
A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 3: systematic review of evidence regarding surgery in compromised patients or specific tumors
Introduction
Several treatment options are available for clinical stage I (cI) non-small cell lung cancer (NSCLC)—lobectomy, segmentectomy, wedge resection, stereotactic body radiotherapy (SBRT) and ablation. Clinicians are faced with selecting the optimal treatment for a spectrum of patients and tumors. Clinical decision-making involves considering multiple outcomes, e.g., short-term treatment-related mortality, morbidity, long-term survival, recurrence and quality-of-life (QOL)—weighing the evidence, the degree of uncertainty and the applicability to an individual patient and setting.
There is a need for better definition of the evidence regarding management of cI NSCLC in a manner that facilitates decision-making for individual patients. We reviewed available evidence with a focus on critically addressing confounders, sources of uncertainty and nuances that impact the confidence in applicability in various circumstances. The project consists of 4 publications: Part 1 concisely summarizes the evidence and provides a framework to guide clinical decision-making (1), Part 2 reviews evidence regarding surgery in generally healthy patients (2), Part 3 (this paper) addresses specific patients and tumors, Part 4 focuses on evidence regarding SBRT and ablation (3).
Methods
General approach
A detailed description of the approach is provided elsewhere (see Methods section of Part 1) (1). Briefly, the subject is stage cIA NSCLC (using the 8th edition nomenclature throughout). Interventions include lobectomy, segmentectomy, wedge resection, SBRT and ablation. The most relevant outcomes were chosen a priori: short-term treatment-related mortality, toxicity/morbidity, pain, QOL and long-term overall survival (OS), lung cancer specific survival (LCSS), freedom from recurrence (FFR), functional status and QOL.
Because few randomized controlled trials (RCTs) are available, we relied heavily on non-randomized comparisons (NRCs) that adjusted for confounders. We critically evaluated how well confounders were accounted for to assess the confidence that observed results reflect the intervention in question. Finally, we explored sources of ambiguity to understand uncertainties and limitations of applicability.
Clinical decision-making for an individual involves weighing multiple outcomes and many aspects of each—e.g., the strength of the evidence, the magnitude of the impact, uncertainty and how well this applies to an individual. The framework presented in the Part 1 paper facilitates identifying the issues with the most impact in a particular setting for a patient. This Part 3 paper provides the foundation, presenting the data in a manner that can at-a-glance provide an aggregate view of an outcome as well as the nuances and uncertainties of the data.
Literature search, selection and assessment
We systematically searched English literature from 2000 –2021; details are provided elsewhere (see App. 1-2 of Part 1) (1). Selected studies provided evidence relevant to the topic, focusing on RCTs and adjusted NRCs. Each evidence table lists specific inclusion and exclusion criteria.
Study quality was assessed using a general tool (4) and an adaption thereof specific to stage I NSCLC (described in App. 2-1 of Part 2) (2). Residual confounding in 7 a priori defined domains is shown in the evidence tables along with the confidence that outcomes reflect the treatment. The domains include non-medical and medical patient-related factors, discrepancies in stage classification, time period, facility factors, treatment quality and favorable tumor selection.
Aggregation of evidence
A quantitative meta-analysis was deemed inappropriate due to variable residual confounding across domains and severity. Instead, thoughtfully structured tables reflecting nuances of the patients, treatments and tumors provide an aggregate impression of the strengths, weaknesses and applicability of the data. We have used color coding, essentially layering a heat map onto the tables to provide an overview without getting overwhelmed by details, aiming to facilitate individualized decision-making through a comprehensive yet nuanced overview. Comparing outcomes is aided by defining what can be considered a clinically meaningful difference (described in Tab. S1-1 of Part 1) (1).
Results
Older patients
Life expectancy in older patients
Life expectancy is ~12–14 years in older US cohorts eligible for lung cancer screening, justifying treatment in most despite the average comorbidities seen in older patients with a smoking history (Table S3-1) (5,6).
Lung cancer remains the dominant cause of death even in older patients with severe comorbidities and localized lung cancer (Figure 1) (7). However, ~1/3 of US patients age 75–84 and ~1/2 of those ≥85 receive no treatment (8,9). Average life-expectancy suggests that age alone or age with low-moderate comorbidities should not preclude lung cancer treatment, at least until age 90 (Figure S3-1) (10).
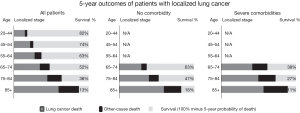
Survival and cause of death in patients with localized lung cancer by age and presence of comorbidities; SEER data 2000–2010. Reproduced with permission from Howlader et al. (7).
Short-term outcomes
Mortality and morbidity
Perioperative mortality in older patients is low (~2–4%, Table 1) (11-28), particularly in recent series. Most studies note no advantage with sublobar resection, with some observing either a benefit or a detriment vs. lobectomy. In a RCT no perioperative mortality benefit was found with sublobar resection among patients ≥70 (14).
Table 1
Ordered by age, time period
Perioperative mortality increases little with increasing age and the difference between sublobar resection and lobectomy widens only slightly (Table 1). An NCDB study [2004–13] reported 90-day mortality for ages 66–70, 71–75, 76–80 and ≥81 of 2.8%, 3.5%, 5.1% and 5.8% for sublobar resection and 3%, 4.5%, 5.8% and 8% for lobectomy, respectively) (15); the difference between sublobar resection and lobectomy in these age cohorts is 0.2%, 1%, 0.7% and 2.2% (marginally clinically meaningful except for age >80).
Most complications in older patients are minor—e.g., atrial fibrillation, hypotension, urinary tract infection and wound infection in the Altorki RCT (14). The severe morbidity rate is ~10–15%; some specific complications may be lower after sublobar resection (14). Among unadjusted NRCs, some observe no difference in morbidity and others suggest a benefit to sublobar resection over lobectomy (Table 1). The limited data leaves the impact of age or resection extent on major morbidity rates unclear.
These studies mostly involved open thoracotomy. The well-documented (29) decrease in perioperative complications with VATS lobectomy in general is also noted in older patients: 50–70% lower morbidity and mortality after VATS (vs. open) lobectomy (28) or segmentectomy (30) in SEER studies of patients ≥65 with thorough adjustment for confounding factors. The impact of VATS may be greater with increasing age: in patients aged ≥80 an adjusted OR of ≤0.5 for mortality or complications after VATS vs. open lobectomy was reported in several studies (31,32). Other reports of patients ≥70 or ≥80 consistently show better short-term results with VATS vs. open lobectomy (operative mortality 0 vs. 2.5–6%, severe complications 0–18% vs. 7–35%, any complication 28–35% vs. 24–63%, respectively) (20,31-33).
Long-term outcomes
OS reflects treatment effectiveness mixed with competing causes of death; LCSS specifically addresses treatment effectiveness. Recurrence (especially locoregional recurrence) is important when potentially suboptimal treatment is in question. Pulmonary function tests (PFTs) serve as an available surrogate for functional capacity.
Survival
The only RCT of resection extent in older patients was initiated in 2016 in China (34). A target of 339 patients were randomized to sublobar resection vs. lobectomy (age ≥70 years, peripheral cIA NSCLC, ≥50% solid). No information is available regarding current accrual, estimated closure or publication date.
Several observations can be made about adjusted NRCs of resection extent in older patients (Table 2 and Figure S3-2) (12,13,18,19,26,35-48). The hazard ratios (HRs) for OS and LCSS fairly consistently favor lobectomy. A benefit for lobectomy is not associated with the type of limited resection, specific age cohorts or lower stage tumors. The OS difference is clinically relevant (5–10%).
