
Translation, cultural adaptation and linguistic validation of the pectus excavatum evaluation questionnaire
Introduction
Pectus excavatum is the most common anterior congenital thoracic wall deformity, characterized by depression of the sternum and adjacent costal cartilage (1). The deformity can impose a significant burden on the patients’ quality of life by interfering with daily routines (e.g., disability to maintain activities of daily living and social interactions) when compared to peers (2-4). This is mainly due to symptoms such as exercise intolerance and cosmetic concerns (5-9). Yet, pectus excavatum remains an underappreciated burden to the quality of life despite the known biopsychosocial effects. To objectively document the problem, patient-reported outcome measures (PROMs) can be used to measure and appreciate outcomes from the patient’s perspective (10-12).
The health-related quality of life (HRQOL) concerns a special category in the domain of PROMs and are classified as generic or disease-specific instruments (12,13). Disease-specific instruments are more sensitive to changes of the concept of interest than generic instruments. Thus, these are better able to measure and detect clinical important changes, e.g., when one wants to compare treatments for a specific disease (11).
A myriad of PROMs for the measurement of HRQOL in pectus excavatum patients exists (2,14-18). The pectus excavatum evaluation questionnaire (PEEQ) is the most used disease specific instrument and was originally designed for the pediatric population (8 to 18 years of age) (2). However, up until now, the PEEQ is only available in English. The Nuss questionnaire modified for adults (NQ-mA) (14), a modified version of the PEEQ, has only two validated translations [i.e., a Swedish (18) and Turkish version (19)]. Since pediatric patients in non-English speaking countries do not have an adequate command of the English language, such a translation is highly desirable for use in clinical practice and research to improve pectus excavatum care. A Dutch version of the PEEQ would have a high impact and applicability as the incidence of pectus excavatum in The Netherlands is estimated at 425 patients per year (20). This study aims to provide a Dutch translation, cultural adaptation and linguistic validation of the PEEQ in order to facilitate research and clinical evaluation on quality of life in this population. Our second goal is to encourage other research teams to develop a translation to other languages following the same translation process to make the PEEQ available to clinicians and researchers worldwide. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-252/rc) (21).
Methods
PEEQ
The PEEQ is a disease-specific PROM to assess the HRQOL of pediatric pectus excavatum patients. The questionnaire was developed and tested in a pilot study by Lawson et al. (2). After further refinement, the developers used it in a large multi-center study to demonstrate that body image and perceived ability for physical activity improve after surgical repair of pectus excavatum (17). This 22-item instrument can be broken down into 11 items which directly address the patient while the remaining 11 items focus on the patient’s parents or legal guardian. In the patient’s part of the questionnaire, the instrument measures the HRQOL in 2 domains (i.e., body image perception and physical difficulties attributable to pectus excavatum). In the parents’ part, the HRQOL is measured in 3 domains (i.e., emotional difficulties, social self-consciousness and physical difficulties attributable to pectus excavatum). The items are scored on a 4-point Likert scale. Depending on the content of the item, the score reflects the frequency of a specific feeling (i.e., very happy to very unhappy) or behavior (i.e., very often to never) (2,17).
Translation process
This prospective study was conducted at Zuyderland Medical Centre, Heerlen, The Netherlands. The translation process of the PEEQ questionnaire was performed according to the World Health Organization (WHO) guideline for the process of translation and adaptation of instruments (22), and the International Society for Pharmacoeconomics and Outcome Research’s (ISPOR) guideline of principles of good practice for the translation and cultural adaptation process for PROMs (23). Patients involved in the translation process were recruited at the outpatient department of thoracic surgery during their routine visits. All pectus excavatum patients aged between 12 to 18 years were eligible for inclusion. Patients were excluded if the patient or his/her parents were not willing to fill in their part of the questionnaire.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (24). The study was approved by the local ethics committee of Zuyderland Medical Center, Heerlen, The Netherlands (registration number: METCZ20210182; date of approval: December 6, 2021). Written informed consent was obtained prior to inclusion from the patients and if applicable, from the patients’ parent(s) or legal guardian(s).
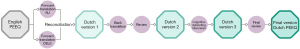
The following consecutive steps were taken (Figure 1):
- The necessary permission of the developers of the PEEQ to translate the questionnaire to the Dutch language was obtained.
