Transthoracic needle biopsy versus surgical diagnosis for solid pulmonary nodules
Introduction
A solitary pulmonary nodule (SPN) is defined as a relatively well-defined round or oval pulmonary parenchymal lesion equal to or smaller than 30 mm in diameter. It is surrounded by pulmonary parenchyma and/or visceral pleura and is not associated with lymphadenopathy, atelectasis or pneumonia (1). Morphologically, nodules are classified as solid or subsolid; subsolid nodules are subdivided into pure ground-glass nodules and part-solid nodules (2). The incidence of cancer in patients with SPN ranges from 10% to 70% (3), and the major goal of the investigation is to exclude or confirm malignancy.
The 2013 American College of Chest Physicians (ACCP) guidelines for the evaluation of the SPN recommend basing the assessment of nodules on their density, size and probability of malignancy (4). In the individual with a solid, indeterminate nodule that measures >8 mm in diameter, they suggest that clinicians estimate the pretest probability of malignancy either qualitatively by using their clinical judgment and/or quantitatively by using a validated model, such as the Brock model (5), or the Mayo Clinic model (6). When the clinical probability of malignancy is very low (<5%), the ACCP guidelines recommend surveillance with serial chest tomography (CT) scans (4). When the probability is low to moderate (10–60%), non-surgical biopsy should be performed, either with transthoracic needle biopsy (TTNB) or bronchoscopy with various guidance tools (fluoroscopy, endobronchial ultrasound, electromagnetic navigation, virtual navigation (4). However, when the probability of malignancy is high (>65%), surgical diagnosis is recommended (grade 2C) (4). If the nodule proves to be a primary lung cancer, diagnosis, staging and therapeutic resection are often completed in a single operative procedure.
The British Thoracic Society (BTS) and the National Comprehensive Cancer Network (NCCN) guidelines also state that patients with a strong clinical suspicion of stage I or II lung cancer (based on risk factors and radiologic appearance) do not require a biopsy before surgery (7,8). They argue that a biopsy adds time, costs, and procedural risk and may not be needed for treatment decisions. Exceptions might be for cases where a non-lung cancer diagnosis is strongly suspected or when an intraoperative diagnosis appears difficult or very risky; then, a preoperative biopsy may be appropriate.
The diagnostic accuracy of CT-guided TTNB has been reported to be high, with an overall sensitivity and specificity of up to 90% and 95%, respectively (7). However, in other series, approximately 15–30% of TTNB yield non-specific or non-diagnostic results (9,10), and additional procedures may be necessary. TTNB also comes with significant complications, such as pneumothorax (15–25%), pneumothorax requiring chest tube (4–6%), and hemorrhage (clinically significant ~1%) (11). In comparison with TTNB, the reported yields of bronchoscopy with guidance are lower (65–84% for electromagnetic navigation bronchoscopy and 46–77% for radial endobronchial ultrasound), and even lower for lesions <2 cm in the peripheral third of the lung (7). Furthermore, these techniques are not available in all centers.
Guidelines regarding the investigation of SPN, the estimation of the probability of malignancy and the use of TTNB, are not always followed by clinicians. A survey of Canadian physicians showed that specialists tended to overestimate the probability of malignancy (12). Also, 53% of physicians would order a TTNB in a medically fit patient presenting with a very high probability of malignancy, when the result was unlikely to affect patient management (12).
Given the paucity of data directly comparing TTNB and surgical diagnosis for the investigation of SPN, and the variability of conducts among physicians, this study reports the proportion of patients with a solid SPN >8 mm but ≤3 cm undergoing TTNB vs. surgical diagnosis, as well as histopathological results and complications from these procedures. Our primary objective was to evaluate the proportion of patients with a SPN >8 mm to ≤3 cm in diameter undergoing a TTNB vs. a surgical diagnosis. Secondary outcomes were to examine the distribution of final diagnoses (malignancy vs. benign lesion), to evaluate the proportion of patients undergoing a TTNB that would yield a benign diagnosis and permit to avoid surgery, to evaluate if delays from imaging to surgery were longer when preoperative TTNB was performed, and to evaluate if operative times were longer in patients without prior TTNB. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-35/rc).