Table 2
Ordered by stage, degree of confidence that results reflect the effect of the treatment, age
Two studies deserve highlighting—rated as very high/high confidence that confounders have been addressed (12,36). Figure 2 shows results in multivariate-adjusted and propensity-matched patients from Shirvani et al. (12) A significant downside for sublobar resection was upheld in multiple sensitivity analyses (VATS/open, segment, age >75, size ≤2 cm). Zhang et al. (36) also found significant downsides with segmentectomy vs. lobectomy in patients ≥70 with cIA tumors; adjustment and additional analyses rendered residual confounding unlikely.
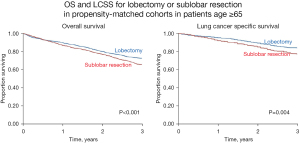
Survival of patients with cI-IIA NSCLC in the SEER-Medicare database 2003–09, age ≥65, extensively propensity-matched (19 factors, 4 sensitivity analyses). Reproduced with permission from Shirvani et al. (12). OS, overall survival; LCSS, lung cancer specific survival.
Stiles et al. analyzed a propensity-matched subgroup of patients who had ≥9 nodes sampled (5% of their original sample) (35). Worse survival after sublobar resection (primarily wedge) in the larger matched group disappeared in this subgroup. The authors speculated that the lower survival generally observed with wedge vs. lobectomy was primarily due to inaccurate stage assessment during wedge resection. Although this study was categorized as “very high confidence” that results reflect the treatment, the small highly selected subgroup undergoing sublobar resection with ≥9 nodes sampled leaves some uneasiness. In contrast, worse survival for sublobar resection persisted in other NRCs despite adjustment for specific numbers (36,39,49-53) or by average number of nodes sampled (37,41,42,54-57) with rare exceptions (58).
Recurrence
Only 1 adjusted NRC evaluated recurrence in older patients (239 patients, age ≥75, cI-IIA NSCLC, rated as low confidence that confounders are addressed) (19). The unadjusted recurrence rate (overall 23% vs. 19%; locoregional 13% vs. 2%) and the adjusted HR for disease-free survival (DFS, 1.43) were worse for sublobar resection than lobectomy, respectively (P= NS).
Functional capacity
Studies addressing functional capacity or PFTs in older patients by resection extent were not identified.
Pain and QOL
There is no data on pain or QOL specifically in older patients and by resection extent. Data for patients in general suggests no major difference in pain or QOL between sublobar resection and lobectomy (see Part 2 paper) (2); the use of VATS vs. thoracotomy appears to have greater impact. QOL studies comparing older and younger cohorts found no difference by age (after mostly open lobectomy) (59-62). Thus, indirect evidence classified as speculative extrapolation (63) suggests that while surgery negatively impacts QOL (especially initially and after thoracotomy), this is probably not worse in older patients and not diminished after lesser resection.
Sources of ambiguity and nuances of applicability
How older patients were selected for surgery is not well-defined. The proportion of cI-IIA NSCLC patients treated surgically is ~70–90% for age 65–74 and ~30–50% for age >75–80 (12,64). A performance status (PS) of 0 is reported in 75–85% of older early-stage surgical patients (12,65); ~15–60% have Charlson comorbidity scores of 0 and ~15-30% a score of ≥2 (12,13,15,37,39). Chronic obstructive pulmonary disease (COPD) is reported in approximately half of surgical patients, and ~5–10% have a history of congestive heart failure, myocardial infarction or cerebrovascular accident (12,13,65). The rates of less favorable Charlson score, PS, or specific comorbidities are ~10% higher among patients undergoing sublobar resection (vs. lobectomy).
Studies of predictors of morbidity and mortality are not well parsed to older patients and by resection extent. Among older patients, male sex was predictive of morbidity by multivariate analysis in 2 studies (66,67) (corroborated in another study including all ages) (68). More sporadic predictors of morbidity include Charlson score of ≥2, larger tumor size, age ≥75 (in patients ≥65 undergoing lobectomy) (67) and the presence of any comorbidity (in patients ≥80 undergoing any resection) (26). A large prospective study (JACS1303) of patients ≥80 identified the following risk factors for severe complications: male sex, impaired memory, diabetes, albumin <3.8 ng/mL, and forced vital capacity <90%; sublobar resection or VATS approach was beneficial in univariate analysis but not carried forward into the final predictive model (65).
A propensity-matched study suggested decreased aggressiveness of cIA NSCLC in older patients (>65)—noting less N1 or N2 involvement despite the same extent of intraoperative node evaluation (69). However, the impact of worse differentiation and a greater consolidation/tumor ratio (CTR, solid/whole tumor size on lung windows) was far greater—i.e., it may be more important to focus on markers of tumor behavior than age. This supports speculation that more frequent incidental detection in older patients results in a greater proportion of indolent tumors (similar to screening).
Summary of outcomes in older patients
Older patients (age 65–90) have relatively long average life expectancy (~8–20 years). Furthermore, death from lung cancer is the most likely 5-year outcome in older patients with comorbidities and localized lung cancer. This argues that most older lung cancer patients should be treated, unless there are severe comorbidities well beyond those that are typical for older patients.
Reported perioperative mortality among older patients is consistently low (~2–4%), including in population-based series; a slight increase between age 65 and 80 is noted in some series. Segmentectomy and wedge resection has at best only a minor impact on decreasing mortality; in older age cohorts differences are only slightly increased. Most complications in older patients are minor; limited data suggests that sublobar resection may decrease morbidity rates. VATS may be particularly important in older patients to decrease morbidity and mortality rates for both lobectomy and limited resection.
Reported 5-year OS in older cI patients is reasonable (40–65%). Adjusted NRCs of segmentectomy/wedge resection vs. lobectomy mostly show a trend toward lower OS/LCSS with sublobar resection, and several well-adjusted NRCs deemed to have little residual confounding found a statistically significant detriment in OS/LCSS for sublobar resection (12,36). One NRC suggests worse long-term outcomes for wedge vs. segmentectomy. The aggregate long-term data does not suggest that the difference between limited resection and lobectomy is diminished by increasing age.
Resected older patients are clearly selected, but how is not well-defined. Most patients have had an excellent PS; many have had comorbidities (presumably not severe).
The short- and long-term outcomes for segmentectomy/wedge vs. lobectomy in older patients are summarized in Table S3-2A, depicting clinically meaningful differences and the confidence in and consistency of the evidence. This provides a succinct summary that can inform judgment for individual patients, as discussed further in the Part 1 paper (1).
Patients with major comorbidities
Life-expectancy in general US cohorts with high comorbidities remains sufficient (>5 years) to justify aggressive treatment of lung cancer (Figure S3-1), at least up to age 85 (10). The impact on life-expectancy of diabetes is low, of COPD only mild, but more substantial for congestive heart failure. The impact of comorbidities diminishes with increasing age.
Comorbidities are more frequent in lung cancer patients than age-matched general cohorts (Figure S3-3) (70). However, among patients ≥65 with severe comorbidities and localized lung cancer, 40–60% die from lung cancer vs. 20–30% from non-cancer causes over 5 years (Figure 1) (7). This argues for treatment in most patients despite comorbidities that are “usual” for this age group. Only severe comorbidities with a life-expectancy <2 years would seem to justify not treating an early-stage lung cancer. However, the incremental value of one treatment over another diminishes as competing causes of death become increasingly dominant.
This section primarily addresses surgery in patients with severely compromised pulmonary reserve because evidence for other comorbidities or for intermediate degrees of pulmonary compromise is unavailable. Nevertheless, definition of outcomes at the ends of the spectrum (healthy and severely compromised patients) facilitates judgement for individuals falling in-between.
Short-term outcomes
Treatment-related morbidity and mortality
VATS markedly ameliorates the increased perioperative morbidity and mortality associated with declining pulmonary reserve (Figure 3) (71), as consistently demonstrated in multiple studies (71-75).
