
Impact of albumin infusion on prognosis of intensive care unit patients with congestive heart failure-hypoalbuminemia overlap: a retrospective cohort study
Introduction
Serum albumin accounts for more than 50% of plasma protein molecules in healthy individuals and its physiological level is 3.5–5.0 g/dL. Hypoalbuminemia is common in patients with cardiovascular diseases (1-3), and 20–50% of patients with heart failure (HF) have overlapping hypoalbuminemia (4-6). This proportion can be as high as 89% in frail, elderly patients with HF (7). Serum albumin has many physiological properties, including anti-inflammatory, anti-oxidant, anti-platelet aggregation, and anti-coagulant (8-11). More importantly, it provides 75% of plasma colloid oncotic pressure (12). Serum albumin deficiency is associated with the occurrence of HF (13,14), and is related to mortality, rehospitalization, and aggravation in HF patients (4-6).
As an important means of serum albumin supplementation, albumin infusion has been widely used in decompensated cirrhosis patients in clinical practice (15). However, the impact of albumin infusion remains controversial in critically ill patients. Albumin infusion improves organ function in critically ill patients with hypoalbuminemia (16). For the underlying disease, albumin infusion improves the prognosis in acute lung injury and severe sepsis patients (17,18). However, albumin infusion appears to increase mortality in patients with traumatic brain injury (19). For HF patients, albumin infusion could theoretically improve organ perfusion, but might also increase the load on the heart. In clinical practice, we prescribed albumin infusion for congestive heart failure (CHF) patients with severe hypoalbuminemia and edema. In some patients, edema was subsided and organ perfusion was improved. But some patients got worsen. Therefore, the impact of albumin infusion remains unclear. There is lack of research to investigate the impact of albumin infusion on the prognosis of CHF patients complicated with hypoalbuminemia. The purpose of this study was to investigate the impact of albumin infusion on prognosis of Intensive Care Unit (ICU) patients with CHF-hypoalbuminemia overlap. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-648/rc).
Methods
Date source and variables
We performed a retrospective cohort study based on the Medical Information Mart for Intensive Care III (MIMIC-III) database version 1.4, which comprises the characteristics, laboratory outcomes, and medical records of more than 50,000 patients admitted to Beth Israel Deaconess Medical Center from 2001 to 2012 (20). We completed the National Institutes of Health’s web-based course, passed the Protecting Human Research Participants module, and obtained database access (ID: 32540900). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
We used structure query language (SQL) to extract data from the MIMIC-III database, including demographics [age, gender, ethnicity, and body mass index (BMI)], comorbidities [diabetes mellitus, hypertension, acute myocardial infarction (AMI), cardiac arrhythmias, chronic pulmonary disease, renal failure, liver disease, stroke, and malignancy], vital signs [systolic blood pressure (SBP), diastolic blood pressure (DBP)], laboratory results [hemoglobin, glucose, potassium, sodium, serum creatine (SCr), alanine transaminase (ALT), aspartate transaminase (AST), lactate, serum albumin, and left ventricular ejection fraction (LVEF)], scoring system [SAPS-II (simplified acute physiology score II) scores (21) and SOFA (sequential organ failure assessment) scores (22)] and therapy [albumin infusion, beta-blockers (BBs), renin-angiotensin system inhibitors (RASIs), mineralcorticoid receptor antagonists (MRAs), diuretic, vasopressor, renal replacement therapy (RRT), and ventilation].
Inclusion and exclusion criteria
We enrolled all patients whose diagnosis included CHF [ICD-9 (international classification of diseases 9) code =428.0] at first ICU admission from the database. The exclusion criteria were as follows: (I) patients with missing serum albumin values; (II) serum albumin >3.4 g/dL; and (III) patients <18 years old.
Patient stratification
We stratified the patients into two groups based on their exposure to albumin infusion: the albumin group included patients who received albumin infusion, and the non-albumin group comprised patients who did not receive albumin infusion during hospitalization.
Follow-up and outcomes
Patients were followed up from the date of admission to the date of discharge or death. Information on mortality was obtained from the Social Security Death Index records. This study’s primary outcome was in-hospital mortality, and the secondary outcomes were the length of stay in the ICU (ICU LOS) and the hospital (hospital LOS).
Statistical analyses
To unbiasedly ascertain the impact of albumin infusion on in-hospital mortality, we performed a 1:1 propensity-score matching (PSM) between the albumin and non-albumin groups based on the estimated propensity scores, using the nearest neighbor method without replacement (23,24). A caliper width of 0.2 was used. The following variables were used to construct the propensity score model: age, liver disease, malignancy, lactate, albumin, LVEF, SAPS-II scores, SOFA scores, ventilator, RRT, vasopressor, BBs, RASIs, and diuretic.
