
A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 4: systematic review of evidence involving SBRT and ablation
Introduction
Treatment options for stage cI non-small cell lung cancer (NSCLC) have evolved. Detected tumors are smaller and biologically less aggressive. Patients are older and comorbidities more frequent. Choosing the best treatment is complex; multiple short- and long-term outcomes are relevant. The available evidence is suboptimal, confusing, with many confounders—factors that affect both treatment selection and outcome. We need a better understanding of the evidence, sources of uncertainty, and nuances of patients, tumors and settings that affect the applicability thereof.
This project strives to comprehensively evaluate the evidence regarding stage cI NSCLC, critically addressing confounders and limitations. Furthermore, we sought to assemble this in a concise format that enhances clinical decision-making for individual patients. The project consists of 4 publications: Part 1 summarizes the evidence and provides a framework to guide clinical decision-making (1), Part 2 reviews evidence regarding surgery in generally healthy patients (2), Part 3 addresses specific patients and tumors (3), and Part 4 (this paper) focuses on evidence regarding SBRT and ablation.
Methods
General approach
Details of the general approach are provided elsewhere (Methods section of Part 1) (1). Briefly, the focus is patients with stage cIA NSCLC (using the 8th edition nomenclature throughout). Interventions include lobectomy, segmentectomy, wedge resection, SBRT and ablation. Relevant outcomes were chosen a priori: treatment-related mortality, toxicity/morbidity, pain, functional capacity, quality-of-life (QOL), overall survival (OS), lung cancer specific survival (LCSS), and freedom-from-recurrence (FFR).
Because few randomized controlled trials (RCTs) are available for this topic, we relied heavily on non-randomized comparisons (NRCs) that adjusted for confounders. How well confounders were addressed was critically evaluated to judge the confidence that observations could be attributed to the intervention in question. Furthermore, we explored sources of ambiguity to understand uncertainties and limitations of applicability.
Literature search, study selection and evidence assessment
We performed a systematic literature search in PubMed from 2000–2021. Details of the search strategy, selection and review process are provided elsewhere (see App. 1-2 of Part 1) (1). Each table lists specific inclusion and exclusion criteria.
Study quality was assessed using a general tool (4) and an adaption thereof specific to stage I NSCLC (described in App. 2-1 of Part 2) (2). Residual confounding in seven a priori defined domains is shown in the evidence tables along with the confidence that observed results reflect the treatment intervention. The domains include non-medical and medical patient-related factors, discrepancies in stage classification, time period, facility factors, treatment quality and favorable tumor selection.
Aggregation of evidence
A quantitative meta-analysis was deemed inappropriate due to the degree and variability of residual confounding. Instead, thoughtfully structured tables reflecting nuances of the patients, treatments and tumors provide an aggregate impression of the strengths, weaknesses and applicability of the data. We have used color coding, essentially layering a heat map onto the tables to facilitate gaining an overview without getting lost in details. This presents the data in a manner that provides an aggregate view of an outcome at-a-glance as well as nuances and uncertainties of the data. The table structure is noted as a subtitle. We aim to enhance individualized decision-making through this comprehensive yet nuanced presentation.
Results
General results of SBRT vs. surgery
Short-term outcomes
Treatment-related morbidity and mortality
Treatment-related mortality is meaningfully lower for SBRT than surgery (90-day mortality ~1% vs. ~3%, respectively, Table S4-1) (5-14). The difference is more pronounced in adjusted NRCs and slightly diminished with VATS surgery.
Short-term toxicity/morbidity appears lower after SBRT vs. surgery, although direct comparison is hampered by the different nature and timing of complications. Grade ≥3 toxicity within 6–12 months of SBRT is reported in 2–5% (Table S4-2) (15-34). The rate is similar for central vs. peripheral tumors (using appropriate dose-fractionation adjustments). Central tumors (1–2 cm from the proximal tracheobronchial tree) tend to be associated with hemoptysis, pericardial effusion, and esophagitis and peripheral tumors with dermatitis, rib fractures, and chest wall pain. SBRT toxicity accumulates over time; ~10–20% of patients experience grade ≥3 toxicity by ~2 years. The rate of grade ≥3 toxicity appears slightly higher in prospective controlled trials than prospective databases, and in inoperable vs. operable patients. Similar toxicity (and survival/control) rates are seen among generally accepted dose/fractionation schemes (e.g., 1× 30–34 Gy, 3× 18–20 Gy, 5× 10–11 Gy, 8× 7.5 Gy; selected based on tumor location and adjacent tissues at risk) (23,26,35).
Short-term QOL
Approximately 25–30% of patients reported meaningful worsening of QOL at 3 months after SBRT and an equal proportion a meaningful improvement in a large study (34). Similar results were noted at 1 and 4 months in another smaller study (36). QOL averaged across the entire cohort, however, is unchanged after SBRT in multiple studies (see subsequent QOL section).
Long-term outcomes
Survival
Several RCTs comparing SBRT to resection in healthy patients closed after accruing only a few patients (Figure 1). The STARS and ROSEL RCTs compared SBRT and lobectomy in lobectomy-eligible patients with cI-IIA NSCLC (≤4 cm). Both were closed due to poor accrual (STARS after 4 years, ROSEL after 2 years). Pooled results (58 patients, median follow-up 35–40 months) demonstrated better OS after SBRT [hazard ratio (HR) 0.14, P=0.037]; there was no difference in recurrence-free survival (RFS, HR 0.69, P=0.53) or local, regional and distant failure rates (37). There were no apparent imbalances among the patient cohorts [mean age 67, 98% performance status (PS) 0–1, 87% cIA]. The results are provocative; however, the limited accrual limits having confidence in the findings.
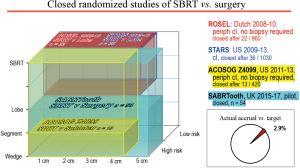
Closed RCTs of SBRT vs. surgery showing the resection extent, tumor size and the type of patients involved, as well as the final accrual. Lobe, lobectomy; Periph, peripheral; SBRT, Stereotactic body radiation therapy.
Several RCTs in good-risk patients are ongoing (Figure 2). The VALOR study (38) compares SBRT to lobectomy or segmentectomy (target accrual 670, results anticipated in 2027). A randomized phase II study of SBRT vs. surgical resection in cIA in China (POSTILV) (39) remains active, seeking to enroll 76 patients from 2012–2021. The prolonged period and limited size of this study raises concerns.
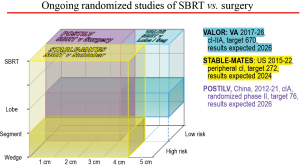
Ongoing RCTs of SBRT vs. surgery showing the resection extent, tumor size and the type of patients involved, with accrual targets and anticipated timeline. Lobe, lobectomy; SBRT, Stereotactic body radiation therapy; Seg, segmentectomy; VA, US Veterans Administration Healthcare System.
Table 1 (7,9,10,13,40-57), Table 2 (8,9,40,42,49,58-63), and Figure S4-1A,S4-1B summarizes adjusted NRCs of SBRT vs. surgery. Surgery involved lobectomy in most studies. OS favors surgery in almost all studies, especially those that adjusted extensively for confounders. This is less true in studies with short follow-up, consistent with the observation that downsides manifest early after surgery and later for SBRT (7). It is unclear if T-stage has an impact; however, most patients had small tumors.