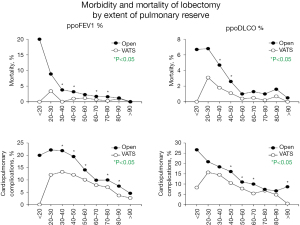
Rates of postoperative mortality and cardiopulmonary complications in propensity-matched VATS and open lobectomy groups, stratified by ppoFEV1% and ppoDLCO%. *, P<0.05. Reproduced with permission from Burt et al. (71). VATS, video-assisted thoracic surgery; ppoFEV1%, predicted postoperative percent of predicted forced expiratory volume in 1 second; ppoDLCO%, percent of predicted diffusing capacity of the lung for carbon monoxide.
Table 3 lists short-term outcomes of resection in patients with pulmonary reserve below thresholds often cited as a contraindication for surgery (71-86). The reported 30-day mortality for lobectomy is surprisingly low (VATS 2–3%, open 3–8%, mixed approach 0–8%). Mortality does not trend higher with greater pulmonary compromise; further nuances in high-risk patients are less well-defined. The 90-day mortality was 2.7% in a prospective trial of sublobar resection in 222 compromised patients (65% VATS, 70% wedge) (84); and 4% in other studies (mixture of lobe/sublobar and open/VATS) (79,81).
Table 3
Ordered by approach, extent of resection, and decreasing pulmonary reserve
Pulmonary complications in compromised patients are lower after VATS (~10–20%) than open (~20–40%) lobectomy. Similar results were found in a meta-analysis (overall morbidity 39% vs. 58%; pulmonary morbidity 26% vs. 46% for VATS vs. open, respectively) (72) and a propensity-matched NRC of VATS vs. open lobectomy (182 patients with COPD and FEV1 <80%) (87). Lower morbidity after VATS vs. open segmentectomy was reported in a propensity-matched NRC (any complication 26% vs. 34%, major complications 6% vs. 12%, pulmonary complications 15% vs. 30%, respectively; unclear proportion of compromised patients) (88).
Little difference in short-term outcomes in compromised patients is reported between segmentectomy and lobectomy, although direct data is limited. A case-matched comparison of segmentectomy vs. lobectomy (primarily via thoracotomy) in 34 patients with a predicted postoperative percentage of predicted forced expiratory volume in 1 second (ppoFEV1) <40% found no difference in mortality (6% each) or any complication (18% each) (89). Comparing across studies of lesser resection or lobectomy in compromised patients suggests little difference in short-term outcomes (also true in healthy patients). Given the surprisingly good results for VATS lobectomy in compromised patients, it seems unlikely that VATS segmentectomy or wedge resection would be meaningfully better. A prospective trial of compromised patients (Z4032) noted less grade ≥3 events with wedge resection vs. segmentectomy (23% vs. 40%) (84).
Compromised patients undergoing resection are clearly selected, but how is not well-defined. Paul et al. noted that 84% of patients were PS 0 despite poor PFTs (81). In the Z4032 sublobar trial (84) 20% of patients were PS 0, 60% PS 1 and 20% PS 2. No information is available on VO2 or formal exercise testing. Preoperative oxygen use was rare (<10%) (79). It appears that most patients in reported studies met only one criterion of poor PFTs, with other parameters being less concerning.
In compromised patients only sporadic and inconsistent predictors have been reported of postoperative complications (any, pulmonary, severe, need for postoperative oxygen) (79,81,84,85,90,91). While lower FEV1, diffusing capacity of the lung for carbon monoxide (DLCO) and older age portend greater risk in large studies involving a wide spectrum of patients (71,74,75), these factors don’t appear predictive among severely compromised (albeit selected) patients.
Short-term QOL
A postoperative new need for oxygen is reported in 10–40% of severely compromised patients. Only ~10% continue to need oxygen after several weeks (77,81,84-86,91,92). One study found that 3 months postoperatively average PS (1.3) was improved over baseline (1.5, 59 patients) (76). No change in QOL 3 months after open lobectomy was noted patients with COPD (SF-36 tool) (93). No other information on short-term postoperative QOL in compromised patients is available.
Long-term outcomes
PFTs
Many studies note that lobectomy has little effect on decreasing PFTs in the presence of COPD (94-100). Calculated ppoFEV1 underestimates observed values 6–12 months later to a greater degree in patients with COPD or lower baseline FEV1 (94-97,99,100). Approximately 1/3rd of patients with COPD experience an improvement in FEV1 and DLCO after lobectomy (96,98). PFTs decrease less (or increase in COPD patients) after an upper vs. lower lobectomy in some studies (98,99), but not in others (perhaps due to a shorter postoperative interval) (95,96).
These general results probably extend to patients with severely limited pulmonary reserve, but it is not well-studied. In severely compromised patients FEV1 appears to decrease after lobectomy, but slightly increase after segmentectomy or wedge resection (86,89,92,101). Approximately 2/3rd of patients experience no change in PFTs, ~20% are worse and ~20% are improved (86,92,101). Fewer patients undergoing upper lobe resections experienced a decline in PFTs in a prospective study (86).
QOL
QOL has not been well-studied in patients with limited pulmonary reserve. In the Z4032 trial of sublobar resection in compromised patients no overall change in QOL was noted (3, 12 and 24 months, SF36 tool) (102). Some patients experienced a clinically meaningful improvement in the physical score at 3 months (17% for VATS vs. 4% open); no patients experienced a decline. For the entire cohort there was no change in dyspnea over 2 years. However, a lower proportion experienced meaningfully worse dyspnea at 12 months after VATS (20%) vs. thoracotomy (39%) and after wedge (22%) vs. segmentectomy (41%) (102). Another study of VATS resection reported that 11% of patients had a decline in PS at 6 months from baseline (90).
Taken together, the available data suggests that resection has little long-term QOL impact from baseline in patients with limited pulmonary reserve (baseline QOL is worse in compromised patients than the general population). A few patients experience a decline in some domains, but others experience an improvement. There may be a meaningful proportion that experience worsening dyspnea, especially after larger resections and an open approach.
Survival and recurrence
A RCT has been launched comparing wedge vs. segmentectomy in high-risk patients with cIA NSCLC (JCOG1909). The definition of high-risk is the same as in the ACOSOG4032 trial. The study aims to enroll 370 patients between 2020 and 2025 (103).
Few adjusted NRCs address resection extent in compromised patients (Table S3-3) (38,104). Salazar et al. analyzed patients with a life expectancy of ≤5 years (based on non-cancer characteristics; 67% age ≥80, 84% Elixhauser comorbidity ≥3) (38). Wedge (vs. lobectomy) was associated with worse LCSS, but fewer non-cancer deaths (90-day mortality excluded). Another NRC, involving patients with CT evidence of interstitial lung disease (ILD), found a trend to better 3-year OS after sublobar resection over lobectomy and no difference in LCSS; the overall high survival suggests a limited degree of ILD (104). Other small NRCs (falling below Table S3-3 inclusion thresholds) reported no difference in adjusted OS between sublobar resection and lobectomy (high degree of residual confounding) (78,82,89). Two small NRCs of wedge resection vs. segmentectomy noted no difference in adjusted OS (high degree of residual confounding) (105,106).
Table 4 summarizes unadjusted long-term outcomes in patients with severely limited pulmonary reserve (76,78-83,89,92,107). In general, 5-year OS is ~50–60%. Most patients were cI and underwent lobectomy. The proportion of unrelated vs. lung cancer deaths is approximately equal (78,91,92,95,107). In summary, available data shows no clear OS difference between segmentectomy/wedge and lobectomy in patients with severely limited pulmonary reserve; whether recurrence is higher after sublobar resection is unclear.