Methods
Study design
This retrospective study included all patients who underwent TTNB and/or surgery for a solid SPN >8 mm but ≤3 cm between January and December 2016, at Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ). Exclusion criteria were: clinical stage other than T1N0M0, the presence of more than 1 nodule >8 mm on CT, a subsolid nodule, a histological diagnosis obtained by flexible bronchoscopy prior to surgery, endobronchial lesions, or a nodule previously irradiated. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Institut Universitaire de Cardiologie et de Pneumologie de Québec (protocol code 2019-3088, 21657, on July 23, 2018). Patient consent was waived due to the fact that it was a retrospective chart review with no impact on patients.
Data collection
Demographic data collected for this study included age, gender, and smoking status. The medical charts were also reviewed for factors that could influence the clinical probability or malignancy and/or the decision to perform TTNB or surgical diagnosis: forced expiratory volume in one second (FEV1), diffusing capacity for carbon monoxide (DLCO), the presence of moderate or severe emphysema on chest CT, a personal history of cancer, a history of disease that might cause lung nodules (granulomatous disease, connective tissue disease), size and location of the nodule, presence of spiculations, standardized uptake value (SUV) on positron emission tomography (PET) scan. The Mayo Clinic model that incorporates age, smoking status, extrathoracic cancer diagnosis at least 5 years prior, nodule diameter, upper lobe location, spiculation and PET uptake, was used to calculate the clinical probability of malignancy for each case (6). From this model, probabilities of cancer <5%, 5–65% and >65% were considered as low, intermediate and high, respectively. Pathology results and complications from TTNB (e.g., pneumothorax and hemorrhage) were collected. For all surgical biopsies, intraoperative consultation with pathology was performed. If malignancy was confirmed, sampling of mediastinal lymph nodes and oncologic resection by lobectomy or segmentectomy were completed. For all surgeries, surgery type (segmentectomy vs. lobectomy) and pathological diagnosis were collected, whereas delay from imaging to surgery and operative time (time between incision and closure of the skin) were calculated.
Statistical analysis
Patient demographics and clinical characteristics were summarized using descriptive methods. Nominal variables were expressed with frequencies and percentage (%) and were analysed using Fisher’s exact test. Continuous variables were reported as median with interquartile range and analyzed with the Wilcoxon rank-sum test. For all statistical analyses, the results were considered significant with P values <0.05.
Results
Patients
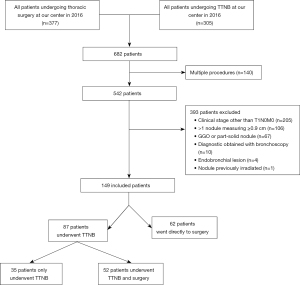
Three-hundred and seventy-seven patients underwent thoracic surgery at our center for a pulmonary nodule in 2016, and 305 patients had TTNB. After taking into account 140 patients that appeared in both groups and/or had multiple procedures, 542 patients were assessed for eligibility. After excluding 393 patients, most often because of a clinical stage other than T1N0M0, 149 patients were included in the analysis (shown in Figure 1). Of these, 87 patients (58%) underwent TTNB; among these patients, 52 had surgery after, while 35 did not. Sixty-two patients (42%) had upfront surgery without a preoperative diagnosis.
Baseline characteristics of all patients and nodules are summarized in Table 1. The population was divided in two groups, with 87 patients who underwent TTNB and 62 patients who had upfront surgery. Patients undergoing TTNB were older than those undergoing surgical diagnosis (median age 69 vs. 64, P<0.01), and their nodule was larger (median size 1.8 vs. 1.5 cm, P=0.03). There were no other statistically significant differences between the two groups. The majority of patients (58%) had a high probability of malignancy according to the Mayo Clinic score, and the median score for the entire cohort was 73% [interquartile range (IQR) 34–92%].