Table 1
Ordered by degree of confidence that results reflect the effect of the treatment, stage
Table 2
Ordered by degree of confidence that results reflect the effect of the treatment, stage
The difference in OS is clinically relevant (20–30% 5-year absolute difference). Figure 3 depicts extensively adjusted OS of patients with a comorbidity score of 0 and eligible for surgery (41). The results were confirmed in patients recommended to have surgery but refused. Worse 5-year OS after SBRT than resection (42% vs. 64%) was similarly found after extensive propensity-matching of patients in whom surgery was recommended but who declined for non-medical reasons in another study (40).
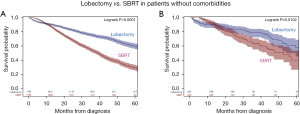
Overall survival in patients with stage cI NSCLC and without comorbidities treated by full-dose SBRT (biologically effective dose of ≥100 Gy) vs. lobectomy. All were surgery-eligible and had a Charlson-Deyo score of 0 (NCDB, 2008-12). (A) Propensity-matched patients and (B) propensity-matched subset who were recommended to have surgery but refused. Reproduced with permission from Rosen et al. (41). SBRT, stereotactic body radiation therapy.
However, addressing confounding is inherently difficult when one treatment is typically selected for robust and another for compromised patients. Better outcomes are consistently reported in operable vs. inoperable patients (24,64-66). Among matched patients in studies reporting this, the proportion of patients with PS ≥2 was 2–59% for SBRT and 0–17% for surgery (44,45,51,54,56,57). The proportion with a Charlson-Deyo score of ≥2 was 14–55% for surgery and 13–61% for SBRT (7-10,40,42,49,51,54,56,58-60,62). Unsuspected node involvement occurred in 14% (range 3–21%) of surgical patients (unknown among SBRT patients) (6,41,44,45,47,49-52,54,56-58,62,67). Furthermore, 0–70%, of the “matched” SBRT patients were designated as “medically inoperable” (44,47,50-52,54,56). Thus, concern of residual confounding remains (e.g., severity of comorbidities, frailty), despite attempts to account for comorbidities. Of note, while LCSS consistently favors surgery, this is less frequently statistically significant.
Recurrence
We think the best measure of recurrence is FFR and locoregional FFR (LR-FFR). Locoregional recurrence is most easily defined similarly for SBRT and surgery (an issue with inherently different treatments). DFS/RFS mixes recurrence and unrelated deaths. Recurrence is affected by the follow-up duration and protocol.
NRCs of recurrence after SBRT vs. resection (Table 3) have generally involved only limited adjustment for confounders (43,44,47,48,50-57,60,61,68-72). Generally, more recurrences are reported after SBRT, but one study found the opposite (despite short follow-up in surgical patients) (51). The number of studies, limited adjustment for confounders and ambiguities of outcome assessment hamper confidently drawing conclusions about recurrence after SBRT vs. resection. A prospective trial of SBRT followed by resection 10 weeks later found viable tumor in 40% of patients—but the relevance of this finding is unclear given the much lower rate of local failure after SBRT (73).
Table 3
Ordered patient type, degree of confidence that results reflect the effect of the treatment, stage
Long-term QOL
Multiple studies show that SBRT has no negative impact on QOL (Table 4) (20,26,34,36,74-87) despite mostly using the more sensitive EORTC assessment (vs. the SF-36). The minimal impact on QOL is seen despite many PS2 patients and most being deemed medically inoperable. Assessing the average for the entire cohort can obscure relevant subsets: 25–30% of patients are meaningfully worse and a similar proportion meaningfully better 3–24 months after SBRT in both global QOL and physical functioning (34). In this study of 382 patients the proportion with worsening physical functioning tended to increase between 12–24 months) (34).
Table 4
Ordered by QOL tool, study size
Long-term toxicity
While short-term toxicity following SBRT is low, prospective studies report 10–30% grade ≥3 late toxicity (Table S4-2A) (17,29,80,84,88-96). Most studies reported treatment-related toxicity, but some adverse events may be attributable to underlying poor health. Approximately 25% of patients had a PS of ≥2. Operable patients may have slightly less late toxicity (Table S4-2B) (24,34,97).
Pulmonary function tests (PFTs)
PFTs were used as a surrogate for functional capacity in the absence of direct data on functional capacity. The reported average long-term decline in PFTs (Table S4-3) (17,29,80,84,88-96) after SBRT is low and not clinically meaningful. However, a substantial proportion of patients experienced a ≥10% decline (~40–50%) or a ≥25% decline (~15–25%). Fewer patients experienced a ≥10% or ≥25% improvement. The average baseline FEV1 (64%) or DLCO (58%) in these SBRT patients was fairly high. As noted in 2 studies, 10–20% of SBRT patients used oxygen pre-treatment, an additional 3% required it later (88,89).
Large observational studies of smokers with moderate chronic obstructive pulmonary disease (COPD) indicate an FEV1 loss of ~50 mL/year or ~1.3%/year (absolute percent-predicted) (98-100). The decline is slower with more severe COPD and markedly diminished after smoking cessation (98-100). While there is individual variability, the chance of a >10% relative FEV1 decline in 1–2 years due to the natural history of COPD is very low, even in active smokers.
Nuances and sources of ambiguity
Modified fractionation schemes (e.g., 5 fractions while decreasing the biologic effective dose) have rendered SBRT for central tumors (1–2 cm from the proximal tracheobronchial tree) as safe and effective as in peripheral tumors (15,17,22). Toxicity concerns remain for ultra-central tumors (≤1 cm from the trachea, mainstem and lobar bronchi), especially with higher doses and fewer fractions (101-104). The HILUS and SUNSET trials are exploring hypofractionated regimens (8–15 fractions) (105,106). Grade 3 toxicity was noted in 22% and grade 5 in 15% in the HILUS trial (105), suggesting that segmentectomy or lobectomy if possible may be better treatment choices for ultra-central tumors.
Factors independently associated with long-term outcomes are not well-defined. Worse outcomes are reported with squamous vs. adenocarcinoma in some studies (multivariable HR ~1.7–2.4) (66,107), but not others (108,109), with rapidly growing tumors (multivariable HR ~1.4–1.5) (109), with high PET-avidity in some studies (multivariable HR ~4–6) (110) but not others (107,111), and larger tumors in some studies (multivariable HR ~1.2–9) (66,108,110,111) but not others (107,109,112). Reasonable outcomes are reported even for tumors >5 cm (113,114).
In conclusion, technical/anatomic factors may impact toxicity and treatment choice. Other tumor-related prognostic factors are not well-defined.
Summary of general evidence for SBRT vs. surgery
Short-term mortality is meaningfully better after SBRT than surgery. While significant acute morbidity/toxicity is low, 10–20% of SBRT patients experience grade ≥3 toxicity by 2 years. Average QOL is not decreased after SBRT. Comparing across studies, this is clearly better than surgery, which causes major short-term QOL impairment, and sustained long-term impairment after open resection (less so after VATS). On average, PFTs are minimally decreased after SBRT, although 20–40% of SBRT patients experience a clinically meaningful decrease after 1–2 years. Preservation of PFTs with SBRT is clinically relevant vs. lobectomy, at most marginally meaningful vs. segmentectomy.
