months for bevacizumab plus chemotherapy and bevacizumab plus erlotinib arm, respectively. Median OS times were 8.6, 12.6, and 13.7 months for the chemotherapy alone, bevacizumab-chemotherapy and bevacizumab-erlotinib arms, with the one-year survival rates of 33.1%, 53.8%, and 57.4%, respectively. However, the difference in PFS or OS between the bevacizumab plus chemotherapy and bevacizumab plus erlotinib arms was not significant.
These findings led to the design of phase III clinical trials utilizing multi-targeted therapy in NSCLC. There are at least two phase III clinical trials combining bevacizumab and erlotinib in NSCLC patients. The ATLAS trial (AVF3671g study) evaluated the efficacy of bevacizumab combined with erlotinib versus bevacizumab alone as a maintenance treatment after four cycles of platinum-based chemotherapy with bevacizumab in patients with stage IIIB/IV NSCLC (42). The ATLAS trial results showed that addition of erlotinib to bevacizumab after chemotherapy improved PFS (4.8 months) when compared with bevacizumab alone (3.7 months). Although ATLAS study was not powered to detect differences in OS, combining bevacizumab and erlotinib may provide OS benefit according to preliminary analysis.
Phase III BeTA Lung trial evaluated the efficacy of erlotinib combined with bevacizumab versus erlotinib and placebo in the second-line treatment setting of advanced NSCLC. Unfortunately the primary point of OS improvement in combination group was not met (43).
|
|
Multitargeting with tyrosine kinase inhibitors in lung cancer
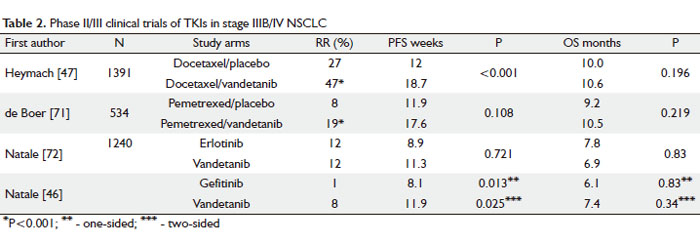
TKIs target the activity of multiple receptors (24, 44). Vandetanib (Zactima, ZD6474) is a small molecule TKI that blocks EGFR, VEGFR-1, -2, -3 and rearranged during transfection (RET) tyrosine kinase (45). In a phase II trial patients with advanced, platinum resistant NSCLC were treated with either vandetanib or erlotinib as a single agent. Vandetanib significantly increased PFS to 11.9 weeks when compared with erlotinib – 8.1 weeks (Table 1). Vandetanib-related side effects like rash, diarrhea and hypertension were manageable and consistent with the general toxicity profile of TKIs. No difference in OS was detected between the study arms (46).
Vandetanib was evaluated in phase II clinical trial in combination with docetaxel for second-line therapy in patients with advanced NSCLC. In patients with previously treated NSCLC, vandetanib 100 mg/day plus docetaxel improved PFS by 6.7 weeks and RR by 20% versus docetaxel alone, 18.7 weeks vs 12 weeks and 47% vs 27%, respectively (Table 2) (47). Additionally, exploratory subgroup analyses showed that women achieved a greater PFS benefit than men with vandetanib 100 mg plus docetaxel versus docetaxel alone. When vandetanib dose was increased from 100 to 300 mg, no improvement in the outcome was seen and the 100 mg dose was used in phase III trial. These potential improvements in outcome were unfortunately not sustained when this combination was tested in a randomized phase III clinical trial. Zactima in combination with Docetaxel in non-small cell lung cancer – ZODIAC trial – was phase III, placebo controlled study that randomized patients with stage IIIB/IV NSCLC to receive either docetaxel alone or combined with 100 mg of vandetanib as second line treatment (48). Median PFS was only 0.8 month better (4.0 months) in patients receiving docetaxel with 100 mg of vandetanib when compared to docetaxel alone arm (3.2 months). There was no improvement in OS.
Exploratory analyses suggest that EGFR gene copy number and EGFR mutation status may have some predictive value in identifying patients who receive the most benefit from combination of vandetanib and docetaxel in second-line treatment of patients with stage IIIB/IV NSCLC (49).
The ZEPHYR trial investigated the clinical efficacy of vandetanib as single agent vs placebo in patients with advanced NSCLC who had previously failed platinum-based chemotherapy and anti-EGFR treatment with TKI (50). Although PFS was better in vandetanib arm, the study did not meet its primary objective of demonstrating an OS benefit of vandetanib over placebo in this heavily pre-treated group of patients.
Ongoing clinical trials with this agent include a combination of vandetanib with carbolpatin and paclitaxel in the neoadjuvant setting in patients with resectable NSCLC as well as maintenance therapy in advanced NSCLC patients following carboplatin and docetaxel chemotherapy. The Phase III clinical trial (Zactima efficacy with Alimta in lung cancer – ZEAL trial) is currently evaluating efficacy of combination of vandetanib and pemetrexed in patients with advanced NSCLC (51).
Sunitinib (Sutent, SU11248), sorafenib (Nexavar, BAY 43-9006) and cediranib (Recentin, AZD2171) are multi-targeting TKIs that block activity of VEGFR-1, -2, -3, as well as PDGF receptors, RET and c-Kit tyrosine kinases (24,52).
The ESCAPE trial investigated the clinical benefit of adding sorafenib to standard platinum doublet chemotherapy (carboplatin/paclitaxel) as a first line treatment of advanced NSCLC. The primary end-point of the study was not met and the trial had to be terminated prematurely after negative effect of adding sorafenib in patients with squamous histology (53). Sorafenib as a single agent, however, showed some clinical activity in phase II clinical trial (E2501) that randomized patients who failed at least two prior chemotherapy treatments to sorafenib vs placebo (54). Recent retrospective analysis showed that patients who carry a specific VEGF gene germ line polymorphism may benefit more from sorafenib treatment than others in this heavily pretreated group (55).