- Two independent forward translations were developed. One translation was performed by Clarity English Language Services (Alkmaar, The Netherlands); a certified translation service, allied to the Dutch Society of Interpreters and Translators. The other translation was carried out by NJ; a native Dutch speaker who is fluent in the English language and familiar with the disease-specific terminology. The certified translator was given background information about the conceptual measure of the PEEQ. A conceptually equivalent and culturally appropriate translation rather than a literal translation was emphasized.
- Reconciliation of the two forward translations into a single forward translation was performed via discussion between the certified forward translator and the second forward translator (NJ). Disagreements were resolved by discussion with an independent translator (ERdL).
- As a double check, a literal back translation was performed by EJvP; a native speaker of the source language who is fluent in the target language.
- Review of the back translation was performed to ensure conceptual equivalence. An expert panel, consisting of the forward translator (NJ), another member of the research team who is also familiar with the terminology of the instrument (JHTD) and four experienced thoracic surgeons (ERdL, KWEH, YJLJ and YLJV), identified and resolved discrepancies between the source instrument and the back translation. The developers were asked for clarification of problematic items.
- The second version of the translated questionnaire was tested by a group of 10 consecutive, prospectively recruited pediatric pectus excavatum patients and their parents. An equal number of preoperative and postoperative patients was included to ensure that that target population of the PEEQ was well represented. The sample size was arbitrarily chosen taking into account the recommendations by the WHO (22) and ISPOR guidelines (23). A single interviewer (NJ) obtained all patient interviews with adherence to an interview protocol that was written in advance by the expert panel. Namely, the participants were asked if there were any unclarities in the questionnaires’ items or whether the items included words or phrases that they were not familiar with or that were not commonly used. If so, they were asked to suggest a different phrasing for the item in question. An expert panel meeting, including the above-mentioned members, was organized to incorporate the patient input into the final version of the questionnaire.
- The final translation was agreed upon by the expert panel.
Statistical analysis
Patient characteristics including sex, age, preoperative Haller index (HI) and allocated treatment for pectus excavatum were documented. The time required to complete the questionnaire was measured. Nominal variables were reported as absolute numbers and percentages. Continuous data was presented as median and interquartile range (IQR).
Data was analyzed using SPSS statistics (IBM Corp. IBM SPSS statistics for MacOS, Version 27.0, Armonk, NY, USA).
Results
The systematic translation process according to WHO and ISPOR guidelines has resulted in a linguistically validated and conceptually equivalent Dutch version of the PEEQ (Tables 1-3).
Table 1
| Hoe voel je je over... | Zeer tevreden | Tevreden | Ontevreden | Zeer ontevreden | |
|---|---|---|---|---|---|
| Q1 | je uiterlijk in het algemeen | 1 | 2 | 3 | 4 |
| Q2 | hoe je eruitziet zonder shirt | 1 | 2 | 3 | 4 |
| Q3 | de rest van je leven doorbrengen met hoe je borstkas er nu uitziet | 1 | 2 | 3 | 4 |
Part of the Dutch version of the PEEQ that is filled out by the child. PEEQ, pectus excavatum evaluation questionnaire; Q, question.
Table 2
| Hoe vaak… | Nooit | Soms | Vaak | Heel vaak | |
|---|---|---|---|---|---|
| Q4 | heb je pijn op de borst of een oncomfortabel gevoel | 1 | 2 | 3 | 4 |
| Q5 | verberg je je borstkas | 1 | 2 | 3 | 4 |
| Q6 | stoor je je aan hoe je borstkas eruitziet | 1 | 2 | 3 | 4 |
| Q7 | voel je je verlegen door je borstkas | 1 | 2 | 3 | 4 |
| Q8 | voel je je slecht over jezelf vanwege je borstkas | 1 | 2 | 3 | 4 |
| Q9 | heb je moeite met sporten | 1 | 2 | 3 | 4 |
| Q10 | veroorzaakte je borstkas kortademigheid | 1 | 2 | 3 | 4 |
| Q11 | veroorzaakte je borstkas vermoeidheid | 1 | 2 | 3 | 4 |
Part of the Dutch version of the PEEQ that is filled out by the child. PEEQ, pectus excavatum evaluation questionnaire; Q, question.