Completed RCTs are inconclusive due to limited accrual. Ongoing RCT results in good risk and high-risk patients are anticipated in 2024–26. Adjusted NRCs quite consistently demonstrate a highly clinically relevant detriment in OS and LCSS for SBRT vs. lobectomy or vs. sublobar resection. This is most apparent in more extensively-adjusted NRCs. Nevertheless, adjustment for confounders is inherently challenging when comparing SBRT and surgery.
SBRT vs. surgery in older patients
Short-term outcomes
Mortality and toxicity
A US National Cancer Database (NCDB) study of post-treatment mortality found little difference in 30- and 90-day mortality for SBRT vs. surgery below age 70 (Figure 4) (5). In older patients there is a clinically meaningful benefit to SBRT. This was confirmed in propensity-matched cohorts (moderate confidence that confounders are accounted for) (5). Similarly, another NCDB study of healthy patients (Charlson score 0) age ≥80 noted better unadjusted 90-day mortality for SBRT (0.7%) vs. lobectomy (3.3% by VATS, 6.7% by thoracotomy, 5.6% total) (6).
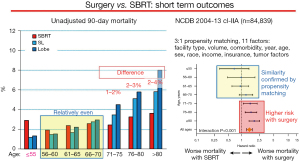
Post-treatment 90-day mortality of early stage lung cancer patients by age cohorts; Unadjusted rates and hazard ratio in propensity-matched groups. Data taken from Stokes et al. (5). Lobe, lobectomy; SBRT, stereotactic body radiotherapy; SL, sublobar resection.
Data regarding toxicity of SBRT has not been parsed to specific age cohorts. However, the average patient age in general studies of SBRT is ~70–75. Comparing across studies suggests less grade ≥3 short-term toxicity after SBRT (5–10%) than surgery (10–20%) in older cohorts (Table S4-2 and see Older Patients section of Part 3) (3).
Long-term outcomes
No RCTs have addressed SBRT vs. resection in older patients. Adjusted NRCs (Table 5 and Figure S4-2) (6,9,11,12,42,58,67-70,115-117) demonstrate worse OS and LCSS after SBRT than surgery (with few exceptions). The difference in adjusted OS is clinically relevant (5–25% absolute difference). Differences were more often statistically significant in the more extensively-adjusted studies. The differences don’t appear to vary by the extent of surgical resection, age cohorts or tumor size. Adjusted NRCs addressing recurrence found worse RFS and higher locoregional recurrence after SBRT than surgery (Table 3) (53,68,70).
Table 5
Ordered by extent of resection, degree of confidence that results reflect the effect of the treatment, stage, age
QOL and long-term toxicity
Data regarding QOL in older SBRT patients was not identified. An adjusted NRC of long-term toxicity in older patients (Figure S4-3, low confidence rating) noted that post-resection complications primarily occur within 1 month; subsequently few additional morbidities develop. In contrast, after SBRT early toxicity is unusual, but a consistent higher incidence of toxicity over time leads to a cumulative equal incidence for SBRT and surgery by 2 years (7).
Summary of SBRT vs. surgery in older patients
SBRT is associated with a clinically meaningful short-term mortality benefit vs. surgery (1–4%). This is more pronounced as age increases, and for open resection (vs. VATS). Morbidity is higher initially after surgery, but late toxicity after SBRT renders the overall incidence relatively equal after 2 years. Surgery (especially open) impairs QOL; SBRT has little impact.
Several extensively adjusted NRCs in older patients suggest meaningfully worse OS after SBRT vs. surgery; often differences were not statistically significant. Age and tumor size do not appear to affect the differences.
SBRT vs. surgery in compromised patients
Short-term outcomes
Short-term outcomes after SBRT have not been specifically addressed in compromised patients. Most of the SBRT patients in the general evidence tables were deemed medically inoperable. However, average reported characteristics (FEV1 >60%, DLCO >50%, PS 0,1 in >75%) leaves uncertainty regarding short-term outcomes in patients with FEV1 or DLCO <40% or PS ≥2. Speculation suggests that outcomes would be worse than the general reported results of SBRT.
Long-term outcomes
Survival and recurrence
Two RCTs in high-risk patients were initiated but had limited accrual (ACOSOG Z4099 (118) and SABRTooth (119), Figure 1). No long-term results have been published, but the limited enrollment leaves little hope that results would be revealing.
The STABLE-Mates trial (120) is ongoing, comparing SBRT to sublobar resection in cI-IIA high risk patients as defined by the ACOSOG criteria (FEV1 or DLCO <50%, or 2 minor criteria including age ≥75, FEV1 or DLCO 51–60%, Figure 2). The target accrual is 272, with results expected in 2024.
Few NRCs with limited adjustment have compared SBRT with surgery in compromised patients (Table 6 and Figure S4-4) (7,9,49,58,71,72,121,122). Results suggest worse OS and LCSS after SBRT than surgery (mostly not statistically significant). The adjusted OS difference was meaningful (10–20%). A multivariable analysis parsed by FEV1% did not suggest greater differences with lower FEV1 or by resection extent—LCSS was consistently worse (mostly statistically non-significant) after SBRT vs. lobectomy (FEV1% 51–80% HR 1.3; 31–50% HR 1.26; ≤30% HR 1.55) and vs. sublobar resection (FEV1% 51–80% HR 1.47; 31–50% HR 2.01; ≤30% HR 1.45) (9). Whether FFR or LR-FFR is worse after SBRT than surgery in compromised patients is unclear (few reported NRCs, Table 3) (71,72).
Table 6
Ordered by extent of resection, degree of confidence that results reflect the effect of the treatment, stage
QOL and PFT studies
QOL after SBRT specifically in compromised patients has not been reported. However, SBRT probably has little average impact because many patients in QOL studies (Table 4) were PS ≥2 or medically inoperable.
Most studies of PFT changes after SBRT (Table S4-3) included a broad spectrum of patients with relatively good PFTs. Limited data specifically on compromised patients suggests that SBRT is well tolerated: no change or a slight improvement was noted in FEV% in patients with GOLD III-IV COPD (88) or cohorts with low baseline FEV1 [average 40% (123) or <50% (89)]. Others report similar findings (29). Multivariable analysis of the RTOG0236 trial revealed no correlation of any PFT parameters and pulmonary toxicity (96). While this is reassuring, the effect of SBRT on severely compromised patients (e.g., FEV1 or DLCO of <40%) is unclear.
Complications/toxicity
Yu et al. compared complications/toxicity after SBRT vs. surgery in propensity-matched high- and low-risk cohorts (7). The cumulative incidence of chest morbidity (cardiopulmonary, esophageal) was nearly double in high- vs. low-risk cohorts with either surgery or SBRT, but the relative benefit of SBRT over surgery was similar in high- and low-risk cohorts (Figure S4-5). Other comparative data was not identified.
Nuances and sources of ambiguity
Interstitial lung disease (ILD), a heterogeneous group of diffuse parenchymal lung diseases, deserves specific discussion. Non-fibrotic ILDs includes multiple inflammatory, multinodular and cystic lung disorders; these are not associated with lung cancer, often acute, and usually respond well to treatment of the underlying etiology (124). Fibrotic ILDs are more common, portend a high risk (10–20%) of developing lung cancer, and a risk of radiation-related toxicity. Fibrotic ILDs may be caused by connective tissue disorders, hypersensitivity pneumonitis, and pneumoconiosis. Most concerning is idiopathic pulmonary fibrosis (IPF): it is frequently progressive, life-limiting and associated with radiation toxicity (124). However, categorization of fibrotic vs. non-fibrotic ILD is imperfect. ILD can overlap with obstructive lung disease (combined pulmonary fibrosis and emphysema)—also associated with development of lung cancer, worse outcomes, and treatment-related complications (125-128). Additionally, some patients have incidentally-noted interstitial lung abnormalities, which may not be progressive or require a unique treatment plan (129).