Continuous variables were summarized using the median [interquartile range (IQR)], and categorical variables were summarized using counts (percentages). Differences in baseline variables between the albumin and non-albumin groups were evaluated using the Mann-Whitney U test for continuous variables and the chi-square test for categorical variables. Logistic regression analysis was used to examine the association between albumin infusion and in-hospital mortality. Odds ratios (ORs) and 95% confidence intervals (CIs) were presented. Variables with a P value <0.1 in the univariate logistic regression analysis model were entered into the multivariate models. A two-sided P value <0.05 was considered to indicate statistical significance in all analyses. Statistical analysis was performed by using STATA 12.0 (STATA Corporation, College Station, USA) and SPSS 22.0 (SPSS Inc, Chicago, IL, USA).
Results
Subject characteristics
Overall, 8,593 ICU patients met the inclusion criteria. Among them, 3,761 patients with missing serum albumin values, 1,641 patients with serum albumin >3.4 g/dL, and one patient younger than 18 years old were excluded. In total, 3,190 patients [median age 75.79 (64.50–83.34) years old, 52.41% males] satisfied the selection criteria (Figure 1). These patients were divided into albumin and non-albumin groups depending on whether they received albumin infusion during hospitalization. A comparison of the baseline clinical characteristics between the two groups is shown in Table 1. Patients in the albumin group tended to have a higher proportion of liver disease, malignancy, MRAs, BBs, diuretic, vasopressor, RRT, ventilation, and a lower proportion of AMI and RASIs. They also tended to be younger, with higher BMI, LVEF, SAPS-II scores, and SOFA scores, and lower SBP, DBP, hemoglobin, sodium, glucose, ALT, lactate, and serum albumin.
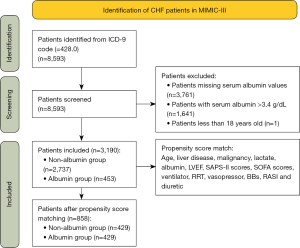
Table 1
| Characteristics | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| Non-albumin group (n=2,737) | Albumin group (n=453) | P | Non-albumin group (n=429) | Albumin group (n=429) | P | ||
| Gender, n (%) | 0.794 | 0.681 | |||||
| Male | 1,432 (52.32) | 240 (52.98) | 233 (54.31) | 227 (52.91) | |||
| Female | 1,305 (47.68) | 213 (47.02) | 196 (45.69) | 202 (47.09) | |||
| Age, years | 76.22 (64.68–83.60) | 72.98 (62.88–81.23) | <0.001 | 75.06 (62.78–82.81) | 73.86 (63.16–81.53) | 0.383 | |
| Ethnicity, n (%) | 0.081 | 0.167 | |||||
| White | 2,035 (74.35) | 352 (77.70) | 324 (75.52) | 332 (77.39) | |||
| Black | 204 (7.45) | 21 (4.64) | 32 (7.46) | 19 (4.43) | |||
| Others | 498 (18.20) | 80 (17.66) | 73 (17.02) | 78 (18.18) | |||
| BMI, kg/m2 | 26.93 (23.19–31.59) | 27.76 (24.61–32.57) | 0.006 | 0.355 | |||
| Comorbidities, n (%) | |||||||
| Diabetes mellitus | 910 (33.25) | 166 (36.64) | 0.157 | 135 (31.47) | 160 (37.30) | 0.072 | |
| Hypertension | 511 (18.67) | 93 (20.53) | 0.349 | 70 (16.32) | 88 (20.51) | 0.113 | |
| AMI | 647 (23.64) | 88 (19.43) | 0.049 | 95 (22.14) | 85 (19.81) | 0.402 | |
| Cardiac arrhythmias | 907 (33.14) | 156 (34.44) | 0.587 | 150 (34.96) | 147 (34.27) | 0.830 | |
| Chronic pulmonary disease | 674 (24.63) | 116 (25.61) | 0.654 | 122 (28.44) | 110 (25.64) | 0.356 | |