Table 4
Ordered by stage, and decreasing pulmonary reserve
Sources of ambiguity and nuances of applicability
VATS markedly diminishes short-term morbidity and mortality in patients with severely limited pulmonary reserve compared to thoracotomy.
Careful patient selection in compromised patients is crucial but not well-defined. Speculative volume estimates suggest that surgery has been used with regularity, but in a minority of compromised patients. Reported series largely precede the acceptance of SBRT as an alternative. The data demonstrates acceptable short- and long-term outcomes in resected patients with PFTs below thresholds traditionally cited as contraindications to surgery. It appears likely that patients selected for surgery had other reassuring characteristics (PS, other PFT/cardiopulmonary exercise results, etc.). Notably, guidelines suggest a ppoFEV1 or ppoDLCO of <30% as relative contraindications to surgery; exercise testing can further risk-stratify (108-111). In summary, if a patient appears otherwise able to undergo resection despite a poor PFT measure one can be reasonably confident in acceptable short- and long-term outcomes, but the selection should be made carefully. The approach (VATS vs. open) appears to have greater impact than the resection extent.
Summary of outcomes after resection in compromised patients
Lung cancer is the cause of death in most patients with major comorbidities and early-stage lung cancer (7), suggesting that treatment of the lung cancer is worthwhile in most of these patients.
The increase in short-term post-operative morbidity and mortality seen with decreasing pulmonary reserve is markedly ameliorated by VATS. In patients with severe pulmonary compromise (below criteria cited as contraindications to surgical resection), 30-day mortality for VATS lobectomy is 2–3% and 3–8% for open lobectomy. Pulmonary complication rates for lobectomy in compromised patients are ~10–20% after VATS vs. ~20–40% after thoracotomy. Limited data suggests little difference in short-term outcomes between segmentectomy vs. lobectomy.
The impact of resection (including lobectomy) on FEV1 is diminished in patients with severely limited pulmonary reserve, and FEV1 is unchanged or even improved in a substantial proportion of patients. Given this variability and the limited data, it is unclear if sublobar resection confers a functional benefit over lobectomy. Limited data suggests little average impact of resection on long-term QOL in patients with limited pulmonary reserve—some patients are better, some worse and many unchanged. A QOL benefit for lesser resection vs. lobectomy has not been demonstrated, but data is limited.
Long-term survival and recurrence in patients with limited reserve has not been addressed in a manner that accounts for confounders. Unadjusted data shows no clear difference between segmentectomy/wedge vs. lobectomy.
Careful selection is crucial in compromised patients, but not well-defined. Good short- and long-term outcomes can be achieved despite limited PFTs, but these patients are likely otherwise robust.
The short- and long-term outcomes for segmentectomy/wedge vs. lobectomy in compromised patients are summarized in Table S3-2B depicting clinically meaningful differences and the confidence in and consistency of the evidence.
Specific tumor characteristics
Non-oncologic outcomes
Certain tumor characteristics presumably correlate with favorable oncologic biology and may indicate candidacy for sublobar resection. These include ground glass (GG), screen-detected, small (≤1 cm), and slow-growing or low positron emission tomography (PET) avidity tumors. Biologic behavior likely affects long-term oncologic outcomes (e.g., OS, LCSS, recurrence). Non-oncologic outcomes (e.g., treatment-related morbidity, mortality, QOL, dyspnea, pain) are presumably unrelated to tumor characteristics; indeed, little evidence for such outcomes by resection extent is available for potentially favorable tumors.
Patients with potentially favorable tumors are typically healthy. Extrapolation of non-oncologic outcomes for healthy patients (covered in the Part 2 paper) (2) to patients with favorable tumors is reasonable. To summarize, RCTs show no difference in morbidity or mortality for sublobar resection vs. lobectomy. Pain and impact on QOL are generally resolved by 3 months after VATS, but open resection results in persistently worse QOL. A QOL benefit to sublobar resection is unclear due to confounding by VATS/open approach. Preservation of lung function is marginally better after a single segmentectomy vs. lobectomy (little difference after multi-segmentectomy). Increased dyspnea may be less often noted after sublobar resection vs. lobectomy.
When a favorable tumor is encountered in an unfavorable patient, the non-oncologic outcomes are presumably similar to those presented in earlier sections (i.e., older or compromised patients).
Long-term outcomes
GG tumors
Major prospective studies involving GG tumors are summarized in Figure 4 (112-118). The JCOG0201 trial involved lobectomy for cIA part-solid tumors, aiming to define imaging features that predict non-invasiveness (as a surrogate for potential appropriateness for sublobar resection). Among mostly GG tumors (CTR ≤0.25, pure GG excluded) ≤2 cm, only 3% were invasive and the 5-year recurrence-free survival (RFS) was 97% (Figure S3-4) (112,113). Larger tumors (2–3 cm) yielded slightly worse results; especially in tumors with greater solid component (CTR >0.5).

Major prospective studies by resection extent, size and ground glass proportion. References: JCOG0201 (112,113), JCOG0802 (114), JCOG1211 (115), Yoshida Trial (116,117), JCOG0804 (118). CTR, consolidation/tumor ratio (size of consolidation on lung windows/total tumor size including ground glass component); DFS, disease-free survival; GG, ground glass; GGO, ground glass opacity; RCT, randomized controlled trial; Seg, segmentectomy.
Further trials built on these results. JCOG1211 used segmentectomy for GG tumors ≤3 cm with a CTR 0.25–0.5 and larger tumors (2–3 cm) that were predominantly GG; we await survival results (115). JCOG0804 used wedge (79%) or segmentectomy for small, nearly pure GG tumors (≤2 cm, CTR ≤0.25) and found a RFS of 99.7% and no local recurrences (118). While lobectomy could hardly yield better results than JCOG0804, an earlier prospective study involving the same tumor characteristics raised concerns (116,117). Although no recurrences were seen at 5 years, 19% of sublobar resection patients exhibited a staple line recurrence (identical genetic profile) by 10 years, despite meticulous intraoperative assurance of an adequate negative margin. Half of these recurrences were re-resected, the other patients had additional distant metastases (117).
JCOG0802 is a RCT of segmentectomy vs. lobectomy for cIA1,2 cancers with a CTR >0.25 (n=1,106; median consolidation diameter 12.5 mm, 51% with a CTR =1) (114). Long-term results were published in 2022 (119). RFS was identical (88%). Five-year OS was better after segmentectomy vs. lobectomy (94% vs. 91%, P=0.008), despite more local recurrences (11% vs. 5%, P=0.0018, median follow-up 7.3 years). At recurrence, re-resection was done more often in the segmentectomy group (19% vs. 4%). There was no difference in lung cancer deaths (4.7% vs. 5.1%) but fewer unrelated deaths after segmentectomy vs. lobectomy (4.9% vs. 9.4%, respectively; mostly cancers other than the index lung cancer). Thus, it appears that a detriment in lung cancer outcomes associated with segmentectomy was effectively countered by a decrease in unrelated cancers following segmentectomy. This study strongly supports segmentectomy as an alternative for cIA1,2 tumors with CTR ≥0.25—when a margin of ≥2 cm or a margin/tumor ratio ≥1 is achieved.
A few details of JCOG0802 deserve highlighting. Due to negative prognostic findings (e.g., positive nodes, insufficient margin), 5% (25/545) in the segmentectomy group underwent lobectomy (but were analyzed with the segmentectomy arm). The trial was designed with partially GG tumors in mind (common in Japan), although inclusion extended up to completely consolidated tumors. We do not know how many of the completely consolidated tumors had a solid component on mediastinal windows. It is unclear how many segmentectomies were “lobe-like” multisegmentectomies. A 2nd primary lung cancer occurred more often in the segmentectomy group (8% vs. 6%) and these were treated surgically more often (74% vs. 53%) (119).