Table 3
| Hoe vaak… | Nooit | Soms | Vaak | Heel vaak | |
|---|---|---|---|---|---|
| Q12 | is het kind geïrriteerd | 1 | 2 | 3 | 4 |
| Q13 | is het kind gefrustreerd | 1 | 2 | 3 | 4 |
| Q14 | is het kind verdrietig | 1 | 2 | 3 | 4 |
| Q15 | is het kind rusteloos | 1 | 2 | 3 | 4 |
| Q16 | verbergt het kind zijn/haar borstkas | 1 | 2 | 3 | 4 |
| Q17 | is het kind terughoudend in zich omkleden in het bijzijn van anderen | 1 | 2 | 3 | 4 |
| Q18 | is het kind terughoudend in het dragen van zwemkleding | 1 | 2 | 3 | 4 |
| Q19 | heeft het kind moeite met sporten | 1 | 2 | 3 | 4 |
| Q20 | heeft het kind pijn op de borst | 1 | 2 | 3 | 4 |
| Q21 | heeft het kind last van kortademigheid | 1 | 2 | 3 | 4 |
| Q22 | heeft het kind last van vermoeidheid | 1 | 2 | 3 | 4 |
Part of the Dutch version of the PEEQ that is filled out by the parents. PEEQ, pectus excavatum evaluation questionnaire; Q, question.
Changes made upon review of the back translation and cognitive debriefing interviews can be found in Table 4. Items that were discussed during the reconciliation process are also listed. All steps undertaken in the translational process are described in more detail below.
Table 4
| Original item | Forward translation | Reconciliation of forward translation | Back translation | Review of back translation | Review of cognitive debriefing interviews |
|---|---|---|---|---|---|
| “Fatigue” | “Vermoeidheid” | “Vermoeidheid” | “Tired” | Accepted due to literal discrepancy | No changes |
| “Chest” | “Borstkas” and “Borst” | “Borstkas” was accepted | “Chest” | Identical to forward translation | No changes |
| “Happy” | “Tevreden” | “Tevreden” | “Satisfied” | Accepted due to cultural nuance | No changes |
| “Bothered because of” | “Last van” and “Storen aan” | “Storen aan” was accepted | “Bothered by” | Accepted due to literal discrepancy | No changes |
| “Sad/depressed” | “Verdrietig/depressief” | “Verdrietig/depressief” | “Sad/depressed” | Identical to forward translation | “Sad” was retained |
All changes made upon review of the back translation and cognitive debriefing interviews are listed. Items that were discussed during the reconciliation process are also provided.
Forward translation and reconciliation
The forward translation process was prone to synonymous translations which resulted in minor literal and diacritical discrepancies between the two different versions. The most culturally and linguistic appropriate translation was adhered to in the reconciliation process. For example, ‘bothered’ in item 6 was translated to ‘last van’ and ‘storen aan’ by the two individual translators respectively. These were ruled conceptually equal and ‘storen aan’ was retained as it is a more correct verb to express appearance issues, while ‘last van’ is more often used to express physical problems.
The forward translation process resulted in one important conceptual conflict between the two forward translations. Namely, the word ‘chest’ was translated to the Dutch word ‘borst’ by the translation service, while NJ had translated it as ‘borstkas’. In the reconciliation process the translation to ‘borstkas’ was accepted. This decision was made in consideration of conceptual equivalence. ‘Borst’ is a semantic equivalent, but can in Dutch also be used to refer to the feminine breast while ‘borstkas’ is both a semantic and conceptual equivalent to the word chest.
The response options for item 1 to 3 were translated as ‘very dissatisfied, dissatisfied, satisfied and very satisfied’ due to cultural nuances. These response options fit better in a Dutch context than the original response options ‘very unhappy, unhappy, happy and very happy’. The semantic equivalent of the latter is in Dutch used for expressing emotions rather than quality of life. The order of the response options of item 1 to 3 was also inverted so the scores per scale can be summed up to obtain a total score for the entire questionnaire. A higher score indicates a higher burden on the patient’s quality of life.
Back translation and review
The back translation process resulted in 6 literal discrepancies (Table 5). For example, where the original questionnaire used the word ‘fatigue’, it was back translated as ‘tired’. However, these words were considered as conceptually equal and no adjustment to the translation was needed. The back translation resulted in one conceptual discrepancy. The word ‘happy’ used in the original questionnaire was back translated as ‘satisfied’. This was expected and had already been discussed when reconciling the forward translation. Therefore, no changes to the translation were made upon review. See Table 5 for the results of the translation process.
Table 5
| Results back translation | PEEQ items, n (%) |
|---|---|
| Identical items | 15 (68.2) |
| Literal discrepancies | 6 (27.3) |
| Conceptual discrepancies resolved through discussion | 1 (4.5) |
| Total items | 22 (100.0) |
Number of identical items, literal and conceptual discrepancies are provided. PEEQ, pectus excavatum evaluation questionnaire.