The first step, establishing whether interstitial imaging findings represent actual ILD, requires a knowledgeable pulmonologist and often a multidisciplinary ILD team. The next step is estimating prognosis—3-year mortality of ILD varies from 10% to 75% (124). Additionally, ~10%/year of IPF patients develop random acute exacerbations, with a 3-month median survival (124).
The third step, treatment selection, is difficult. IPF patients typically have poor DLCO and significant restrictive pulmonary compromise. A recent systematic review of toxicity noted SBRT was associated with high treatment-related toxicity (25%) and mortality (16%, Table S4-4) (130). Treatment-related ILD mortality was 7% in studies that appear to focus on mild ILD vs. 22% in the remainder (130). Surgery had better outcomes, but the patients are likely not comparable. An increased risk of post-operative ILD exacerbation is associated with a history of exacerbations, preoperative steroids, usual interstitial pneumonia pattern, and reduced lung function (131,132). Reported 3-year survival of ILD patients with lung cancer is 50–60% (130).
Other major comorbidities rendering patients compromised are not clearly tied to greater risk or efficacy of any treatment. Tumor characteristics influencing the effectiveness of surgery, SBRT, or ablation are discussed elsewhere in this and the Parts 2 and 3 papers (2,3).
Summary of outcomes in patients with limited pulmonary reserve
Extrapolation from general evidence and older patients suggests a meaningful short-term mortality and morbidity benefit for SBRT over surgery. This may be accentuated in more compromised patients and slightly diminished with VATS resection, less clearly by sublobar resection.
NRCs of compromised patients consistently show long-term downsides for SBRT vs. surgery (10–20% worse 5-year OS). However, studies are limited, only partially adjusted, and results are mostly statistically non-significant. The patients are undoubtedly selected; limited data does not suggest a potential marker to guide treatment selection (e.g., cohorts of Charlson scores or FEV1%) (9).
Methods of ablation
Percutaneous ablation of lung tumors has been used for >20 years, including when there are contraindications to surgery or SBRT (e.g., poor PFTs, ILD, prior radiotherapy, difficult anatomy). It is not clear that one method of ablation is better than another (133); radiofrequency, microwave and cryoablation are most common. While many single-modality reports of lung ablation demonstrate reasonable local control and OS, comparative studies of ablation vs. SBRT or surgery are limited and not well-parsed to specific techniques, patients or tumors. Therefore, this section addresses all methods of percutaneous ablation collectively for all patients.
Short-term outcomes
Treatment-related toxicity
Several large series (>200 patients) (134-136) and systematic reviews (137) report pneumothorax (often presenting after several days) in 10–70% with 10–50% of these requiring a chest tube. Grade ≥3 morbidity is seen in 10–20%, and includes pleuritis, bleeding, lung abscess and pneumonia (each in ~1–3%). Similar frequencies were noted in smaller prospective studies (86,87,138). Larger series report a 30-day mortality of 0.3–0.5% (134-136); but 90-day mortality was 3.8% in a large study (NCDB, 2004–14, 1,009 ablation patients) (139).
Long-term outcomes
Survival
Adjusted NRCs (Table 7 and Figure S4-6) (59,139-149) demonstrate worse long-term outcomes after ablation than resection. The differences appear larger than for SBRT vs. resection (Table 1), but studies are limited and residual confounding makes interpretation difficult. Most studies report an average age of ~75, and a Charlson comorbidity score of ≥2 in 15–20%. Reported OS is low for early-stage NSCLC—likely reflecting both patients’ general health and treatment efficacy (ablation yields worse LCSS than resection).
Table 7
Ordered by degree of confidence that results reflect the effect of the treatment, stage
One adjusted NRC (149) found no difference in DFS or recurrence pattern between microwave ablation vs. lobectomy; extensive residual confounding precludes drawing firm conclusions regarding recurrence.
QOL
Very limited data (Table 4) demonstrates a mild decrease in some parameters 1 month after percutaneous ablation, but no evidence of long-term QOL impairment (85-87).
PFTs
Ablation appears to have limited but variable impact on PFTs. At 3 months, an increase of 2–6% in the average FEV1 has been observed (85,86,138). At 12–24 months, average FEV1 is 1–5% lower in several studies (85,86,150) and increased 5% in one (albeit with frequent missing data); similar results are seen for DLCO (138). Regarding subsets, at 3 months 10–20% of patients experienced a >10% FEV1 increase and a similar proportion a ≥10% decrease (i.e., a meaningful change) (87,138). Similar findings are reported for DLCO (138). Long-term 20–30% of patients experienced a ≥10% increase in FEV1 or DLCO, with a similar proportion experiencing a ≥10% decrease (138).
Nuances and ambiguity
The mechanism of action of specific ablation modalities (radiofrequency, microwave or cryoablation) affects efficacy, technical considerations (ablation size, number of needle punctures, maintenance of tissue architecture, etc.), and risk of complications (151). For example, cryoablation may increase the risk of pneumothorax and bleeding by requiring more needle punctures, while the increased power of microwave can shorten treatment times—these features may weigh more heavily in particular cases. Local expertise with particular ablation modalities is important. Similarly, local expertise with advanced image guidance and percutaneous ablation vs. SBRT should weigh in choosing a treatment approach (152).
Tumor-related factors can impact both efficacy and risks of ablation. Studies report >95% local control with tumors ≤2 cm, but considerably less for tumors >3 cm (153). Larger ablation zones increase the concern of complications; note that 8–10 mm of ablation beyond the tumor is recommended to reduce recurrence (154). Anatomical location, i.e., adjacent to pericardium, bronchus, pulmonary artery, diaphragm or blebs) affects concerns about toxicity. Patient-related factors may increase the risk of complications (e.g., degree of emphysema, ILD) (155).
Logistical issues affect deciding on the best treatment approach. Percutaneous ablation permits biopsy and treatment during the same session. Ablation is convenient, typically involving a single session. However, ablation is usually done under general anesthesia to control respiration and optimize tumor targeting.
Percutaneous ablation is an option for recurrence after prior radiotherapy. Furthermore, unlike radiotherapy or surgery, percutaneous ablation can be repeated as many times as necessary.
Summary of results of ablation vs. surgery or SBRT
Comparing across studies suggest that ablation is associated with a higher rate of short-term complications than SBRT. Short-term (90-day) mortality may be higher after ablation than SBRT comparing across studies (whether the patients are comparable is unclear). Surgery is associated with short-term pain and impairment of QOL in contrast to ablation. However, while some data suggests that 90-day mortality and an overall rate of Gr ≥3 complications is similar after ablation vs. surgery (especially VATS), this may be misleading because it is likely that the surgical patients are more carefully selected.
Adjusted NRCs indicate that OS or LCSS is clinically meaningfully worse after ablation vs. resection, and to a lesser degree after ablation vs. SBRT. However, the number of studies and degree of adjustment for confounders is limited. It is likely that many of the patients in these NRCs are compromised, but this is poorly characterized.
Key drivers of patient selection are avoiding patients likely to experience complications (severe emphysema, tumor surrounded by vessels) and technical factors limiting efficacy (e.g., tumor size).