Table 1
| Characteristics | All patients (n=149) | TTNB (n=87) | Surgical diagnosis (n=62) | P value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Median age, years, [IQR] | 66 (61–73] | 69 (62–74] | 64 (58–70] | <0.01 |
| Male, n [%] | 63 [42] | 35 [40] | 28 [45] | 0.61 |
| Former or current smoker, n [%] | 134 [90] | 76 [87] | 58 [94] | 0.28 |
| Median FEV1, %, [IQR] | 89 [74–103] (n=142) | 90 [72–104] (n=81) | 85 [77–103] (n=61) | 0.34 |
| Median DLCO, %, [IQR] | 86 [67–104] (n=135) | 80 [64–105] (n=76) | 90 [77–104] (n=59) | 0.11 |
| Moderate or severe emphysema on chest CT, n [%] | 40 [27] | 24 [28] | 16 [26] | 0.85 |
| Personal history of cancer | 56 [38] | 31 [36] | 25 [40] | 0.61 |
| History of disease that might cause lung nodules | 19 [13] | 11 [13] | 8 [13] | 1.00 |
| Nodule characteristics | ||||
| Median size, cm, [IQR] | 1.7 [1.3–2.2] | 1.8 [1.4–2.3] | 1.5 [1.2–1.9] | 0.03 |
| Location, n [%] | 0.70 | |||
| RUL | 50 [34] | 27 [31] | 23 [37] | |
| RML | 13 [9] | 6 [7] | 7 [11] | |
| RLL | 29 [19] | 18 [21] | 11 [18] | |
| LUL | 30 [20] | 20 [23] | 10 [16] | |
| LLL | 27 [18] | 16 [18] | 11 [18] | |
| Presence of spiculations | 71 [48] | 40 [46] | 31 [50] | 0.74 |
| Median SUV on PET scan, [IQR] | 3.3 [1.8–5.9] (n=132*) | 3.8 [1.9–7.2] (n=75) | 3.0 [1.7–4.8] (n=57) | 0.07 |
| Median Mayo Clinic score, %, [IQR] | 73 [34–92] | 74 [40–93] | 69 [27–90] | 0.16 |
| Probability of malignancy according to the Mayo Clinic score, n [%] | 0.10 | |||
| Low (<5%) | 2 [1] | 0 | 2 [3] | |
| Intermediate (5–65%) | 60 [41] | 32 [37] | 28 [45] | |
| High (>65%) | 87 [58] | 55 [63] | 32 [52] | |
*, 12 patients had a PET scan, but their fluoro-deoxyglucose (FDG) uptake was described only qualitatively as absent, faint, moderate or intense. Only 5 patients did not have a PET scan. TTNB, transthoracic needle biopsy; IQR, interquartile range; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity for carbon monoxide; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; SUV, standardized uptake value; PET, positron emission tomography.
Transthoracic needle biopsy
Among 87 patients undergoing TTNB, 12 (14%) had a second biopsy after insufficient tissue, non-specific or non-diagnostic results with the first one; hence a total of 99 TTNBs were performed (Table 2). Following these 99 procedures, there were 30 pneumothorax (30%), including 6 requiring a chest tube (6%). There was no significant hemorrhage. Among the 35 patients who only underwent TTNB without subsequent surgery, 19 had a positive biopsy for malignancy but were not surgical candidates and were treated with stereotactic body radiation therapy (SBRT). Three patients had results that were suspicious for malignancy but not diagnostic, were presumed to have cancer, were not surgical candidates, and were also treated with SBRT. The other 13 patients had a specific benign (n=5) or non-specific diagnosis (n=8) on TTNB, and underwent surveillance with serial CT scans after discussion with their physician. They were all followed for at least 24 months with serial imaging, and their nodule remained stable. Hence, they could be classified after this 2-year stability period as having benign disease, as demonstrated by Swensen et al., as well as the Fleischner Society (2,6).