| Renal failure | 627 (22.91) | 115 (25.39) | 0.248 | 93 (21.68) | 108 (25.17) | 0.227 | |
| Liver disease | 126 (4.60) | 66 (14.57) | <0.001 | 43 (10.02) | 51 (11.89) | 0.382 | |
| Stroke | 152 (5.55) | 25 (5.52) | 0.976 | 26 (6.06) | 25 (5.83) | 0.885 | |
| Malignancy | 532 (19.44) | 119 (26.27) | 0.001 | 113 (26.34) | 114 (26.57) | 0.938 | |
| Vital signs, mmHg | |||||||
| SBP | 119 (103–137) | 113 (98–132) | <0.001 | 117 (100.5–134.5) | 113 (98–133) | 0.094 | |
| DBP | 59 (49–71) | 57 (48–67) | 0.008 | 59 (50–69) | 57 (49–66) | 0.069 | |
| Laboratory results | |||||||
| Hemoglobin, g/dL | 10.7 (9.5–12) | 10.4 (9.2–11.8) | 0.001 | 10.4 (9.4–11.8) | 10.5 (9.3–11.8) | 0.832 | |
| Sodium, mEq/L | 139 (136–142) | 139 (135–141) | 0.005 | 139 (136–141) | 139 (135–141) | 0.216 | |
| Potassium, mEq/L | 4.1 (3.7–4.6) | 4.2 (3.8–4.6) | 0.175 | 4.2 (3.7–4.6) | 4.2 (3.8–4.6) | 0.741 | |
| Glucose, mg/dL | 128 (103–170) | 124 (99–159) | 0.017 | 129 (101.5–175) | 123 (100–157) | 0.057 | |
| SCr, mg/dL | 1.2 (0.9–2) | 1.2 (0.9–2) | 0.650 | 1.2 (0.8–2.1) | 1.2 (0.9–1.9) | 0.980 | |
| ALT, IU/L | 31 (17–72) | 26 (14–59) | 0.005 | 30 (16–65) | 26 (14–58) | 0.096 | |
| AST, IU/L | 41 (23–93) | 41 (23–83) | 0.961 | 44 (24–96.5) | 41 (23–83) | 0.282 | |
| Lactate, mmol/L | 1.9 (1.3–2.5) | 1.7 (1.2–2.5) | 0.016 | 1.8 (1.3–2.5) | 1.7 (1.2–2.4) | 0.089 | |
| Serum albumin, g/dL | 2.9 (2.6–3.2) | 2.7 (2.3–3.1) | <0.001 | 2.7 (2.3–3.1) | 2.7 (2.4–3.1) | 0.414 | |
| LVEF, n (%) | 0.017 | 0.166 | |||||
| >50% | 1,226 (44.79) | 233 (51.43) | 236 (55.01) | 216 (50.35) | |||
| 40–50% | 418 (15.27) | 68 (15.01) | 56 (13.05) | 65 (15.15) | |||
| 30–40% | 490 (17.90) | 79 (17.44) | 57 (13.29) | 77 (17.95) | |||
| <30% | 603 (22.03) | 73 (16.11) | 80 (18.65) | 71 (16.55) | |||
| Scoring system | |||||||
| SAPS-II | 41 (33–50) | 46 (36–55) | <0.001 | 44 (36–56) | 46 (36–55) | 0.729 | |
| SOFA | 5 (3–7) | 6 (4–9) | <0.001 | 6 (4–9) | 6 (4–9) | 0.957 | |
| Therapy, n (%) | |||||||
| RASIs | 1,155 (42.02) | 147 (32.45) | <0.001 | 136 (31.70) | 147 (34.27) | 0.424 | |
| MRAs | 163 (5.96) | 49 (10.82) | <0.001 | 32 (7.46) | 42 (9.79) | 0.224 | |
| BBs | 1,935 (70.70) | 374 (82.56) | <0.001 | 355 (82.75) | 354 (82.52) | 0.928 | |
| Diuretic | 1,995 (72.89) | 406 (89.62) | <0.001 | 378 (88.11) | 383 (89.28) | 0.590 | |
| Vasopressor | 1,241 (45.34) | 327 (72.19) | <0.001 | 310 (72.26) | 303 (70.63) | 0.597 | |
| RRT | 96 (3.51) | 69 (15.23) | <0.001 | 48 (11.19) | 50 (11.66) | 0.830 | |
| Ventilation | 1,509 (55.13) | 350 (77.26) | <0.001 | 334 (77.86) | 328 (76.46) | 0.626 | |
For categorical variables, n (%) is presented. For continuous variables, median [interquartile range (IQR)] is presented. BMI, body mass index; AMI, acute myocardial infarction; SBP, systemic blood pressure; DBP, diastolic blood pressure; SCr, serum creatine; ALT, alanine transaminase; AST, aspartate transaminase; LVEF, left ventricular ejection fraction; SAPS-II, simplified acute physiology score II; SOFA, sequential organ failure assessment; RASIs, renin-angiotensin system inhibitors; MRAs, mineralcorticoid receptor antagonist; BBs, beta-blockers; RRT, renal replacement therapy.