Overall summary of SBRT or ablation vs. surgery
Outcomes for SBRT or ablation vs. lobectomy or sublobar resection are summarized in Table S4-5A-S4-5C. A benefit or detriment is qualitatively depicted relative to clinically meaningful differences, together with the confidence in and consistency of the evidence. This provides a succinct summary that can inform judgment for individual patients, as discussed in the Part 1 paper (1).
Conclusions
It is a major asset to have several treatment options for stage I NSCLC. In general, the short-term benefits of SBRT and ablation over surgery are clinically meaningful (e.g., mortality, morbidity/toxicity, QOL). This is offset by a clinically meaningful downside in long-term outcomes. In older patients the short-term benefits of SBRT and ablation are marginally increased, and the long-term downsides slightly diminished. In seriously compromised patients there is limited evidence, but it appears that short-term benefits are increased and long-term downsides diminished vs. surgery. Selection based on patient characteristics is poorly defined; tumor characteristics that influence technical feasibility of particular modalities are important considerations. ILD is particularly problematic due to the interplay of accurately diagnosing ILD, estimating relative prognosis of the ILD and lung cancer, and significant treatment-related toxicity and mortality.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation”. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1826/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1826/coif). The series “A Guide for Managing Patients with Stage I NSCLC: Deciding between Lobectomy, Segmentectomy, Wedge, SBRT and Ablation” was commissioned by the editorial office without any funding or sponsorship. FCD served as the unpaid Guest Editor of the series. HSP serves as an unpaid editorial board member of Journal of Thoracic Disease. HSP reports research funding from RefleXion Medical; consulting fees from AstraZeneca; honoraria and speaking fees from Bristol Myers Squibb; and advisory board fees from Galera Therapeutics; all unrelated to current work. DCM reports that he is the lead for an early career educational course on microwave ablation that is sponsored by Johnson & Johnson. BCB reports in the past 36 months, he receives grants from Veterans Affairs Central Office, American Cancer Society, Yale SPORE in Lung Cancer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Blasberg JD, Woodard GA, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation—part 1: a guide to decision-making. J Thorac Dis 2022; [Crossref]
- Detterbeck FC, Mase VJ Jr, Li AX, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation— part 2: systematic review of evidence regarding resection extent in generally healthy patients. J Thorac Dis 2022; [Crossref]
- Bade BC, Blasberg JD, Mase VJ Jr, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation— part 3: systematic review of evidence regarding surgery in compromised patients or specific tumors. J Thorac Dis 2022; [Crossref]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Stokes WA, Bronsert MR, Meguid RA, et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:642-51. [Crossref] [PubMed]
- Razi SS, Kodia K, Alnajar A, et al. Lobectomy Versus Stereotactic Body Radiotherapy in Healthy Octogenarians With Stage I Lung Cancer. Ann Thorac Surg 2021;111:1659-65. [Crossref] [PubMed]
- Yu JB, Soulos PR, Cramer LD, et al. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer 2015;121:2341-9. [Crossref] [PubMed]
- Mayne NR, Lin BK, Darling AJ, et al. Stereotactic Body Radiotherapy Versus Delayed Surgery for Early-stage Non-small-cell Lung Cancer. Ann Surg 2020;272:925-9. [Crossref] [PubMed]
- Bryant AK, Mundt RC, Sandhu AP, et al. Stereotactic Body Radiation Therapy Versus Surgery for Early Lung Cancer Among US Veterans. Ann Thorac Surg 2018;105:425-31. [Crossref] [PubMed]
- Boyer MJ, Williams CD, Harpole DH, et al. Improved Survival of Stage I Non-Small Cell Lung Cancer: A VA Central Cancer Registry Analysis. J Thorac Oncol 2017;12:1814-23. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [Crossref] [PubMed]
- Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014;147:1183-1191; discussion 1191-2. [Crossref] [PubMed]
- Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013;145:692-9. [Crossref] [PubMed]
- Park HS, Harder EM, Mancini BR, et al. Central versus Peripheral Tumor Location: Influence on Survival, Local Control, and Toxicity Following Stereotactic Body Radiotherapy for Primary Non-Small-Cell Lung Cancer. J Thorac Oncol 2015;10:832-7. [Crossref] [PubMed]
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012;82:967-73. [Crossref] [PubMed]
- Mangona VS, Aneese AM, Marina O, et al. Toxicity after central versus peripheral lung stereotactic body radiation therapy: a propensity score matched-pair analysis. Int J Radiat Oncol Biol Phys 2015;91:124-32. [Crossref] [PubMed]
- Sun B, Brooks ED, Komaki RU, et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer 2017;123:3031-9. [Crossref] [PubMed]
- Claude L, Morelle M, Mahé MA, et al. A comparison of two modalities of stereotactic body radiation therapy for peripheral early-stage non-small cell lung cancer: results of a prospective French study. Br J Radiol 2020;93:20200256. [Crossref] [PubMed]
- Nestle U, Adebahr S, Kaier K, et al. Quality of life after pulmonary stereotactic fractionated radiotherapy (SBRT): Results of the phase II STRIPE trial. Radiother Oncol 2020;148:82-8. [Crossref] [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gaspar LE, et al. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol 2019;37:1316-25. [Crossref] [PubMed]
- Stephans KL, Woody NM, Reddy CA, et al. Tumor Control and Toxicity for Common Stereotactic Body Radiation Therapy Dose-Fractionation Regimens in Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2018;100:462-9. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019;20:494-503. [Crossref] [PubMed]
- Singh AK, Gomez-Suescun JA, Stephans KL, et al. One Versus Three Fractions of Stereotactic Body Radiation Therapy for Peripheral Stage I to II Non-Small Cell Lung Cancer: A Randomized, Multi-Institution, Phase 2 Trial. Int J Radiat Oncol Biol Phys 2019;105:752-9. [Crossref] [PubMed]
- Cheung P, Faria S, Ahmed S, et al. Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1-3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J Natl Cancer Inst 2014;106:dju164. [Crossref] [PubMed]
- Inoue T, Katoh N, Ito YM, et al. Stereotactic body radiotherapy to treat small lung lesions clinically diagnosed as primary lung cancer by radiological examination: A prospective observational study. Lung Cancer 2018;122:107-12. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer - a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol 2008;88:359-67. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [Crossref] [PubMed]
- Modh A, Rimner A, Williams E, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;90:1168-76. [Crossref] [PubMed]
- Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7:1148-54. [Crossref] [PubMed]