Cognitive debriefing and final review
All eligible patients agreed to participate in the study and completed the patient interviews. Patient characteristics of the 10 included patients and results of the cognitive debriefing are displayed in Table 6. Median age of the patients was 15 years (IQR, 15–16) and the vast majority were boys (90%). Five patients were scheduled for the Nuss procedure and 5 patients already had undergone surgical correction of their pectus excavatum by the Nuss procedure. The questionnaire was well received by the patients and their parents. Median completing time of the PEEQ was 1.5 (1.0–1.7) minutes. None reported the length of the survey as inconvenient.
Table 6
| Patient characteristics | PEEQ pilot test |
|---|---|
| Patients, n (%) | 10 (100.0) |
| Preoperatively, n (%) | 5 (50.0) |
| Postoperatively, n (%) | 5 (50.0) |
| Type of planned or performed procedure, n (%) | |
| Nuss procedure | 10 (100.0) |
| Sex, n (%) | |
| Male | 9 (90.0) |
| Age, years, median (IQR) | 15 (15–16) |
| BMI, kg/m2, median (IQR) | 19.1 (16.8–20.3) |
| Preoperative HI, median (IQR) | 3.30 (3.12–3.71) |
| Completion time, minutes, median (IQR) | 1.5 (1.0–1.7) |
| Changes made upon comments, n | 1 |
| Participants rated survey content as pleasant, n (%) | 10 (100.0) |
| Participants reported survey length as pleasant, n (%) | 10 (100.0) |
Patient characteristics as well as the results from the cognitive debriefing interviews are shown. PEEQ, pectus excavatum evaluation questionnaire; IQR, interquartile range; BMI, body mass index; HI, Haller index.
Participant input led to the revision of item 14. Parents reported that they interpreted the Dutch translation of the term “sadness/depression” in the pilot version as sadness in the context of a depressive disorder. The expert panel resolved this issue by proposing a new translation. Clarification was asked from the developers to ensure correct interpretation and translation of the item.
No spelling, diacritical or grammatical errors were detected during proofreading of the final version.
Discussion
Pectus excavatum patients report physical complaints and psychosocial distress which can both lead to a diminished quality of life. As there is no correlation between the anatomical severity of the deformity and the level of impairment in HRQOL, it is important to measure outcomes in pectus excavatum from the patient’s perspective (17). Body image disturbances can indicate surgical correction regardless of the presence of cardiopulmonary compression or the HI, and therefore it is even more important to quantify the level of decrease in HRQOL.
A disease specific PROM is the best instrument for measuring the HRQOL in this patient population as the specific physical and psychosocial complaints that form a burden to these patients are not incorporated in generic PROMs like the 36-item short form health survey (SF-36) (25) or the EuroQol 5 dimensions 5 levels instrument (EQ-5D-5L) (26).
The PEEQ, developed by a team of clinical experts with years of experience and a psychologist, is the most used PROM for pediatric pectus excavatum patients. Currently, the PEEQ is only available in English. Especially pediatric patients who live in non-English speaking countries have a poor proficiency of the English language, while pediatric patients form the majority of the patient population. Thus, the PEEQ is not practically applicable in those countries.
Availability of this instrument is of value for both clinicians and researchers to improve pectus excavatum care. As our research team is located in The Netherlands, the aim of this study was to perform a Dutch translation to make the PEEQ available for measurement of the HRQOL in the patient population of The Netherlands. However, one member of the expert panel (YJLJ) is a surgeon from Belgium and ensured during the expert panel meetings that the questionnaire would be suitable for the Dutch speaking population of Belgium. Though we did only include Dutch patients originating from The Netherlands in the cognitive debriefing interviews, our translation of the PEEQ is also applicable to Dutch speaking patients who attend the public health care system in Belgium.
In this study we adhered to all recommendations by the WHO and ISPOR guidelines to produce a Dutch version of the questionnaire that is conceptually equal to the original questionnaire, linguistically validated and culturally adapted to the Dutch population. The core of the translation process is to ensure that the PROM in the target language will function the same at measuring the same constructs as the PROM in the source language (23). Though construct equivalence is measured post translational by a set of psychometric tests, it is important to minimize the threats to construct equivalence during the translation process. Disease specific PROMs should be translated with the same precautions as generic PROMs as it is not guaranteed that that both a conceptual and semantic equivalent is present in the target language for each disease specific item. Moreover, disease specific questionnaires often contain questions addressing more generic issues like body image perception. If the concept of interest exists in the target language, but not the expression then the expression can be substituted. If the expression exists, but not the concept, then the concept is culturally specific. If neither the expression, nor the concept exist, then it is not possible to translate that specific item (23,27).