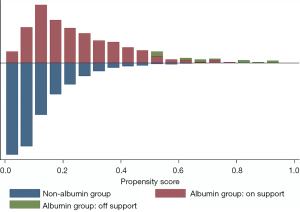
Subsequently, PSM was performed to control for measurable confounding factors. The mean propensity score of all patients was 0.142. The mean scores of the albumin and non-albumin groups were 0.206 and 0.122, with minimum and maximum scores ranging from 0.013–0.925 and 0.006–0.803, respectively. A graphical representation of the propensity score is displayed in Figure 2. After matching, 429 pairs of patients [median age 74.33 (63.07–82.12) years old, 53.61% male] were included in the final analysis (Figure 1). There were no statistically significant differences in the baseline characteristics between the two groups (Table 1).
Outcomes
Before PSM, there were 762 (23.89%) in-hospital deaths. The in-hospital mortality of the albumin group was markedly higher than that of the non-albumin group (36.42% vs. 21.81%, P<0.001). After matching, there were 267 (31.12%) deaths, and the in-hospital mortality was still higher in the albumin group compared to the non-albumin group (34.97% vs. 27.27%, P=0.015) (Figure 3A).

Before matching, the ICU LOS [median 6.93 (3.39–14.82) vs. 3.84 (1.96–8.00) days, P<0.001] and hospital LOS [median 17.46 (11.45–28.33) vs. 10.92 (6.81–18.00) days, P<0.001] were longer in the albumin group than the non-albumin group. After matching, the ICU LOS [median 8.43 (4.33–16.28) vs. 6.43 (3.07–13.66) days, P<0.001] and hospital LOS [median 16.92 (11.27–28.06) vs. 13.33 (8.00–21.10) days, P<0.001] remained longer in the albumin group than the non-albumin group (Figure 3B,3C).
Before matching, the serum albumin levels were similar between admission and discharge in the albumin group [median 2.7 (2.3–3.1) vs. 2.7 (2.3–3.1) g/dL, P=0.629]. In the non-albumin group, the serum albumin level was 2.9 (2.6–3.2) g/dL at admission and 2.8 (2.4–3.1) g/dL at discharge (P<0.0001 vs. admission). After matching, the serum albumin levels remained similar between admission and discharge in the albumin group [median 2.7 (2.4–3.1) vs. 2.7 (2.3–3.1) g/dL, P=0.838]. In the non-albumin group, the serum albumin level was 2.7 (2.3–3.1) g/dL at admission and 2.6 (2.3–3.0) g/dL at discharge (P=0.019 vs. admission) (Figure 4).

Albumin infusion and in-hospital mortality
Table 2 shows the results of the logistic regression analysis before PSM. In the univariate analysis, age, ethnicity (Other), cardiac arrhythmias, liver disease, stroke, potassium, SCr, ALT, AST, lactate, LVEF (<30%), SAPS-II scores, SOFA scores, vasopressor, RRT, ventilation, and albumin infusion were associated with an increased risk of in-hospital mortality. Meanwhile, BMI, diabetes mellitus, SBP, DBP, hemoglobin, sodium, serum albumin, RASIs, BBs, and diuretic were associated with a decreased risk of in-hospital mortality.
Table 2
| Variables | P | OR | 95% CIs | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | <0.001 | 1.019 | 1.012 | 1.025 |
| Ethnicity | ||||
| White | Ref. | Ref. | Ref. | Ref. |
| Black | 0.098 | 0.741 | 0.520 | 1.057 |
| Other | <0.001 | 1.588 | 1.301 | 1.940 |
| BMI | 0.082 | 0.990 | 0.980 | 1.001 |
| Diabetes mellitus | 0.008 | 0.789 | 0.662 | 0.941 |
| Cardiac arrhythmias | 0.001 | 1.325 | 1.119 | 1.570 |
| Liver disease | 0.002 | 1.648 | 1.206 | 2.250 |
| Stroke | 0.003 | 1.643 | 1.189 | 2.271 |
| SBP | <0.001 | 0.994 | 0.991 | 0.997 |
| DBP | <0.001 | 0.990 | 0.985 | 0.995 |
| Hemoglobin | 0.035 | 0.955 | 0.915 | 0.997 |
| Sodium | 0.092 | 0.987 | 0.971 | 1.002 |
| Potassium | 0.093 | 1.094 | 0.985 | 1.214 |
| SCr | 0.036 | 1.052 | 1.003 | 1.103 |