Table 2
| Results and complications | n | % |
|---|---|---|
| Pathological diagnosis (n=87)* | ||
| Specific benign diagnosis** | 5 | 6 |
| Non-specific diagnosis§ | 12 | 14 |
| Insufficient or undetermined | 2 | 2 |
| Suspicious | 10 | 11 |
| Malignant | 58 | 67 |
| Adenocarcinoma | 35 | |
| Squamous cell carcinoma | 12 | |
| NSCLC NOS | 5 | |
| Other¶ | 6 | |
| Complications (n=99) | ||
| Any pneumothorax | 30 | 30 |
| Pneumothorax requiring a chest tube | 6 | 6 |
*, if a patient underwent two TTNBs, the result of the second procedure is reported; **, non-necrosing granuloma (n=2), necrosing granuloma (n=1), hamartoma (n=1), follicular lymphoid hyperplasia (n=1); §, normal parenchyma (n=7), fibroinflammatory changes (n=4), non-specific chronic inflammation (n=1); ¶, metastatic lesion from colorectal cancer (n=2), metastatic lesion from breast cancer (n=1), carcinoid tumor (n=1), small cell lung cancer (n=1), low grade epithelioid tumor (n=1). NSCLC NOS, non-small cell lung cancer not otherwise specified.
Surgical diagnosis
Surgery was performed on 114 patients; among these, 52 (46%) underwent prior TTNB, while 62 (54%) had upfront surgery (Table 3). There was a tendency for a longer delay from imaging to surgery in patients with prior TTNB, but the difference between the two groups was not statistically significant (median 92 vs. 80 days, P=0.09). There was no significant difference in the operative time between the two groups (median 129 vs. 131 minutes, P=0.94). The majority of patients (59%) had a lobectomy. Among the 114 surgical procedures, 108 were performed by with video-assisted thoracoscopic surgery (VATS), 4 were conversions to thoracotomy from VATS, and 2 were upfront thoracotomies. In the group without prior TTNB, 5 patients (8%) had a final benign pathological diagnosis. Of note, 4 patients who had prior TTNB showing a benign or non-specific diagnosis also underwent surgery. Among these, 2 were found to have malignant disease: 1 patient with non-specific chronic inflammation at TTNB was diagnosed with carcinoid tumor at surgery, and 1 patient with normal parenchyma at TTNB was diagnosed with intrapulmonary solitary fibrous tumor. For the 2 other patients, a benign diagnosis was found: 1 patient with normal parenchyma at TTNB was diagnosed with necrosing granulomatosis at surgery, and 1 patient with non-necrosing granuloma at TTNB was diagnosed with necrotising pneumonia caused by Aspergillus at surgery.
Table 3
| Outcomes | All patients who underwent surgery (n=114) | Patients with prior TTNB (n=52) | Patients without prior TTNB (n=62) | P value |
|---|---|---|---|---|
| Median delay from imaging to surgery, days [IQR] | 85 [64–136] | 92 [72–141] | 80 [56–135] | 0.09 |
| Median operative time, minutes [IQR] | 130 [95–160] | 129 [98–161] | 131 [95–160] | 0.94 |
| Surgery type, n [%] | ||||
| Segmentectomy | 47 [41] | 21 [40] | 26 [42] | 1.00 |
| Lobectomy | 67 [59] | 31 [60] | 36 [58] | |
| Pathological diagnosis, n [%] | ||||
| Benign | 8 [7] | 3 [6]* | 5 [8]** | 0.73 |
| Malignant | 106 [93] | 49 [94] | 57 [92] | |
| Adenocarcinoma | 74 [69] | 34 [70] | 40 [70] | |
| Squamous cell carcinoma | 11 [10] | 6 [12] | 5 [9] | |
| Carcinoid tumor | 7 [7] | 3 [6] | 4 [7] | |
| Metastatic lesion | 7 [7] | 3 [6] | 4 [7] | |
| Other§ | 7 [7] | 3 [6] | 4 [7] | |
*, coccidioidomycosis (n=1), prior TTNB was undetermined; necrosing granulomatosis (n=1), prior TTNB showed normal parenchyma; necrotising pneumonia caused by Aspergillus (n=1), prior TTNB showed non-necrosing granuloma; **, necrosing granulomatosis (n=1), fibrocicatricial changes (n=1), hamartoma (n=1), osseous metaplasia with alveolar hemorrhage (n=1), aspergilloma (n=1); §, adenosquamous carcinoma (n=2), mixed small and large cell neuroendocrine carcinoma (n=1), intrapulmonary solitary fibrous tumor (n=1), small cell lung cancer (n=1), mucosa-associated lymphoid tissue (MALT) lymphoma (n=1), pleomorphic carcinoma (n=1). TTNB, transthoracic needle biopsy; IQR, interquartile range.