By providing an insight in our translation process, we want to encourage other researchers to perform translations to other languages to make the PEEQ available to clinicians and researchers worldwide.
The PEEQ was originally designed for the pediatric population, which constitutes the majority of the pectus excavatum population (7). For the sake of external applicability we therefore involved pediatric patients and their parents during the cognitive debriefing process (2,17).
Krasopoulos and colleagues (14) modified the PEEQ as published by Lawson et al. (2) to fit the adult population and named their adjusted questionnaire the NQ-mA. They replaced the word ‘children’ by ‘people’ in item 4 (14). However, this question was not retained after further refinement of the PEEQ (17). Also, in the NQ-mA the part of the PEEQ that is filled out by the parents is omitted (14). We therefore advocate that the parent’s part could be left out of the Dutch version of the PEEQ, developed by the present study, if one wants to use the translation for the adult population.
The content of the PEEQ possibly applies to patients with other thoracic wall deformities like pectus carinatum or pectus arcuatum. The PEEQ was developed by a psychologist and a group of clinical experts having experience with pectus excavatum, but it is unknown if they have a considerable experience with other thoracic wall deformities. Also, patient input from only pectus excavatum patients was used in the development process. The questionnaire was thus not developed and validated for other patient groups. Therefore, we cannot ensure that all topics of interest for other patient groups are included in our Dutch translation of the PEEQ, that the items are formulated in a manner that suits those patient groups best and that the measure functions the same across different patient groups. Future research should prove whether the current questionnaire can be extrapolated to other thoracic wall diseases with minimal changes.
Strengths and limitations
The required sample size for the pilot testing phase of a translation study is not unanimously established. The ISPOR guideline (23) recommends 5 to 8 patients while the WHO guideline (22) advises a minimum of 10 patients to participate in the pilot test. We chose to select the highest required number of 10 patients to enhance scientific value. Our study sample adequately represents the target population of the PEEQ in terms of age and male to female ratio, as established by large cohort studies (7,17). By including an equal number of preoperative and postoperative patients the instrument is applicable to patients across the full range of their treatment (23).
During the cognitive debriefing, the questionnaire proved to be easy to understand and only one item required revision. The risk of selection bias was considered minimal as the participants were consecutively recruited and all eligible patients agreed to participate in the study. There was no response bias in the current study as all included patients completed the questionnaires and interviews. No bias due to missing data was present given the prospective nature of this study. Interviews were obtained by a single interviewer to prevent interviewer bias. An interview protocol was written in advance by the expert panel.
The present study was designed to perform a Dutch translation of the PEEQ questionnaire. The study was not designed to evaluate what the optimal frequency of taking the questionnaire is to optimally detect changes in the HRQOL, as well as to minimize respondent fatigue. Therefore, we recommend for further studies to establish guidelines about the optimal frequency for the PEEQ to be administered. For now, we advise clinicians and researchers to take the questionnaire when changes in quality of life are expected or when the patient reports changes in variables that could influence the quality of life.
Moreover, before the PEEQ can be implemented in clinical practice and research, further psychometric validation (i.e., test-retest, internal consistency and responsiveness) as well as cross-cultural evaluation by applying Rash methodology is warranted. Our research team intends to perform such psychometric validation and evaluation soon.