Adjusted NRCs of lesser resections vs. lobectomy in GG tumors (Table 5 and Figure S3-5) (120-127) suggest little difference in OS, but the data is limited. The widely disparate HRs in the Zang et al. study for CTR ≥ vs. <0.5 (and other aspects of the analysis) suggest residual confounding (123). The 5-year OS (94%) and DFS (91%) in a multi-institutional study of 1,737 healthy patients who underwent segmentectomy (63%) or wedge for a pI GG tumor (CTR ≤0.25 in 47%, 91% ≤2 cm, 1992–2012) (128) is essentially identical to JCOG0201 (OS 95%, DFS 92%) which involved lobectomy for very similar tumors and time period. Additionally, institutional series of sublobar resection for mostly GG tumors demonstrate similar good survival (122,129-133). Based on this data, some clinical guidelines suggest wedge or segmentectomy can be an alternative for predominantly GG cIA1,2 tumors (134,135) but others don’t in non-compromised patients (110).
Table 5
Ordered by degree of confidence that results reflect the effect of the treatment, resection type, stage
Limited data shows no clear difference in loco-regional recurrence after lesser resection vs. lobectomy in GG tumors (Table 6) (121,122,124-126,132,136). Differences are statistically insignificant, the direction of trends is variable, and unadjusted recurrence rates are generally low. Only Nishio et al. (136) report higher loco-regional recurrences, despite selecting patients with favorable tumor location and using “extended” segmentectomies—but involved tumors with CTR >0.5. The JCOG0802 report noted higher locoregional recurrence after segmentectomy vs. lobectomy (119).
Table 6
Ordered by degree of confidence that results reflect the effect of the treatment, resection type, stage
In summary, many retrospective and prospective studies report such good survival after sublobar resection of GG tumors that lobectomy can hardly be better and a large RCT confirms excellent OS after segmentectomy. However, concern has been raised that late recurrence may be an issue. We await the results of other prospective studies.
Screen-detected cancers
Screening inherently causes a “spectrum shift” (a.k.a. length-time and overdiagnosis bias), meaning that a higher proportion of screen-detected cancers manifest low aggressiveness than normal-care-detected cancers (137-139). Most of these “well-behaved” lung cancers in the lung cancer screening experience are GG tumors (139-142).
An adjusted NRC of screen-detected solid tumors noted good OS difference for both sublobar resection and lobectomy, although the HR favored lobectomy (Table 5) (120). The good survival in these solid tumors underscores the spectrum-shift phenomenon (unspecified mixture of prevalence and incidence scans). For screen-detected GG tumors, it is reasonable to extrapolate from GG tumors in general. This argument is supported by a study of wedge or segmentectomy for mostly screen-detected GG tumors that reported results similar to GG tumors in general (143).
Other potential markers of low aggressiveness
No analysis of lesser resection vs. lobectomy in slow-growing or low PET avidity tumors was identified. Speculative extrapolation of the evidence for GG tumors suggests that limited resection and lobectomy may yield similar outcomes in such tumors.
Small solid tumors (≤1 cm) are not a reliably favorable group (Table S3-4, Figure S3-6). Adjusted NRCs report HRs for OS and LCSS favoring lobectomy (moderate to very low confidence that confounders are accounted for) (40,50,144-146).
Nuances and sources of ambiguity
While excellent outcomes are reported for GG tumors with no or minimal solid component regardless of resection extent, it is arguable whether treatment is needed at all. Many studies demonstrate that most such tumors change minimally over many years, and surveillance is safe (147-151). Furthermore, progression may be so indolent that the cancer is inconsequential considering the patient’s longevity. It is also arguable whether distinguishing pre-invasive and invasive cancer is an appropriate surrogate to define when lobectomy is necessary. This concept is based on rationale, countered by emerging data (129,147,152).
There is an unresolved conflict between the extensive data demonstrating excellent 5-year outcomes in favorable tumors, and the report of late staple line recurrences in an earlier prospective study (116,117). The study involved sublobar resection for Noguchi A or B tumors, with a margin ≥1 cm in all, and a meticulous process to evaluate the margin. Furthermore, data suggests margin distance may be unimportant with predominantly GG tumors (2). These recurrences raise the question of a potential impact of STAS (spread through air spaces)—unknown during the study accrual period. However, STAS is rare in GG tumors.
Incidentally-detected cancers appear more similar to screen-detected than symptom-detected cancers (153,154)—worth noting given the increasing prevalence of CT imaging.
Summary of outcomes for specific tumor characteristics
Non-oncologic outcomes are likely similar for lesser resection vs. lobectomy for favorable tumors (extrapolating from evidence in generally healthy patients). GG and screen-detected tumors have very favorable long-term outcomes—equally true for sublobar resection and lobectomy. However, data is limited and some concerns about late recurrence have been raised.
Reasonable speculation suggests that tumors exhibiting low PET-activity or slow progression may have long-term outcomes similar to GG tumors—i.e., excellent after both sublobar resection and lobectomy. However, small solid tumors (<1 cm) tend to have worse outcomes after sublobar resection than lobectomy in adjusted NRCs.
The short- and long-term outcomes for segmentectomy/wedge vs. lobectomy for potentially favorable tumors are summarized in Table S3-2C depicting clinically meaningful differences and the confidence in and consistency of the evidence.
Conclusions
This detailed assessment of outcomes by resection extent in specific patients (i.e., increasing age and pulmonary compromise) and for potentially less aggressive tumors can inform individualized clinical decision-making. In general, short- and intermediate-term benefits of a sublobar resection in older or compromised patients are marginal, countered by a somewhat meaningful detriment in long-term outcomes. Lesser resection does not meaningfully diminish long-term outcomes for most less aggressive tumors.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation”. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1825/prf
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1825/coif). The series “A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation” was commissioned by the editorial office without any funding or sponsorship. FCD served as the unpaid Guest Editor of the series. HSP serves as an unpaid editorial board member of Journal of Thoracic Disease. BCB reports in the past 36 months, he receives grants from Veterans Affairs Central Office, American Cancer Society, Yale SPORE in Lung Cancer. HSP reports research funding from RefleXion Medical; consulting fees from AstraZeneca; honoraria and speaking fees from Bristol Myers Squibb; and advisory board fees from Galera Therapeutics; all unrelated to current work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Blasberg JD, Woodard GA, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 1: a guide to decision-making. J Thorac Dis 2022; [Crossref]
- Detterbeck FC, Mase VJ Jr, Li AX, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 2: systematic review of evidence regarding resection extent in generally healthy patients. J Thorac Dis 2022; [Crossref]
- Park HS, Detterbeck FC, Madoff DC, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation— part 4: systematic review of evidence involving SBRT and ablation. J Thorac Dis 2022; [Crossref]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Arias E. United States Life Tables, 2017. Natl Vital Stat Rep 2019;68:1-66. [PubMed]
- Howard DH, Richards TB, Bach PB, et al. Comorbidities, smoking status, and life expectancy among individuals eligible for lung cancer screening. Cancer 2015;121:4341-7. [Crossref] [PubMed]
- Howlader N, Mariotto AB, Woloshin S, et al. Providing clinicians and patients with actual prognosis: cancer in the context of competing causes of death. J Natl Cancer Inst Monogr 2014;2014:255-64. [Crossref] [PubMed]