Overall, after considering TTNB and surgical biopsies results, 128 patients (86% of the entire cohort) had a final malignant diagnosis (either proven or strongly suspected).
Probability of malignancy and procedure performed
Fifty-five patients (63% of the TTNB group) had a high probability of malignancy and underwent TTNB. Of these, 18 were not surgical candidates and TTNB was performed to prove malignancy before SBRT. Six patients had a benign (n=1) or non-specific diagnosis (n=5) after TTNB, and the physician decided for radiological surveillance. One patient had a benign diagnosis after TTNB and still underwent surgery for confirmation. For the remaining 30 patients, TTNB confirmed malignancy and did not affect management as they went to surgery after.
Thirty patients (48% of the surgical group) had a low or intermediate probability of malignancy and underwent surgical diagnosis without prior TTNB. Among these, 26 were diagnosed with cancer after surgery, and only 4 had a benign diagnosis.
Discussion
In this monocentric cohort of patients with a solid SPN >8 mm but ≤3 cm, 58% of patients underwent TTNB, while 42% of patients had a surgical diagnosis. We could not find studies similar to ours to evaluate and compare this proportion with other centers. This study being retrospective, it was not easy to understand the reason why the clinician chose one procedure over the other. Patients undergoing TTNB were older than patients undergoing surgical diagnosis. We have limited information regarding comorbidities, but 22 out of 87 patients undergoing TTNB (25%) were not surgical candidates. Also, the median size of the nodule was larger in the TTNB group, and maybe some of the nodules were too small or not accessible for TTNB in the surgical diagnosis group. The complication rates after TTNB (30% of pneumothorax, 6% of pneumothorax requiring a chest tube, no significant hemorrhage), were comparable to what is reported in the literature (11).
One hundred and twenty-eight patients (86% of the entire cohort) had a final malignant diagnosis (either proven with TTNB and/or surgery, or strongly suspected), which is higher than the malignancy rates reported for SPN in the literature (3). By using TTNB only, a definitive benign or malignant diagnosis was obtained in 63 out of 87 patients (yield of 72.4%). Still, thirteen patients out of the 87 biopsied (15%), could avoid surgery owing to their biopsy result, either because of a specific benign diagnosis (n=5), or because of a non-specific diagnosis and a physician reassured enough to decide for radiological surveillance (n=8). These latter patients were all diagnosed with benign nodules after a stability of 24 months on serial imaging. This means that the number of TTNB needed to avoid 1 surgery would be 6.7. Again, we did not identify any randomized controlled trials comparing TTNB with other diagnostic approaches, but a 2002 study used case vignettes from 114 patients with SPN (71% malignant) to determine the frequency with which TTNB results changed management (13). In this study, the addition of TTNB results to clinical history and chest CT scan findings reduced the frequency of unnecessary surgery for a benign lesion from 39% to 15%, which is higher than the rate we observed in our retrospective study (7% of all surgeries performed had a final benign pathological diagnosis).