- Videtic GM, Paulus R, Singh AK, et al. Long-term Follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2019;103:1077-84. [Crossref] [PubMed]
- Jain S, Poon I, Soliman H, et al. Lung stereotactic body radiation therapy (SBRT) delivered over 4 or 11 days: a comparison of acute toxicity and quality of life. Radiother Oncol 2013;108:320-5. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
-
VALOR Veterans Affairs Lung Cancer or Stereotactic Radiotherapy 2019 Available online: https://clinicaltrials.gov/show/NCT02984761 - Kong FM. Radical Resection vs. Ablative Stereotactic Radiotherapy in Patients with Operable Stage I NSCLC (POSTILIV): National Library of Medicine 2019 [cited 2020]. Available online: https://clinicaltrials.gov/show/NCT01753414
- Khorfan R, Kruser TJ, Coughlin JM, et al. Survival of Primary Stereotactic Body Radiation Therapy Compared With Surgery for Operable Stage I/II Non-small Cell Lung Cancer. Ann Thorac Surg 2020;110:228-34. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Chi A, Fang W, Sun Y, et al. Comparison of Long-term Survival of Patients With Early-Stage Non-Small Cell Lung Cancer After Surgery vs Stereotactic Body Radiotherapy. JAMA Netw Open 2019;2:e1915724. [Crossref] [PubMed]
- Chang JY, Mehran RJ, Feng L, et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol 2021;22:1448-57. [Crossref] [PubMed]
- Sebastian NT, Merritt RE, Abdel-Rasoul M, et al. Recurrence After Stereotactic Body Radiation Therapy Versus Lobectomy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2020;110:998-1005. [Crossref] [PubMed]
- Spencer KL, Kennedy MPT, Lummis KL, et al. Surgery or radiotherapy for stage I lung cancer? An intention-to-treat analysis. Eur Respir J 2019;53:1801568. [Crossref] [PubMed]
- Tomita N, Okuda K, Osaga S, et al. Surgery versus stereotactic body radiotherapy for clinical stage I non-small-cell lung cancer: propensity score-matching analysis including the ratio of ground glass nodules. Clin Transl Oncol 2021;23:638-47. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015;99:1122-9. [Crossref] [PubMed]
- Dong B, Zhu X, Shu Z, et al. Video-Assisted Thoracoscopic Lobectomy Versus Stereotactic Body Radiotherapy Treatment for Early-Stage Non-Small Cell Lung Cancer: A Propensity Score-Matching Analysis. Front Oncol 2020;10:585709. [Crossref] [PubMed]
- Puri V, Crabtree TD, Bell JM, et al. Treatment Outcomes in Stage I Lung Cancer: A Comparison of Surgery and Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1776-84. [Crossref] [PubMed]
- Lin Q, Sun X, Zhou N, et al. Outcomes of stereotactic body radiotherapy versus lobectomy for stage I non-small cell lung cancer: a propensity score matching analysis. BMC Pulm Med 2019;19:98. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Cornwell LD, Echeverria AE, Samuelian J, et al. Video-assisted thoracoscopic lobectomy is associated with greater recurrence-free survival than stereotactic body radiotherapy for clinical stage I lung cancer. J Thorac Cardiovasc Surg 2018;155:395-402. [Crossref] [PubMed]
- Dong B, Wang J, Xu Y, et al. Comparison of the Efficacy of Stereotactic Body Radiotherapy versus Surgical Treatment for Early-Stage Non-Small Cell Lung Cancer after Propensity Score Matching. Transl Oncol 2019;12:1032-7. [Crossref] [PubMed]
- van den Berg LL, Klinkenberg TJ, Groen HJM, et al. Patterns of Recurrence and Survival after Surgery or Stereotactic Radiotherapy for Early Stage NSCLC. J Thorac Oncol 2015;10:826-31. [Crossref] [PubMed]
- Albano D, Bilfinger T, Nemesure B. 1-, 3-, and 5-year survival among early-stage lung cancer patients treated with lobectomy vs SBRT. Lung Cancer (Auckl) 2018;9:65-71. [Crossref] [PubMed]
- Mokhles S, Verstegen N, Maat AP, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis. Lung Cancer 2015;87:283-9. [Crossref] [PubMed]
- Kastelijn EA, El Sharouni SY, Hofman FN, et al. Clinical Outcomes in Early-stage NSCLC Treated with Stereotactic Body Radiotherapy Versus Surgical Resection. Anticancer Res 2015;35:5607-14. [PubMed]
- Yerokun BA, Yang CJ, Gulack BC, et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:675-686.e4. [Crossref] [PubMed]
- Wu J, Bai HX, Chan L, et al. Sublobar resection compared with stereotactic body radiation therapy and ablation for early stage non-small cell lung cancer: A National Cancer Database study. J Thorac Cardiovasc Surg 2020;160:1350-1357.e11. [Crossref] [PubMed]
- Dong B, Zhu X, Jin J, et al. Comparison of the outcomes of sublobar resection and stereotactic body radiotherapy for stage T1-2N0M0 non-small cell lung cancer with tumor size ≤ 5 cm: a propensity score matching analysis. J Thorac Dis 2020;12:5934-54. [Crossref] [PubMed]
- Yuan XS, Chen WC, Lin QR, et al. A propensity-matched analysis of stereotactic body radiotherapy and sublobar resection for stage I non-small cell lung cancer in patients at high risk for lobectomy: the results in a Chinese population. J Thorac Dis 2021;13:1822-32. [Crossref] [PubMed]
- Ajmani GS, Wang CH, Kim KW, et al. Surgical quality of wedge resection affects overall survival in patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;156:380-391.e2. [Crossref] [PubMed]
- Iguchi T, Hiraki T, Matsui Y, et al. Survival Outcomes of Treatment with Radiofrequency Ablation, Stereotactic Body Radiotherapy, or Sublobar Resection for Patients with Clinical Stage I Non-Small-Cell Lung Cancer: A Single-Center Evaluation. J Vasc Interv Radiol 2020;31:1044-51. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Kann BH, Verma V, Stahl JM, et al. Multi-institutional analysis of stereotactic body radiation therapy for operable early-stage non-small cell lung carcinoma. Radiother Oncol 2019;134:44-9. [Crossref] [PubMed]
- Baine MJ, Verma V, Schonewolf CA, et al. Histology significantly affects recurrence and survival following SBRT for early stage non-small cell lung cancer. Lung Cancer 2018;118:20-6. [Crossref] [PubMed]
- Detillon DDEMA, Aarts MJ, De Jaeger K, et al. Video-assisted thoracic lobectomy versus stereotactic body radiotherapy for stage I nonsmall cell lung cancer in elderly patients: a propensity matched comparative analysis. Eur Respir J 2019;53:1801561. [Crossref] [PubMed]
- Tamura M, Matsumoto I, Tanaka Y, et al. Comparison Between Stereotactic Radiotherapy and Sublobar Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1544-50. [Crossref] [PubMed]
- Dong B, Wang J, Zhu X, et al. Comparison of the outcomes of stereotactic body radiotherapy versus surgical treatment for elderly (≥70) patients with early-stage non-small cell lung cancer after propensity score matching. Radiat Oncol 2019;14:195. [Crossref] [PubMed]
- Wang P, Zhang D, Guo XG, et al. A propensity-matched analysis of surgery and stereotactic body radiotherapy for early stage non-small cell lung cancer in the elderly. Medicine (Baltimore) 2016;95:e5723. [Crossref] [PubMed]
- Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer 2014;50:2932-8. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Palma DA, Nguyen TK, Louie AV, et al. Measuring the Integration of Stereotactic Ablative Radiotherapy Plus Surgery for Early-Stage Non-Small Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019;5:681-8. [Crossref] [PubMed]