Conclusions
This study provides a step-by-step Dutch translation, cultural adaptation, and linguistic validation of the PEEQ designed for pediatric patients. Further psychometric validation of this PROM is warranted before implementation in clinical practice and research. We provide a structural approach that can be used by other research groups to perform translations of the PEEQ to additional languages to make it available for clinicians and their patients worldwide and to bridge the gap of HRQOL evaluation in non-English speaking countries.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-252/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-252/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-252/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committee of Zuyderland Medical Center, Heerlen, The Netherlands (registration number: METCZ20210182; date of approval: December 6, 2021). Written informed consent was obtained prior to inclusion from the patients and if applicable, from patients’ parent(s) or legal guardian(s).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fokin AA, Steuerwald NM, Ahrens WA, et al. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg 2009;21:44-57. [Crossref] [PubMed]
- Lawson ML, Cash TF, Akers R, et al. A pilot study of the impact of surgical repair on disease-specific quality of life among patients with pectus excavatum. J Pediatr Surg 2003;38:916-8. [Crossref] [PubMed]
- Sacco Casamassima MG, Gause C, Goldstein SD, et al. Patient Satisfaction After Minimally Invasive Repair of Pectus Excavatum in Adults: Long-Term Results of Nuss Procedure in Adults. Ann Thorac Surg 2016;101:1338-45. [Crossref] [PubMed]
- Kuru P, Bostanci K, Ermerak NO, et al. Quality of life improves after minimally invasive repair of pectus excavatum. Asian Cardiovasc Thorac Ann 2015;23:302-7. [Crossref] [PubMed]
- Ewert F, Syed J, Kern S, et al. Symptoms in Pectus Deformities: A Scoring System for Subjective Physical Complaints. Thorac Cardiovasc Surg 2017;65:43-9. [Crossref] [PubMed]
- Fonkalsrud EW, Dunn JC, Atkinson JB. Repair of pectus excavatum deformities: 30 years of experience with 375 patients. Ann Surg 2000;231:443-8. [Crossref] [PubMed]
- Kelly RE Jr, Shamberger RC, Mellins RB, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg 2007;205:205-16. [Crossref] [PubMed]
- Steinmann C, Krille S, Mueller A, et al. Pectus excavatum and pectus carinatum patients suffer from lower quality of life and impaired body image: a control group comparison of psychological characteristics prior to surgical correction. Eur J Cardiothorac Surg 2011;40:1138-45. [Crossref] [PubMed]
- Miller KA, Woods RK, Sharp RJ, et al. Minimally invasive repair of pectus excavatum: a single institution's experience. Surgery 2001;130:652-7; discussion 657-9. [Crossref] [PubMed]
- Salsburg DS. Measuring Disease. Errors, Blunders, Lies. 1st edition. New York, NY, USA: Chapman and Hall/CRC, 2016:37-44. ISBN-13: 978-1498795784.
- Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346:f167. [Crossref] [PubMed]
- Weldring T, Smith SM. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights 2013;6:61-8. [Crossref] [PubMed]
- Kingsley C, Patel S. Patient-reported outcome measures and patient-reported experience measures. BJA Educ 2017;17:137-44. [Crossref]
- Krasopoulos G, Dusmet M, Ladas G, et al. Nuss procedure improves the quality of life in young male adults with pectus excavatum deformity. Eur J Cardiothorac Surg 2006;29:1-5. [Crossref] [PubMed]
- Kim HK, Shim JH, Choi KS, et al. The quality of life after bar removal in patients after the nuss procedure for pectus excavatum. World J Surg 2011;35:1656-61. [Crossref] [PubMed]
- Metzelder ML, Kuebler JF, Leonhardt J, et al. Self and parental assessment after minimally invasive repair of pectus excavatum: lasting satisfaction after bar removal. Ann Thorac Surg 2007;83:1844-9. [Crossref] [PubMed]
- Kelly RE Jr, Cash TF, Shamberger RC, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 2008;122:1218-22. [Crossref] [PubMed]
- Norlander L, Karlsson J, Anderzén-Carlsson A, et al. Translation and psychometric evaluation of the Swedish versions of the Nuss Questionnaire modified for Adults and the Single Step Questionnaire. J Patient Rep Outcomes 2021;5:21. [Crossref] [PubMed]
- Bahadir AT, Kuru P, Afacan C, et al. Validity and reliability of the Turkish version of the nuss questionnaire modified for adults. Korean J Thorac Cardiovasc Surg 2015;48:112-9. [Crossref] [PubMed]
- Zuidema WP, van der Steeg AFW, Oosterhuis JWA, et al. Trends in the Treatment of Pectus Excavatum in the Netherlands. Eur J Pediatr Surg 2021;31:261-5. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- WHO. Tools guideline (Process of translation and adaptation of instruments). World Heal Organ 2020. Available online: https://www.mhinnovation.net/sites/default/files/files/WHO%20Guidelines%20on%20Translation%20and%20Adaptation%20of%20Instruments.docx
- Wild D, Grove A, Martin M, et al. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005;8:94-104. [Crossref] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Ware JE Jr, Gandek B. The SF-36 Health Survey: Development and Use in Mental Health Research and the IQOLA Project. Int J Ment Health 1994;23:49-73. [Crossref]
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. [Crossref] [PubMed]
- Patrick DL, Wild DJ, Johnson ES, et al. Cross-cultural validation of quality of life measures. In: Orley J, Kuyken W. editors. Quality of Life Assessment: International Perspectives. Heidelberg: Springer-Verlag, 1994:19-32.