| ALT | 0.022 | 1.000 | 1.000 | 1.000 |
| AST | 0.018 | 1.000 | 1.000 | 1.000 |
| Lactate | <0.001 | 1.225 | 1.174 | 1.278 |
| Serum albumin | <0.001 | 0.446 | 0.374 | 0.531 |
| LVEF | ||||
| >55 | Ref. | Ref. | Ref. | Ref. |
| 40–55 | 0.855 | 0.977 | 0.762 | 1.253 |
| 30–40 | 0.276 | 1.135 | 0.903 | 1.426 |
| <30 | <0.001 | 1.469 | 1.194 | 1.806 |
| SAPS-II | <0.001 | 1.062 | 1.055 | 1.069 |
| SOFA | <0.001 | 1.226 | 1.195 | 1.258 |
| RASIs | <0.001 | 0.344 | 0.286 | 0.415 |
| BBs | <0.001 | 0.402 | 0.339 | 0.478 |
| Diuretic | <0.001 | 0.584 | 0.489 | 0.699 |
| Vasopressor | <0.001 | 2.919 | 2.455 | 3.470 |
| RRT | <0.001 | 5.492 | 3.972 | 7.592 |
| Ventilation | <0.001 | 2.492 | 2.081 | 2.985 |
| Albumin infusion | <0.001 | 2.054 | 1.662 | 2.538 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; SBP, systemic blood pressure; DBP, diastolic blood pressure; SCr, serum creatine; ALT, alanine transaminase; AST, aspartate transaminase; LVEF, left ventricular ejection fraction; SAPS-II, simplified acute physiology score II; SOFA, sequential organ failure assessment; RASIs, renin-angiotensin system inhibitors; BBs, beta-blockers; RRT, renal replacement therapy.
In the multivariate analysis, albumin infusion (OR, 1.509; 95% CI: 1.164–1.957; P=0.002), age (OR, 1.020; 95% CI: 1.011–1.028; P<0.001), ethnicity (other; OR, 1.521; 95% CI: 1.205–1.921; P<0.001), cardiac arrhythmias (OR, 1.437; 95% CI: 1.174–1.759; P<0.001), liver disease (OR, 1.507; 95% CI: 1.020–2.227; P=0.040), stroke (OR, 1.915; 95% CI: 1.314–2.789; P=0.001), lactate (OR, 1.105; 95% CI: 1.053–1.160; P<0.001), LVEF (<30%; OR, 1.571; 95% CI: 1.227–2.012; P<0.001), SAPS-II scores (OR, 1.038; 95% CI: 1.031–1.046; P<0.010), vasopressor (OR, 1.358; 95% CI: 1.087–1.697; P=0.007), RRT (OR, 2.642; 95% CI: 1.813–3.852; P<0.001), and ventilation (OR, 1.619; 95% CI: 1.285–2.040; P<0.001) were independently associated with an increased risk of in-hospital mortality, while sodium (OR, 0.979; 95% CI: 0.962–0.996; P=0.017), serum albumin (OR, 0.682; 95% CI: 0.555–0.838; P<0.001), RASIs (OR, 0.514; 95% CI: 0.412–0.640; P<0.001), and BBs (OR, 0.456; 95% CI: 0.368–0.566; P<0.001) were significantly associated with a decreased risk of in-hospital mortality (Table 3).
Table 3
| Variables | P | OR | 95% CIs | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | <0.001 | 1.020 | 1.011 | 1.028 |
| Ethnicity | ||||
| White | Ref. | Ref. | Ref. | Ref. |
| Black | 0.594 | 0.893 | 0.589 | 1.355 |
| Other | 0.000 | 1.521 | 1.205 | 1.921 |
| Cardiac arrhythmias | <0.001 | 1.437 | 1.174 | 1.759 |
| Liver disease | 0.040 | 1.507 | 1.020 | 2.227 |
| Stroke | 0.001 | 1.915 | 1.314 | 2.789 |
| Sodium | 0.017 | 0.979 | 0.962 | 0.996 |
| Lactate | <0.001 | 1.105 | 1.053 | 1.160 |
| Serum albumin | <0.001 | 0.682 | 0.555 | 0.838 |
| LVEF | ||||
| >55 | Ref. | Ref. | Ref. | Ref. |
| 40–55 | 0.694 | 0.945 | 0.711 | 1.255 |
| 30–40 | 0.164 | 1.206 | 0.926 | 1.571 |
| <30 | <0.001 | 1.571 | 1.227 | 2.012 |
| SAPS-II | <0.001 | 1.038 | 1.031 | 1.046 |
| RASIs | <0.001 | 0.514 | 0.412 | 0.640 |
| BBs | <0.001 | 0.456 | 0.368 | 0.566 |
| Vasopressor | 0.007 | 1.358 | 1.087 | 1.697 |
| RRT | <0.001 | 2.642 | 1.813 | 3.852 |
| Ventilation | <0.001 | 1.619 | 1.285 | 2.040 |
| Albumin infusion | 0.002 | 1.509 | 1.164 | 1.957 |
OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; SAPS-II, simplified acute physiology score II; RASIs, renin-angiotensin system inhibitors; BBs, beta-blockers; RRT, renal replacement therapy.