- Schwartz RM, Alpert N, Rosenzweig K, et al. Changes in quality of life after surgery or radiotherapy in early-stage lung cancer. J Thorac Dis 2019;11:154-61. [Crossref] [PubMed]
- Widder J, Postmus D, Ubbels JF, et al. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e291-7. [Crossref] [PubMed]
- Farrugia MK, Yu H, Videtic GM, et al. A Principal Component of Quality-of-Life Measures Is Associated with Survival: Validation in a Prospective Cohort of Lung Cancer Patients Treated with Stereotactic Body Radiation Therapy. Cancers (Basel) 2021;13:4542. [Crossref] [PubMed]
- Nugent SM, Golden SE, Hooker ER, et al. Longitudinal Health-related Quality of Life among Individuals Considering Treatment for Stage I Non-Small-Cell Lung Cancer. Ann Am Thorac Soc 2020;17:988-97. [Crossref] [PubMed]
- Jeppesen SS, Matzen LE, Brink C, et al. Impact of comprehensive geriatric assessment on quality of life, overall survival, and unplanned admission in patients with non-small cell lung cancer treated with stereotactic body radiotherapy. J Geriatr Oncol 2018;9:575-82. [Crossref] [PubMed]
- Rutkowski J, Szymanik M, Blok M, et al. Prospective evaluation of anxiety, depression and quality of life in medically inoperable early stage non-small cell lung cancer patients treated with stereotactic ablative radiotherapy. Rep Pract Oncol Radiother 2017;22:217-22. [Crossref] [PubMed]
- Mathieu D, Campeau MP, Bahig H, et al. Long-term quality of life in early-stage non-small cell lung cancer patients treated with robotic stereotactic ablative radiation therapy. Pract Radiat Oncol 2015;5:e365-73. [Crossref] [PubMed]
- Alberts L, Wolff HB, Kastelijn EA, et al. Patient-reported Outcomes After the Treatment of Early Stage Non-small-cell Lung Cancer With Stereotactic Body Radiotherapy Compared With Surgery. Clin Lung Cancer 2019;20:370-377.e3. [Crossref] [PubMed]
- Ubels RJ, Mokhles S, Andrinopoulou ER, et al. Quality of life during 5 years after stereotactic radiotherapy in stage I non-small cell lung cancer. Radiat Oncol 2015;10:98. [Crossref] [PubMed]
- van der Voort van Zyp NC, Prévost JB, van der Holt B, et al. Quality of life after stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2010;77:31-7. [Crossref] [PubMed]
- Videtic GM, Reddy CA, Sorenson L. A prospective study of quality of life including fatigue and pulmonary function after stereotactic body radiotherapy for medically inoperable early-stage lung cancer. Support Care Cancer 2013;21:211-8. [Crossref] [PubMed]
- Chen T, Jin J, Chen S. Clinical assessment of computed tomography guided radiofrequency ablation in the treatment of inoperable patients with pulmonary tumors. J Thorac Dis 2017;9:5131-42. [Crossref] [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. [Crossref] [PubMed]
- Palussière J, Chomy F, Savina M, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg 2018;13:91. [Crossref] [PubMed]
- Takeda A, Enomoto T, Sanuki N, et al. Reassessment of declines in pulmonary function ≥1 year after stereotactic body radiotherapy. Chest 2013;143:130-7. [Crossref] [PubMed]
- Stone B, Mangona VS, Johnson MD, et al. Changes in Pulmonary Function Following Image-Guided Stereotactic Lung Radiotherapy: Neither Lower Baseline Nor Post-SBRT Pulmonary Function Are Associated with Worse Overall Survival. J Thorac Oncol 2015;10:1762-9. [Crossref] [PubMed]
- Regnery S, Eichkorn T, Weykamp F, et al. Progression of Pulmonary Function and Correlation with Survival Following Stereotactic Body Radiotherapy of Central and Ultracentral Lung Tumors. Cancers (Basel) 2020;12:2862. [Crossref] [PubMed]
- Guckenberger M, Klement RJ, Kestin LL, et al. Lack of a dose-effect relationship for pulmonary function changes after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;85:1074-81. [Crossref] [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009;4:838-44. [Crossref] [PubMed]
- Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 2011;80:1343-9. [Crossref] [PubMed]
- Navarro-Martin A, Aso S, Cacicedo J, et al. Phase II Trial of SBRT for Stage I NSCLC: Survival, Local Control, and Lung Function at 36 Months. J Thorac Oncol 2016;11:1101-11. [Crossref] [PubMed]
- Ferrero C, Badellino S, Filippi AR, et al. Pulmonary function and quality of life after VMAT-based stereotactic ablative radiotherapy for early stage inoperable NSCLC: a prospective study. Lung Cancer 2015;89:350-6. [Crossref] [PubMed]
- Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early- stage peripheral non-small cell lung cancer: an analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014;88:1092-9. [Crossref] [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol 2018;4:1263-6. [Crossref] [PubMed]
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002;166:675-9. [Crossref] [PubMed]
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645-8. [Crossref] [PubMed]
- Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis 2012;7:95-9. [Crossref] [PubMed]
- Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012;366:2327-9. [Crossref] [PubMed]
- Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with "Ultracentral" Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1081-9. [Crossref] [PubMed]
- Raman S, Yau V, Pineda S, et al. Ultracentral Tumors Treated With Stereotactic Body Radiotherapy: Single-Institution Experience. Clin Lung Cancer 2018;19:e803-10. [Crossref] [PubMed]
- Lindberg K, Bergström P, Brustugun OT, et al. OA24.05 The Nordic HILUS-Trial - First Report of a Phase II Trial of SBRT of Centrally Located Lung Tumors. J Thorac Oncol 2017;12:S340. [Crossref]
- Lindberg K, Grozman V, Karlsson K, et al. The HILUS-Trial-a Prospective Nordic Multicenter Phase 2 Study of Ultracentral Lung Tumors Treated With Stereotactic Body Radiotherapy. J Thorac Oncol 2021;16:1200-10. [Crossref] [PubMed]
- Giuliani M, Mathew AS, Bahig H, et al. SUNSET: Stereotactic Radiation for Ultracentral Non-Small-Cell Lung Cancer-A Safety and Efficacy Trial. Clin Lung Cancer 2018;19:e529-32. [Crossref] [PubMed]
- Woody NM, Stephans KL, Andrews M, et al. A Histologic Basis for the Efficacy of SBRT to the lung. J Thorac Oncol 2017;12:510-9. [Crossref] [PubMed]
- Rodrigues I, Figueiredo T, Gagean J, et al. Prognostic factors and clinical outcomes after stereotactic radiotherapy for primary lung tumors. Rep Pract Oncol Radiother 2020;25:943-50. [Crossref] [PubMed]
- Atallah S, Cho BC, Allibhai Z, et al. Impact of pretreatment tumor growth rate on outcome of early-stage lung cancer treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;89:532-8. [Crossref] [PubMed]
- Lee DS, Kim YS, Yoo IeR, et al. Long-term clinical experience of high-dose ablative lung radiotherapy: high pre-treatment 18Ffluorodeoxyglucose-positron emission tomography maximal standardized uptake value of the primary tumor adversely affects treatment outcome. Lung Cancer 2013;80:172-8. [Crossref] [PubMed]
- Burdick MJ, Stephans KL, Reddy CA, et al. Maximum standardized uptake value from staging FDG-PET/CT does not predict treatment outcome for early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1033-9. [Crossref] [PubMed]
- Allibhai Z, Taremi M, Bezjak A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;87:1064-70. [Crossref] [PubMed]