However, among 62 patients who underwent upfront surgery, 5 (8%) had a benign diagnosis, which means the number of upfront surgeries without prior TTNB needed to ‘harm’ would be 12.5. Of note, these 5 patients were operated by VATS, and complications with this technique for the diagnosis of lung nodules are rare (14). Also, the definitive benign diagnosis with surgery eliminates the need for surveillance with serial CT scans, and can decrease patient anxiety. Our proportion of negative surgical biopsies compares favorably with other studies, reporting ranges from 12% to 62% of ‘futile’ surgeries (15-19), which are not always futile as they can show an unsuspected diagnosis or cause a treatment course change in up to 85% of cases (19). Of note, most of these studies were conducted prior to the availability of PET scans.
One of the arguments for performing surgery without prior biopsy is to avoid adding time to the investigation of patients. In our study, there was a tendency for a longer delay (11 more days) from imaging to surgery in patients with prior TTNB, but the difference between the two groups was not statistically significant. Another relevant question is whether performing frozen sections in patients without prior diagnosis increases the operative time. In our cohort, there was no significant difference in the median operative time between patients with prior TTNB and patients without.
Our study also showed that adherence to guidelines by physicians is poor. In 30 patients (43% of the TTNB group), TTNB was performed despite a high probability of malignancy and no contraindications to surgery. Also, 48% of patients who had surgical diagnosis had a low or intermediate probability of malignancy and should have undergone prior TTNB. Our results are comparable to results from a Canadian survey (12). In our cohort, even among these patients who had a low or intermediate probability of malignancy, the malignancy rate was very high (87%). The Mayo Clinic model has been shown by some authors to underestimate the pre-test probability, especially in practice settings where the prevalence of malignant nodules is high like in ours (20,21). It is also questionable if all patients with an intermediate probability of malignancy should be viewed and treated the same, since the interval is broad (5–65%), and the discussion with the patient might be very different depending on the exact risk.
The question of our study is relevant. We could not find other studies comparing TTNB and surgical diagnosis for the investigation of SPN. Our cohort is contemporary and 97% of our patients underwent PET scans, which could help in calculating the probability of malignancy.
Limitations
Our results are limited by the retrospective nature of the study, by a small sample and by the fact that the study is unicentric. Although the adherence from clinicians to guidelines might seem low, information on patients’ values, preferences and comorbidities is lacking and the proportion of nodules that could not be submitted to TTNB because of their location is unknown. We had multiple exclusion criteria which limit the extrapolation of results to all patients with SPN. These results cannot apply to patients with subsolid nodules, multiple nodules, nodules ≤8 mm, or clinical stage other than T1N0M0. Our findings might not be applicable to all populations, as our malignancy rate was high (86%) and our patients had multiple risk factors: 90% were former or current smokers, 38% had a personal history of cancer, and 58% had a high probability of malignancy.
Conclusions
In this unicentric retrospective cohort of patients who were investigated for a SPN >8 mm but ≤3 cm, 58% of patients underwent TTNB, while 42% of patients had upfront surgery. The malignancy rate was 86%, which is higher than malignancy rates reported for SPN in the literature, and which seemed to limit the applicability of prediction models such as the Mayo Clinic model. Still, TTNB seemed useful, as 15% of patients who underwent this procedure could avoid surgery, either because of a specific benign diagnosis or because of a non-specific diagnosis and a physician reassured enough to decide for radiological surveillance. Among patients who underwent upfront surgery, only 8% were found to have a benign diagnosis, which is an acceptable rate of what could be considered as ‘futile’ surgeries, although we can argue that these surgeries are of value to have a definitive diagnosis and for patient reassurance. Adherence to guidelines for the investigation of SPN seemed suboptimal, and maybe physicians would need more education about the calculation of the probability of malignancy and about algorithms for the investigation of SPN. However, prediction models might not be suitable for all practice settings, especially if the local prevalence of malignancy is high. As shown in our cohort, even in the patients with a low to intermediate probability of malignancy, the malignancy rate was very high. Obviously, patient preferences should always be included in clinical decisions.