Table 4 shows the results of the logistic regression analysis after PSM. In the univariate analysis, age, liver disease, SCr, lactate, SAPS-II scores, SOFA scores, vasopressor, RRT, ventilation, and albumin infusion were associated with an increased risk of in-hospital mortality. Meanwhile, diabetes mellitus, SBP, DBP, hemoglobin, sodium, serum albumin, RASIs, BBs, and diuretic were associated with a decreased risk of in-hospital mortality.
Table 4
| Variables | P | OR | 95% CIs | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.011 | 1.015 | 1.003 | 1.026 |
| Diabetes mellitus | 0.033 | 0.711 | 0.520 | 0.972 |
| Liver disease | 0.069 | 1.507 | 0.969 | 2.344 |
| SBP | 0.006 | 0.992 | 0.986 | 0.998 |
| DBP | 0.004 | 0.986 | 0.977 | 0.996 |
| Hemoglobin | 0.006 | 0.896 | 0.829 | 0.969 |
| Sodium | 0.080 | 0.975 | 0.948 | 1.003 |
| SCr | 0.009 | 1.138 | 1.034 | 1.254 |
| Lactate | <0.001 | 1.249 | 1.145 | 1.362 |
| Serum albumin | <0.001 | 0.512 | 0.383 | 0.684 |
| SAPS-II | <0.001 | 1.049 | 1.038 | 1.061 |
| SOFA | <0.001 | 1.216 | 1.163 | 1.271 |
| RASIs | <0.001 | 0.377 | 0.267 | 0.532 |
| BBs | <0.001 | 0.234 | 0.162 | 0.338 |
| Diuretic | <0.001 | 0.242 | 0.156 | 0.375 |
| Vasopressor | <0.001 | 2.070 | 1.458 | 2.937 |
| RRT | <0.001 | 4.015 | 2.599 | 6.201 |
| Ventilation | 0.009 | 1.634 | 1.131 | 2.360 |
| Albumin infusion | 0.015 | 1.434 | 1.072 | 1.917 |
OR, odds ratio; CI, confidence interval; SBP, systemic blood pressure; DBP, diastolic blood pressure; SCr, serum creatine; SAPS-II, simplified acute physiology score II; SOFA, sequential organ failure assessment; RASIs, renin-angiotensin system inhibitors; BBs, beta-blockers; RRT, renal replacement therapy.
In the multivariate analysis, albumin infusion (OR, 1.594; 95% CI: 1.143–2.223; P=0.006), age (OR, 1.024; 95% CI: 1.009–1.039; P=0.001), SAPS-II scores (OR, 1.022; 95% CI: 1.005–1.039; P=0.010), SOFA scores (OR, 1.082; 95% CI: 1.014–1.156; P=0.018), and RRT (OR, 3.196; 95% CI: 1.933–5.282; P<0.001) were independently associated with an increased risk of in-hospital mortality, while serum albumin (OR, 0.582; 95% CI: 0.418–0.812; P=0.001), RASIs (OR, 0.525; 95% CI: 0.357–0.773; P=0.001), BBs (OR, 0.331; 95% CI: 0.214–0.512; P<0.001), and diuretic (OR, 0.560; 95% CI: 0.333–0.942; P=0.029) were significantly associated with a decreased risk of in-hospital mortality (Table 5).
Table 5
| Variables | P | OR | 95% CIs | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.001 | 1.024 | 1.009 | 1.039 |
| Serum albumin | 0.001 | 0.582 | 0.418 | 0.812 |
| SAPS-II | 0.010 | 1.022 | 1.005 | 1.039 |
| SOFA | 0.018 | 1.082 | 1.014 | 1.156 |
| RASIs | 0.001 | 0.525 | 0.357 | 0.773 |
| BBs | <0.001 | 0.331 | 0.214 | 0.512 |
| Diuretic | 0.029 | 0.560 | 0.333 | 0.942 |
| RRT | <0.001 | 3.196 | 1.933 | 5.282 |
| Albumin infusion | 0.006 | 1.594 | 1.143 | 2.223 |
OR, odds ratio; CI, confidence interval; SAPS-II, simplified acute physiology score II; SOFA, sequential organ failure assessment; RASIs, renin-angiotensin system inhibitors; BBs, beta-blockers; RRT, renal replacement therapy.