- Wang S, Wong ML, Hamilton N, et al. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol 2012;30:1447-55. [Crossref] [PubMed]
- Nadpara PA, Madhavan SS, Tworek C, et al. Guideline-concordant lung cancer care and associated health outcomes among elderly patients in the United States. J Geriatr Oncol 2015;6:101-10. [Crossref] [PubMed]
- Cho H, Klabunde CN, Yabroff KR, et al. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med 2013;159:667-76. [Crossref] [PubMed]
- Zhang Z, Feng H, Zhao H, et al. Sublobar resection is associated with better perioperative outcomes in elderly patients with clinical stage I non-small cell lung cancer: a multicenter retrospective cohort study. J Thorac Dis 2019;11:1838-48. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Billmeier SE, Ayanian JZ, Zaslavsky AM, et al. Predictors and outcomes of limited resection for early-stage non-small cell lung cancer. J Natl Cancer Inst 2011;103:1621-9. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Stokes WA, Bronsert MR, Meguid RA, et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:642-51. [Crossref] [PubMed]
- Liu T, Liu H, Li Y. Early lung cancer in the elderly: sublobar resection provides equivalent long-term survival in comparison with lobectomy. Contemp Oncol (Pozn) 2014;18:111-5. [Crossref] [PubMed]
- Rostad H, Naalsund A, Strand TE, et al. Results of pulmonary resection for lung cancer in Norway, patients older than 70 years. Eur J Cardiothorac Surg 2005;27:325-8. [Crossref] [PubMed]
- Tsutani Y, Tsubokawa N, Ito M, et al. Postoperative complications and prognosis after lobar resection versus sublobar resection in elderly patients with clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2018;53:366-71. [Crossref] [PubMed]
- Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today 2016;46:1370-82. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8. [Crossref] [PubMed]
- Dell'Amore A, Monteverde M, Martucci N, et al. Early and long-term results of pulmonary resection for non-small-cell lung cancer in patients over 75 years of age: a multi-institutional study. Interact Cardiovasc Thorac Surg 2013;16:250-6. [Crossref] [PubMed]
- Okami J, Ito Y, Higashiyama M, et al. Sublobar resection provides an equivalent survival after lobectomy in elderly patients with early lung cancer. Ann Thorac Surg 2010;90:1651-6. [Crossref] [PubMed]
- Linden PA, D'Amico TA, Perry Y, et al. Quantifying the safety benefits of wedge resection: a society of thoracic surgery database propensity-matched analysis. Ann Thorac Surg 2014;98:1705-11; discussion 1711-2. [Crossref] [PubMed]
- Dell'Amore A, Monteverde M, Martucci N, et al. Lobar and sub-lobar lung resection in octogenarians with early stage non-small cell lung cancer: factors affecting surgical outcomes and long-term results. Gen Thorac Cardiovasc Surg 2015;63:222-30. [Crossref] [PubMed]
- Zhang J, Xue ZQ, Chu XY, et al. Surgical treatment and prognosis of octogenarians with non-small cell lung cancer. Asian Pac J Trop Med 2012;5:465-8. [Crossref] [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Dominguez-Ventura A, Allen MS, Cassivi SD, et al. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg 2006;82:1175-9. [Crossref] [PubMed]
- Ezer N, Kale M, Sigel K, et al. Outcomes after Video-assisted Thoracoscopic Lobectomy versus Open Lobectomy for Early-Stage Lung Cancer in Older Adults. Ann Am Thorac Soc 2018;15:76-82. [Crossref] [PubMed]
- Detterbeck F, Antonicelli A, Okada M. Results of Video-Assisted Techniques for Resection of Lung Cancer. In: Pass H, Ball D, Scagliotti G. editors. Thoracic Oncology: The IASLC Multidisciplinary Approach (2nd Edition): IASLC, 2018.
- Smith CB, Kale M, Mhango G, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol 2014;9:383-9. [Crossref] [PubMed]
- Port JL, Mirza FM, Lee PC, et al. Lobectomy in octogenarians with non-small cell lung cancer: ramifications of increasing life expectancy and the benefits of minimally invasive surgery. Ann Thorac Surg 2011;92:1951-7. [Crossref] [PubMed]
- Pagès PB, Mariet AS, Madelaine L, et al. Impact of video-assisted thoracic surgery approach on postoperative mortality after lobectomy in octogenarians. J Thorac Cardiovasc Surg 2019;157:1660-7. [Crossref] [PubMed]
- Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-5; discussion 235-6. [Crossref] [PubMed]
- Yang F, Sui X, Chen X, et al. Sublobar resection versus lobectomy in Surgical Treatment of Elderly Patients with early-stage non-small cell lung cancer (STEPS): study protocol for a randomized controlled trial. Trials 2016;17:191. [Crossref] [PubMed]
- Stiles BM, Mao J, Harrison S, et al. Extent of lymphadenectomy is associated with oncological efficacy of sublobar resection for lung cancer ≤2 cm. J Thorac Cardiovasc Surg 2019;157:2454-2465.e1. [Crossref] [PubMed]
- Zhang Y, Yuan C, Zhang Y, et al. Survival following segmentectomy or lobectomy in elderly patients with early-stage lung cancer. Oncotarget 2016;7:19081-6. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [Crossref] [PubMed]
- Salazar MC, Canavan ME, Walters SL, et al. The Survival Advantage of Lobectomy over Wedge Resection Lessens as Health-Related Life Expectancy Decreases. JTO Clin Res Rep 2021;2:100143. [Crossref] [PubMed]
- Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol 2015;33:3447-53. [Crossref] [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [Crossref] [PubMed]
- Moon MH, Moon YK, Moon SW. Segmentectomy versus lobectomy in early non-small cell lung cancer of 2 cm or less in size: A population-based study. Respirology 2018;23:695-703. [Crossref] [PubMed]
- Razi SS, John MM, Sainathan S, et al. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res 2016;200:683-9. [Crossref] [PubMed]
- Zhang B, Liu R, Ren D, et al. Comparison of Lobectomy and Sublobar Resection for Stage IA Elderly NSCLC Patients (≥70 Years): A Population-Based Propensity Score Matching's Study. Front Oncol 2021;11:610638. [Crossref] [PubMed]
- Wang W, Sun Y, Li H, et al. Surgical modality for stage IA non-small cell lung cancer among the elderly: analysis of the Surveillance, Epidemiology, and End Results database. J Thorac Dis 2020;12:6731-42. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [Crossref] [PubMed]
- Stiles BM, Mao J, Harrison S, et al. Sublobar resection for node-negative lung cancer 2-5 cm in size. Eur J Cardiothorac Surg 2019;56:858-66. [Crossref] [PubMed]
- Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45. [Crossref] [PubMed]
- Smith CB, Swanson SJ, Mhango G, et al. Survival after segmentectomy and wedge resection in stage 1 non-small-cell lung cancer. J Thorac Oncol 2013;8:73-8. [Crossref] [PubMed]
- Qu X, Wang K, Zhang T, et al. Long-term outcomes of stage I NSCLC (≤3 cm) patients following segmentectomy are equivalent to lobectomy under analogous extent of lymph node removal: a PSM based analysis. J Thorac Dis 2017;9:4561-73. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Invasive adenocarcinoma with bronchoalveolar features: a population-based evaluation of the extent of resection in bronchoalveolar cell carcinoma. J Thorac Cardiovasc Surg 2012;143:591-600.e1. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (≤ 2 cm) non-small cell lung cancers--a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Zhao ZR, Situ DR, Lau RWH, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol 2017;12:890-6. [Crossref] [PubMed]
- Koike T, Kitahara A, Sato S, et al. Lobectomy Versus Segmentectomy in Radiologically Pure Solid Small-Sized Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1354-60. [Crossref] [PubMed]
- Subramanian M, McMurry T, Meyers BF, et al. Long-Term Results for Clinical Stage IA Lung Cancer: Comparing Lobectomy and Sublobar Resection. Ann Thorac Surg 2018;106:375-81. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Yu Y, Huang R, Wang P, et al. Sublobectomy versus lobectomy for long-term survival outcomes of early-stage non-small cell lung cancer with a tumor size ≤2 cm accompanied by visceral pleural invasion: a SEER population-based study. J Thorac Dis 2020;12:592-604. [Crossref] [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. [Crossref] [PubMed]
- Schulte T, Schniewind B, Walter J, et al. Age-related impairment of quality of life after lung resection for non-small cell lung cancer. Lung Cancer 2010;68:115-20. [Crossref] [PubMed]
- Burfeind WR Jr, Tong BC, O'Branski E, et al. Quality of life outcomes are equivalent after lobectomy in the elderly. J Thorac Cardiovasc Surg 2008;136:597-604. [Crossref] [PubMed]