Unfortunately, recently announced results of phase II trial that investigated the benefit of adding cediranib to the first line carboplatin/gemcitabine chemotherapy showed no significant improvement in RR, PFS or OS (56).
A number of small molecule TKIs with predominantly VEGFR blocking activity such as pazopanib, axitinib, motesanib (AMG 706) are currently being evaluated at present in early phase clinical trials in patients with NSCLC. The early clinical data from some of these trials is encouraging and will hopefully shed some more light on the complex issues of molecular targeted therapy of lung cancer (57,58).
The combined toxicities associated with multitargeted agents approach are clinically significant and cannot be ignored in the design of new trials. Recently a study of sunitinib in combination with bevacizumab, carboplatin and paclitaxel in patients with advanced NSCLC (SABRE-L trial) had to be terminated prematurely since the combination was not well tolerated (www.clinicaltrials.gov).
 |
|
Vascular disrupting agents in lung cancer
Vascular disrupting agents (VDAs) target endothelial cells and pericytes of the already established tumor vessels. VDAs are divided into two types: ligand-directed VDAs and small molecules. Clinical efficacy of ligand-directed VDAs which have linked targeting and effector moieties is limited because of high toxicity and lack of specificity. Small molecules comprise two classes: flavonoids, which induce local cytokine production, and the tubulin-binding agents (59,60).
The FALCON trial investigated the efficacy of CA4P (fosbretabulin tromethamine) when combined with carboplatin/paclitaxel/bevacizumab regimen. Fifty patients with stage IIIB/IV NSCLC were randomized to chemotherapy/bevacizumab/CA4P arm vs chemotherapy/bevacizumab/placebo. Preliminary data suggests survival benefit in the group treated with CA4P, however mature data is not available (61).
The ADVANCE study randomized patients with stage IIIB/IV NSCLC who progressed on prior chemotherapy to docetaxel alone or in combination with VDA – plinabulin (NPI-2358) (62). Although recruitment is still ongoing, some preliminary analysis showed promising results of 22% partial response rate in combination group vs 5% in docetaxel only group.
Multiple clinical trials are ongoing at the moment that investigate clinical efficacy of combination of chemotherapy with VDAs in advanced NSCLC (63).
|
|
Future directions
In the 1970’s the 5-year survival rate for all patients diagnosed with NSCLC was 10%. In the past 40 years we were able to increase it only to 15%. There is no simple answer to the question why the numbers of those who survive the disease are so small. Biology and natural history of the disease probably contributes significantly to the fact that the vast majority of patients are diagnosed at advanced/metastatic stage when cure is not possible.
It was postulated many years ago that focus is necessary on other treatments than cytotoxic chemotherapy in NSCLC if we want to make a change in the clinical outcome (64). So far one phase III clinical trial showed survival benefit of adding anti-angiogenic agent to standard platinum-based chemotherapy in patients with stage IIIB/IV NSCLC. Selected groups of patients respond to anti-angiogenic therapies that result in tumor shrinkage and disease stabilization but the results are far from being clinically meaningful in most of the trials that were conducted so far (65). At present, nearly 100 new agents are being tested in more than 600 clinical trials in patients with advanced NSCLC (www.clinicaltrials.gov). The oncology community is confronted with the early phase data from enormous amount of small clinical trials investigating multiple agents that have some anti-angiogenic activity. We still do not know which of these new promising agents should be tested in large scale phase III trials, and what is even more important, which patients will benefit from them the most.
The new, personalized approach to management of advanced NSCLC has become more relevant in the recent years. When planning systemic chemotherapy, we now consider the histological subtype of NSCLC (adenocarcinoma vs squamous), presence or absence of specific mutation within EGFR tyrosine kinase domain or presence or absence of EML-ALK4 fusion gene in the tumor tissue (66-68). Unfortunately, the number of patients for whom personalized therapies are recommended are still small at present, however ongoing basic and translational research helps to identify future potential molecular targets (69,70). Although the clinical benefits of targeting tumor angiogenesis are not satisfying for many oncologists and their patients, they provide an alternative to the sole cytotoxic approach in patients with advanced NSCLC and selected patients may derive limited survival benefit. Currently bevacizumab, monoclonal antibody against VEGF, is approved to combine with platinum-based chemotherapy in patients with stage IIIB/IV NSCLC who have good performance status, non-squamous histology and no clinically relevant haemoptysis. Erlotinib, which is a TKI with anti-angioenic properties, is approved as a second line regimen for patients with NSCLC who progressed on platinum-based systemic chemotherapy. Limited clinical activity of antiangiogenic drugs in treatment of unresectable/metastatic NSCLC may be explained partially by the redundancy of tumor angiogenic growth factors. Targeting a single angiogenic factor then, may not be an optimal way to make a significant clinical impact on the tumor progression. Combining drugs, which target different pro-angiogenic factors sequentially, or combining drugs with a distinct mode of action (i.e. monoclonal antibodies with TKIs) may help to target tumor angiogenesis more efficiently.
We hope that the ongoing research into identifying the targeted therapies will improve markedly survival of patients with NSCLC in the years to come.
|
|
References
Cite this article as: Korpanty G, Smyth E, Carney DN. Update on anti-angiogenic therapy in non-small cell lung cancer: Are we making progress? J Thorac Dis 2011;3(1):19-29. doi: 10.3978/j.issn.2072-1439.2010.11.11
|