Discussion
Among the 4,832 patients with serum albumin values, the proportion of patients with overlapping hypoalbuminemia was as high as 66.04% (3,191/4,832), which is higher than that reported in previous studies (4-6). Among the 3,190 eligible patients, the proportion of patients receiving albumin infusion was only 14.20% (453/3,190). Hypoalbuminemia is widespread in ICU patients with CHF. However, due to the absence of evidence, clinicians are cautious about albumin infusion. Therefore, it is particularly important to investigate the impact of albumin infusion on prognosis of ICU patients with CHF-hypoalbuminemia overlap. ICU patients with CHF-hypoalbuminemia overlap. The present real-world PSM study is the first to verify that albumin infusion significantly increased the in-hospital mortality, ICU LOS, and hospital LOS of ICU patients with CHF-hypoalbuminemia overlap, after adjusting for multiple covariates, including age, diabetes mellitus, liver disease, SBP, DBP, hemoglobin, sodium, SCr, lactate, serum albumin, SAPS-II scores, SOFA scores, RASIs, BBs, diuretic, vasopressor, RRT, and ventilation.
HF is a complex clinical syndrome caused by structural or functional impairment of ventricular filling or ejection of blood and is characterized by pulmonary and peripheral circulation congestion (25). Chronic peripheral circulation congestion can impair liver and kidney function, which inhibits albumin synthesis and promotes albumin loss. Meanwhile, peripheral circulation congestion increases hydrostatic venous pressure, which in turn increases vascular endothelial permeability, leading to albumin escape to the extravascular space through the venous end of the microvasculature (26). In addition, intestinal congestion not only increases albumin enteric losses (27) but also reduces the absorption of nutrients and leads to malnutrition, which inhibits albumin synthesis (28). HF is a systemic inflammatory disease in which activated monocytes promote the release of IL (interleukin)-1, IL-6, and TNF (tumor necrosis factor)-α (29). These cytokines not only act against the hepatic synthesis of albumin but also increase vascular endothelial permeability and albumin escape (28,30). Low serum albumin is a downstream biomarker of HF syndrome severity but is not a determinant of poorer prognosis. Zhuang et al. found that serum albumin is inversely associated with the occurrence of HF; however, Mendelian randomization analyses did not show evidence of serum albumin in the etiology of HF (31). This partially supports our view, especially given the absence of etiological studies of low albumin and HF prognosis.
Human albumin is used to correct hypoalbuminemia, volume expansion, and resuscitation (18). In practice, clinicians are cautious about albumin infusion. Clinicians may prescribe albumin infusion for patients with severe hypoalbuminemia or severe edema. However, Margarson et al. found that a 20% albumin infusion in sepsis patients led to a significantly faster decrease in serum albumin compared with healthy controls (32). Lewis et al. reported that due to the increased vascular endothelial permeability, the formation of edema was governed more by hydrostatic venous pressure than colloid oncotic pressure (33). Therefore, the impact of albumin infusion on hypoalbuminemia and edema is uncertain, but it will increase the workload of the heart, which may explain the results of our study.
For ICU patients with CHF-hypoalbuminemia overlap, treatment of upstream pathological processes such as congestion, inflammation, and malnutrition is crucial. Diuretics can alleviate congestion and reduce the transcapillary escape rate of albumin (26). RASIs, BBs, and omega-3 polyunsaturated acids may attenuate the inflammatory responses in HF patients (34-36). Exogenous amino acid supplementation may be a better option for correcting malnutrition (37).
Limitations
Our study also had shortcomings that should be noted. Firstly, this was a retrospective cohort study; we demonstrated a correlation between exposure and outcome, but not causation. Secondly, our study was based on the MIMIC database, from which the opportunity and dose of albumin infusion were difficult to confirm. This affected our assessment of the impact of albumin infusion. Finally, BNP (brain natriuretic polypeptide) and pro-BNP were excluded from our analysis due to their missing values being more than 50%, which may have affected the reliability and accuracy of our results.