- Salati M, Brunelli A, Xiumè F, et al. Quality of life in the elderly after major lung resection for lung cancer. Interact Cardiovasc Thorac Surg 2009;8:79-83. [Crossref] [PubMed]
- Möller A, Sartipy U. Changes in quality of life after lung surgery in old and young patients: are they similar? World J Surg 2010;34:684-91. [Crossref] [PubMed]
- Detterbeck FC, Gould MK, Lewis SZ, et al. Extending the Reach of Evidence-Based Medicine: A Proposed Categorization of Lower-Level Evidence. Chest 2018;153:498-506. [Crossref] [PubMed]
- Okami J. Treatment strategy and decision-making for elderly surgical candidates with early lung cancer. J Thorac Dis 2019;11:S987-97. [Crossref] [PubMed]
- Saji H, Ueno T, Nakamura H, et al. A proposal for a comprehensive risk scoring system for predicting postoperative complications in octogenarian patients with medically operable lung cancer: JACS1303. Eur J Cardiothorac Surg 2018;53:835-41. [Crossref] [PubMed]
- Hino H, Karasaki T, Yoshida Y, et al. Risk factors for postoperative complications and long-term survival in lung cancer patients older than 80 years. Eur J Cardiothorac Surg 2018;53:980-6. [Crossref] [PubMed]
- Rueth NM, Parsons HM, Habermann EB, et al. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg 2012;143:1314-23. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Deng HY, Zhou J, Wang RL, et al. Age-different extent of resection for clinical IA non-small cell lung cancer: analysis of nodal metastasis. Sci Rep 2020;10:9587. [Crossref] [PubMed]
- Cho H, Mariotto AB, Mann BS, et al. Assessing non-cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol 2013;178:339-49. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28, dicussion 28-29.e1.
- Zhang R, Ferguson MK. Video-Assisted versus Open Lobectomy in Patients with Compromised Lung Function: A Literature Review and Meta-Analysis. PLoS One 2015;10:e0124512. [Crossref] [PubMed]
- Sandri A, Papagiannopoulos K, Milton R, et al. High-risk patients and postoperative complications following video-assisted thoracic surgery lobectomy: a case-matched comparison with lower-risk counterparts†. Interact Cardiovasc Thorac Surg 2015;21:761-5. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Berry MF, Villamizar-Ortiz NR, Tong BC, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044-51; discussion 1051-2. [Crossref] [PubMed]
- Wang W, Xu Z, Xiong X, et al. Video-assisted thoracoscopic lobectomy for non-small cell lung cancer in patients with severe chronic obstructive pulmonary disease. J Thorac Dis 2013;5:S253-9. [PubMed]
- Kachare S, Dexter EU, Nwogu C, et al. Perioperative outcomes of thoracoscopic anatomic resections in patients with limited pulmonary reserve. J Thorac Cardiovasc Surg 2011;141:459-62. [Crossref] [PubMed]
- Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient--the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13. [Crossref] [PubMed]
- Taylor MD, LaPar DJ, Isbell JM, et al. Marginal pulmonary function should not preclude lobectomy in selected patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:738-44; Discussion 744-6. [Crossref] [PubMed]
- Puri V, Crabtree TD, Bell JM, et al. National cooperative group trials of "high-risk" patients with lung cancer: are they truly "high-risk"? Ann Thorac Surg 2014;97:1678-83; discussion 1683-5. [Crossref] [PubMed]
- Paul S, Andrews WG, Nasar A, et al. Outcomes of lobectomy in patients with severely compromised lung function (predicted postoperative diffusing capacity of the lung for carbon monoxide % ≤ 40%). Ann Am Thorac Soc 2013;10:616-21. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Surgical resection for clinical-Stage I radiological pure-solid lung cancer that met the current high risk criteria. Jpn J Clin Oncol 2017;47:630-8. [Crossref] [PubMed]
- Sancheti MS, Melvan JN, Medbery RL, et al. Outcomes After Surgery in High-Risk Patients With Early Stage Lung Cancer. Ann Thorac Surg 2016;101:1043-50; Discussion 1051. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg 2011;142:1143-51. [Crossref] [PubMed]
- Linden PA, Bueno R, Colson YL, et al. Lung resection in patients with preoperative FEV1 < 35% predicted. Chest 2005;127:1984-90. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. The impact of adjuvant brachytherapy with sublobar resection on pulmonary function and dyspnea in high-risk patients with operable disease: preliminary results from the American College of Surgeons Oncology Group Z4032 trial. J Thorac Cardiovasc Surg 2011;142:554-62. [Crossref] [PubMed]
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. [Crossref] [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [Crossref] [PubMed]
- Garzon JC, Ng CS, Sihoe AD, et al. Video-assisted thoracic surgery pulmonary resection for lung cancer in patients with poor lung function. Ann Thorac Surg 2006;81:1996-2003. [Crossref] [PubMed]
- Cerfolio RJ, Allen MS, Trastek VF, et al. Lung resection in patients with compromised pulmonary function. Ann Thorac Surg 1996;62:348-51. [Crossref] [PubMed]
- Magdeleinat P, Seguin A, Alifano M, et al. Early and long-term results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg 2005;27:1099-105. [Crossref] [PubMed]
- Pompili C, Brunelli A, Refai M, et al. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010;37:525-30. [Crossref] [PubMed]
- Baldi S, Ruffini E, Harari S, et al. Does lobectomy for lung cancer in patients with chronic obstructive pulmonary disease affect lung function? A multicenter national study. J Thorac Cardiovasc Surg 2005;130:1616-22. [Crossref] [PubMed]
- Sekine Y, Iwata T, Chiyo M, et al. Minimal alteration of pulmonary function after lobectomy in lung cancer patients with chronic obstructive pulmonary disease. Ann Thorac Surg 2003;76:356-61; discussion 362. [Crossref] [PubMed]
- Brunelli A, Xiumé F, Refai M, et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: a prospective follow-up analysis. Chest 2007;131:141-7. [Crossref] [PubMed]
- Brunelli A, Refai M, Salati M, et al. Predicted versus observed FEV1 and DLCO after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007;83:1134-9. [Crossref] [PubMed]
- Kushibe K, Takahama M, Tojo T, et al. Assessment of pulmonary function after lobectomy for lung cancer--upper lobectomy might have the same effect as lung volume reduction surgery. Eur J Cardiothorac Surg 2006;29:886-90. [Crossref] [PubMed]
- Kushibe K, Kawaguchi T, Kimura M, et al. Influence of the site of lobectomy and chronic obstructive pulmonary disease on pulmonary function: a follow-up analysis. Interact Cardiovasc Thorac Surg 2009;8:529-33. [Crossref] [PubMed]
- Korst RJ, Ginsberg RJ, Ailawadi M, et al. Lobectomy improves ventilatory function in selected patients with severe COPD. Ann Thorac Surg 1998;66:898-902. [Crossref] [PubMed]
- Kent MS, Mandrekar SJ, Landreneau R, et al. Impact of Sublobar Resection on Pulmonary Function: Long-Term Results from American College of Surgeons Oncology Group Z4032 (Alliance). Ann Thorac Surg 2016;102:230-8. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg 2015;149:718-25; discussion 725-6. [Crossref] [PubMed]
- Shimoyama R, Tsutani Y, Wakabayashi M, et al. A multi-institutional randomized phase III trial comparing anatomical segmentectomy and wedge resection for clinical stage IA non-small cell lung cancer in high-risk operable patients: Japan Clinical Oncology Group Study JCOG1909 (ANSWER study). Jpn J Clin Oncol 2020;50:1209-13. [Crossref] [PubMed]
- Tsutani Y, Mimura T, Kai Y, et al. Outcomes after lobar versus sublobar resection for clinical stage I non-small cell lung cancer in patients with interstitial lung disease. J Thorac Cardiovasc Surg 2017;154:1089-1096.e1. [Crossref] [PubMed]
- Hattori A, Takamochi K, Matsunaga T, et al. Oncological outcomes of sublobar resection for clinical-stage IA high-risk non-small cell lung cancer patients with a radiologically solid appearance on computed tomography. Gen Thorac Cardiovasc Surg 2016;64:18-24. [Crossref] [PubMed]
- Tsutani Y, Kagimoto A, Handa Y, et al. Wedge resection versus segmentectomy in patients with stage I non-small-cell lung cancer unfit for lobectomy. Jpn J Clin Oncol 2019;49:1134-42. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol 2014;32:2456-62. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger K, et al. Physiologic Evaluation of the Patient with Lung Cancer Being Considred for Resectional Surgery: Diagnosis and Management of Lung Cancer 3 rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:e166S-e90S.