More real-world prospective studies are needed to compare non-surgical biopsies, including bronchoscopy with various guidance tools, to surgical biopsies, to assess their diagnostic yield, their complication rates, as well as the implications for patients in terms of anxiety and quality of life. There is also a need for simpler nodule evaluation algorithms, possibly including new diagnostic modalities, such as liquid biopsies and biomarkers.
Acknowledgments
The authors acknowledge Serge Simard (M.Sc Stats) from the Centre de recherche de l’Institut Universitaire de cardiologie et de pneumologie de Québec, Université Laval, Québec, QC, Canada for statistical analyses.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-35/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-35/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-35/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-35/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Winer-Muram HT. The solitary pulmonary nodule. Radiology 2006;239:34-49. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [Crossref] [PubMed]
- Baldwin DR, Callister MEGuideline Development Group. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practive guidelines: Non-Small Cell Lung Cancer. Version 4. 2021. Accessed on March 18, 2021. Available online: https://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf
- Fontaine-Delaruelle C, Souquet PJ, Gamondes D, et al. Negative Predictive Value of Transthoracic Core-Needle Biopsy: A Multicenter Study. Chest 2015;148:472-80. [Crossref] [PubMed]
- Lee KH, Lim KY, Suh YJ, et al. Nondiagnostic Percutaneous Transthoracic Needle Biopsy of Lung Lesions: A Multicenter Study of Malignancy Risk. Radiology 2019;290:814-23. [Crossref] [PubMed]
- Wiener RS, Wiener DC, Gould MK. Risks of Transthoracic Needle Biopsy: How High? Clin Pulm Med 2013;20:29-35. [Crossref] [PubMed]
- Lacasse Y, Plante J, Martel S, et al. Transthoracic needle biopsy in the diagnosis of solitary pulmonary nodules: a survey of Canadian physicians. J Thorac Cardiovasc Surg 2003;126:761-8. [Crossref] [PubMed]
- Baldwin DR, Eaton T, Kolbe J, et al. Management of solitary pulmonary nodules: how do thoracic computed tomography and guided fine needle biopsy influence clinical decisions? Thorax 2002;57:817-22. [Crossref] [PubMed]
- Murasugi M, Onuki T, Ikeda T, et al. The role of video-assisted thoracoscopic surgery in the diagnosis of the small peripheral pulmonary nodule. Surg Endosc 2001;15:734-6. [Crossref] [PubMed]
- Allen MS, Deschamps C, Lee RE, et al. Video-assisted thoracoscopic stapled wedge excision for indeterminate pulmonary nodules. J Thorac Cardiovasc Surg 1993;106:1048-52. [Crossref] [PubMed]
- Mack MJ, Hazelrigg SR, Landreneau RJ, et al. Thoracoscopy for the diagnosis of the indeterminate solitary pulmonary nodule. Ann Thorac Surg 1993;56:825-30; discussion 830-2. [Crossref] [PubMed]
- Marchevsky AM, Changsri C, Gupta I, et al. Frozen section diagnoses of small pulmonary nodules: accuracy and clinical implications. Ann Thorac Surg 2004;78:1755-9. [Crossref] [PubMed]
- Kuo E, Bharat A, Bontumasi N, et al. Impact of video-assisted thoracoscopic surgery on benign resections for solitary pulmonary nodules. Ann Thorac Surg 2012;93:266-72; discussion 272-3. [Crossref] [PubMed]
- Grogan EL, Weinstein JJ, Deppen SA, et al. Thoracic operations for pulmonary nodules are frequently not futile in patients with benign disease. J Thorac Oncol 2011;6:1720-5. [Crossref] [PubMed]
- Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax 2008;63:335-41. [Crossref] [PubMed]
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18-F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490-6. [Crossref] [PubMed]