Conclusions
Low serum albumin is a downstream biomarker of HF syndrome severity but is not a determinant of poorer prognosis. Albumin infusion increased the in-hospital mortality, ICU LOS, and hospital LOS in ICU patients with CHF-hypoalbuminemia overlap.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-648/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-648/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- González-Pacheco H, Amezcua-Guerra LM, Sandoval J, et al. Prognostic Implications of Serum Albumin Levels in Patients With Acute Coronary Syndromes. Am J Cardiol 2017;119:951-8. [Crossref] [PubMed]
- Oduncu V, Erkol A, Karabay CY, et al. The prognostic value of serum albumin levels on admission in patients with acute ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis 2013;24:88-94. [Crossref] [PubMed]
- Plakht Y, Gilutz H, Shiyovich A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI-II) project. Int J Cardiol 2016;219:20-4. [Crossref] [PubMed]
- Horwich TB, Kalantar-Zadeh K, MacLellan RW, et al. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883-9. [Crossref] [PubMed]
- Bonilla-Palomas JL, Gámez-López AL, Moreno-Conde M, et al. Hypoalbuminemia in acute heart failure patients: causes and its impact on hospital and long-term mortality. J Card Fail 2014;20:350-8. [Crossref] [PubMed]
- Uthamalingam S, Kandala J, Daley M, et al. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J 2010;160:1149-55. [Crossref] [PubMed]
- Arques S, Roux E, Sbragia P, et al. Usefulness of serum albumin concentration for in-hospital risk stratification in frail, elderly patients with acute heart failure. Insights from a prospective, monocenter study. Int J Cardiol 2008;125:265-7. [Crossref] [PubMed]
- Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res 2002;55:820-9. [Crossref] [PubMed]
- Roche M, Rondeau P, Singh NR, et al. The antioxidant properties of serum albumin. FEBS Lett 2008;582:1783-7. [Crossref] [PubMed]
- Lam FW, Cruz MA, Leung HC, et al. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res 2013;132:69-76. [Crossref] [PubMed]
- Paar M, Rossmann C, Nusshold C, et al. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS One 2017;12:e0182997. [Crossref] [PubMed]
- Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology 2005;41:1211-9. [Crossref] [PubMed]
- Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, et al. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am Heart J 2010;160:279-85. [Crossref] [PubMed]
- Filippatos GS, Desai RV, Ahmed MI, et al. Hypoalbuminaemia and incident heart failure in older adults. Eur J Heart Fail 2011;13:1078-86. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- Dubois MJ, Orellana-Jimenez C, Melot C, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit Care Med 2006;34:2536-40. [Crossref] [PubMed]
- Martin GS, Moss M, Wheeler AP, et al. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med 2005;33:1681-7. [Crossref] [PubMed]
- SAFE Study Investigators. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 2011;37:86-96. [Crossref] [PubMed]
- SAFE Study Investigators. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 2007;357:874-84. [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. [Crossref] [PubMed]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Zhu S, Ge T, Hu J, et al. Prognostic value of surgical intervention in advanced lung adenocarcinoma: a population-based study. J Thorac Dis 2021;13:5942-53. [Crossref] [PubMed]
- WRITING COMMITTEE MEMBERS. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327. [PubMed]
- Hesse B, Parving HH, Lund-Jacobsen H, et al. Transcapillary escape rate of albumin and right atrial pressure in chronic congestive heart failure before and after treatment. Circ Res 1976;39:358-62. [Crossref] [PubMed]
- Battin DL, Ali S, Shahbaz AU, et al. Hypoalbuminemia and lymphocytopenia in patients with decompensated biventricular failure. Am J Med Sci 2010;339:31-5. [Crossref] [PubMed]
- Omran ML, Morley JE. Assessment of protein energy malnutrition in older persons, Part II: Laboratory evaluation. Nutrition 2000;16:131-40. [Crossref] [PubMed]
- Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 2011;13:1161-71. [Crossref] [PubMed]
- Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr 2001;20:271-3. [Crossref] [PubMed]
- Zhuang XD, Zhang SZ, Liao LZ, et al. Serum Albumin and Incident Heart Failure: Insights From Epidemiological and Mendelian Randomization Studies. Circ Genom Precis Med 2020;13:e002989. [Crossref] [PubMed]
- Margarson MP, Soni NC. Changes in serum albumin concentration and volume expanding effects following a bolus of albumin 20% in septic patients. Br J Anaesth 2004;92:821-6. [Crossref] [PubMed]
- Lewis CA, Martin GS. Understanding and managing fluid balance in patients with acute lung injury. Curr Opin Crit Care 2004;10:13-7. [Crossref] [PubMed]
- Anand IS, Latini R, Florea VG, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation 2005;112:1428-34. [Crossref] [PubMed]
- Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223-30. [Crossref] [PubMed]
- Ohtsuka T, Hamada M, Hiasa G, et al. Effect of beta-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol 2001;37:412-7. [Crossref] [PubMed]
- Pasini E, Aquilani R, Gheorghiade M, et al. Malnutrition, muscle wasting and cachexia in chronic heart failure: the nutritional approach. Ital Heart J 2003;4:232-5. [PubMed]
(English Language Editor: A. Kassem)