- National Institute for Health and Clinical Excellence. The Diagnosis and Treatment of Lung Cancer (Update) Cardiff UK: © National Collaborating Centre for Cancer, 2011 [updated Apr 2011].
- National Institute for Health and Care Excellence. Lung cancer: diagnosis and management - NICE Guideline [ng122] London: © Nice 2019; 2019 [updated Mar; cited 2019]. Available online: nice.org.uk/guidance/ng122
- Brunelli A, Charloux A, Bolliger CT, et al. The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients with lung cancer. Eur J Cardiothorac Surg 2009;36:181-4. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Yoshida J, Ishii G, Yokose T, et al. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol 2010;5:546-50. [Crossref] [PubMed]
- Nakao M, Yoshida J, Goto K, et al. Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J Thorac Oncol 2012;7:1563-6. [Crossref] [PubMed]
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; Discussion 762-4. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Zhang C, He Z, Cheng J, et al. Surgical Outcomes of Lobectomy Versus Limited Resection for Clinical Stage I Ground-Glass Opacity Lung Adenocarcinoma 2 Centimeters or Smaller. Clin Lung Cancer 2021;22:e160-e168. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Chiang XH, Hsu HH, Hsieh MS, et al. Propensity-Matched Analysis Comparing Survival After Sublobar Resection and Lobectomy for cT1N0 Lung Adenocarcinoma. Ann Surg Oncol 2020;27:703-15. [Crossref] [PubMed]
- Okada M, Mimae T, Tsutani Y, et al. Segmentectomy versus lobectomy for clinical stage IA lung adenocarcinoma. Ann Cardiothorac Surg 2014;3:153-9. [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Yano M, Yoshida J, Koike T, et al. Survival of 1737 lobectomy-tolerable patients who underwent limited resection for cStage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;47:135-42. [Crossref] [PubMed]
- Son JY, Lee HY, Lee KS, et al. Quantitative CT analysis of pulmonary ground-glass opacity nodules for the distinction of invasive adenocarcinoma from pre-invasive or minimally invasive adenocarcinoma. PLoS One 2014;9:e104066. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Neither Maximum Tumor Size nor Solid Component Size Is Prognostic in Part-Solid Lung Cancer: Impact of Tumor Size Should Be Applied Exclusively to Solid Lung Cancer. Ann Thorac Surg 2016;102:407-15. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Importance of Ground Glass Opacity Component in Clinical Stage IA Radiologic Invasive Lung Cancer. Ann Thorac Surg 2017;104:313-20. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. [Crossref] [PubMed]
- Sagawa M, Oizumi H, Suzuki H, et al. A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur J Cardiothorac Surg 2018;53:849-56. [Crossref] [PubMed]
- National Comprehensive CN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Small Cell Lung Cancer Version 7.2019 - August 30, 2019 2019.
- Howington JA, Blum MG, Chang AC, et al. Treatment of Stage I and II Non-small Cell Lung Cancer. Chest 2013;143:e278S-e313S. [Crossref] [PubMed]
- Nishio W, Yoshimura M, Maniwa Y, et al. Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1702-10. [Crossref] [PubMed]
- Detterbeck FC, Gibson CJ. Turning gray: the natural history of lung cancer over time. J Thorac Oncol 2008;3:781-92. [Crossref] [PubMed]
- Detterbeck FC. Cancer, concepts, cohorts and complexity: avoiding oversimplification of overdiagnosis. Thorax 2012;67:842-5. [Crossref] [PubMed]
- Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269-74. [Crossref] [PubMed]
- Lindell RM, Hartman TE, Swensen SJ, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology 2007;242:555-62. [Crossref] [PubMed]
- Wilson DO, Ryan A, Fuhrman C, et al. Doubling times and CT screen–detected lung cancers in the Pittsburgh Lung Screening Study. Am J Respir Crit Care Med 2012;185:85-9. [Crossref] [PubMed]
- Sone S, Nakayama T, Honda T, et al. CT findings of early-stage small cell lung cancer in a low-dose CT screening programme. Lung Cancer 2007;56:207-15. [Crossref] [PubMed]
- Sugi K, Kobayashi S, Sudou M, et al. Long-term prognosis of video-assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 2010;37:456-60. [PubMed]
- Cao J, Yuan P, Wang Y, et al. Survival Rates After Lobectomy, Segmentectomy, and Wedge Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:1483-91. [Crossref] [PubMed]
- Fan X, Liang Y, Bai Y, et al. Conditional survival rate estimates of lobectomy, segmentectomy and wedge resection for stage IA1 non-small cell lung cancer: A population-based study. Oncol Lett 2020;20:1607-18. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Mase VJ Jr, Detterbeck FC. Approach to the Subsolid Nodule. Clin Chest Med 2020;41:99-113. [Crossref] [PubMed]
- Sawada S, Yamashita N, Sugimoto R, et al. Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. Chest 2017;151:308-15. [Crossref] [PubMed]
- Kobayashi Y, Fukui T, Ito S, et al. How long should small lung lesions of ground-glass opacity be followed? J Thorac Oncol 2013;8:309-14. [Crossref] [PubMed]
- Lee SW, Leem CS, Kim TJ, et al. The long-term course of ground-glass opacities detected on thin-section computed tomography. Respir Med 2013;107:904-10. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Oncological Characteristics of Radiological Invasive Adenocarcinoma with Additional Ground-Glass Nodules on Initial Thin-Section Computed Tomography: Comparison with Solitary Invasive Adenocarcinoma. J Thorac Oncol 2016;11:729-36. [Crossref] [PubMed]
- Schabath MB, Massion PP, Thompson ZJ, et al. Differences in Patient Outcomes of Prevalence, Interval, and Screen-Detected Lung Cancers in the CT Arm of the National Lung Screening Trial. PLoS One 2016;11:e0159880. [Crossref] [PubMed]
- Hanagiri T, Sugio K, Mizukami M, et al. Postoperative prognosis in patients with non-small cell lung cancer according to the method of initial detection. J Thorac Oncol 2007;2:907-11. [Crossref] [PubMed]