- Verma V, Simone CB 2nd. Approaches to stereotactic body radiation therapy for large (≥5 centimeter) non-small cell lung cancer. Transl Lung Cancer Res 2019;8:70-7. [Crossref] [PubMed]
- Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer 2017;123:688-96. [Crossref] [PubMed]
- Paul S, Lee PC, Mao J, et al. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ 2016;354:i3570. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [Crossref] [PubMed]
- Ezer N, Veluswamy RR, Mhango G, et al. Outcomes after Stereotactic Body Radiotherapy versus Limited Resection in Older Patients with Early-Stage Lung Cancer. J Thorac Oncol 2015;10:1201-6. [Crossref] [PubMed]
- Surgery with or without Internal Radiation Therapy Compared with Stereotactic Body Radiation Therapy in Treating Patients with High-Risk Stage I Non-Small Cell Lung Cancer 2017. Available online: https://clinicaltrials.gov/show/NCT01336894
- Franks KN, McParland L, Webster J, et al. SABRTooth: a randomised controlled feasibility study of stereotactic ablative radiotherapy (SABR) with surgery in patients with peripheral stage I nonsmall cell lung cancer considered to be at higher risk of complications from surgical resection. Eur Respir J 2020;56:2000118. [Crossref] [PubMed]
- JoLT-Ca Sublobar Resection (SR) Versus Stereotactic Ablative Radiotherapy (SAbR) for Lung Cancer (STABLE-MATES). 2020. Available online: https://clinicaltrials.gov/show/NCT02468024
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [Crossref] [PubMed]
- Ackerson BG, Tong BC, Hong JC, et al. Stereotactic body radiation therapy versus sublobar resection for stage I NSCLC. Lung Cancer 2018;125:185-91. [Crossref] [PubMed]
- Guckenberger M, Kestin LL, Hope AJ, et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? J Thorac Oncol 2012;7:542-51. [Crossref] [PubMed]
- Goodman CD, Nijman SFM, Senan S, et al. A Primer on Interstitial Lung Disease and Thoracic Radiation. J Thorac Oncol 2020;15:902-13. [Crossref] [PubMed]
- Kwak N, Park CM, Lee J, et al. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir Med 2014;108:524-30. [Crossref] [PubMed]
- Girard N, Marchand-Adam S, Naccache JM, et al. Lung cancer in combined pulmonary fibrosis and emphysema: a series of 47 Western patients. J Thorac Oncol 2014;9:1162-70. [Crossref] [PubMed]
- Wong AW, Liang J, Cottin V, et al. Diagnostic Features in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review. Ann Am Thorac Soc 2020;17:1333-6. [Crossref] [PubMed]
- Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015;147:157-64. [Crossref] [PubMed]
- Antoniou KM, Tzilas V, Vasarmidi E, et al. Interstitial lung abnormalities: ignotum per ignotius. Lancet Respir Med 2019;7:376-8. [Crossref] [PubMed]
- Chen H, Senan S, Nossent EJ, et al. Treatment-Related Toxicity in Patients With Early-Stage Non-Small Cell Lung Cancer and Coexisting Interstitial Lung Disease: A Systematic Review. Int J Radiat Oncol Biol Phys 2017;98:622-31. [Crossref] [PubMed]
- Sato T, Watanabe A, Kondo H, et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015;149:64-9, 70.e1-2.
- Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-1611.e3. [Crossref] [PubMed]
- Chi J, Ding M, Shi Y, et al. Comparison study of computed tomography-guided radiofrequency and microwave ablation for pulmonary tumors: A retrospective, case-controlled observational study. Thorac Cancer 2018;9:1241-8. [Crossref] [PubMed]
- de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987-91. [Crossref] [PubMed]
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol 2011;197:W576-80. [Crossref] [PubMed]
- Zheng A, Wang X, Yang X, et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg 2014;98:243-8. [Crossref] [PubMed]
- Bi N, Shedden K, Zheng X, et al. Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Pooled Analysis. Int J Radiat Oncol Biol Phys 2016;95:1378-90. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Baine MJ, Sleightholm R, Neilsen BK, et al. Stereotactic Body Radiation Therapy Versus Nonradiotherapeutic Ablative Procedures (Laser/Cryoablation and Electrocautery) for Early-Stage Non-Small Cell Lung Cancer. J Natl Compr Canc Netw 2019;17:450-8. [Crossref] [PubMed]
- Lam A, Yoshida EJ, Bui K, et al. A National Cancer Database Analysis of Radiofrequency Ablation versus Stereotactic Body Radiotherapy in Early-Stage Non-Small Cell Lung Cancer. J Vasc Interv Radiol 2018;29:1211-1217.e1. [Crossref] [PubMed]
- Ager BJ, Wells SM, Gruhl JD, et al. Stereotactic body radiotherapy versus percutaneous local tumor ablation for early-stage non-small cell lung cancer. Lung Cancer 2019;138:6-12. [Crossref] [PubMed]
- Li M, Xu X, Qin Y, et al. Radiofrequency ablation vs. stereotactic body radiotherapy for stage IA non-small cell lung cancer in nonsurgical patients. J Cancer 2021;12:3057-66. [Crossref] [PubMed]
- Liang L, Li G, Xie S, et al. Choice of Treatment for Stage IA Non-small Cell Lung Cancer Patients Ineligible for Surgery: Ablation or Stereotactic Body Radiotherapy? J Cancer 2020;11:1634-40. [Crossref] [PubMed]
- Uhlig J, Mehta S, Case MD, et al. Effectiveness of Thermal Ablation and Stereotactic Radiotherapy Based on Stage I Lung Cancer Histology. J Vasc Interv Radiol 2021;32:1022-1028.e4. [Crossref] [PubMed]
- Uhlig J, Ludwig JM, Goldberg SB, et al. Survival Rates after Thermal Ablation versus Stereotactic Radiation Therapy for Stage 1 Non-Small Cell Lung Cancer: A National Cancer Database Study. Radiology 2018;289:862-70. [Crossref] [PubMed]
- Kwan SW, Mortell KE, Talenfeld AD, et al. Thermal ablation matches sublobar resection outcomes in older patients with early-stage non-small cell lung cancer. J Vasc Interv Radiol 2014;25:1-9.e1. [Crossref] [PubMed]
- Hu H, Zhai B, Liu R, et al. Microwave Ablation Versus Wedge Resection for Stage I Non-small Cell Lung Cancer Adjacent to the Pericardium: Propensity Score Analyses of Long-term Outcomes. Cardiovasc Intervent Radiol 2021;44:237-46. [Crossref] [PubMed]
- Zeng C, Lu J, Tian Y, et al. Thermal Ablation Versus Wedge Resection for Stage I Non-small Cell Lung Cancer Based on the Eighth Edition of the TNM Classification: A Population Study of the US SEER Database. Front Oncol 2020;10:571684.
- Yao W, Lu M, Fan W, et al. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: a propensity score analysis. Int J Hyperthermia 2018;34:1329-36. [Crossref] [PubMed]
- Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160-6. [Crossref] [PubMed]
- Palussière J, Cazayus M, Cousin S, et al. Is There a Role for Percutaneous Ablation for Early Stage Lung Cancer? What Is the Evidence? Curr Oncol Rep 2021;23:81. [Crossref] [PubMed]
- Lam A, Yoshida EJ, Bui K, et al. Patient and Facility Demographics Related Outcomes in Early-Stage Non-Small Cell Lung Cancer Treated with Radiofrequency Ablation: A National Cancer Database Analysis. J Vasc Interv Radiol 2018;29:1535-1541.e2. [Crossref] [PubMed]
- de Baère T, Palussière J, Aupérin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology 2006;240:587-96. [Crossref] [PubMed]
- Beland MD, Wasser EJ, Mayo-Smith WW, et al. Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology 2010;254:301-7. [Crossref] [PubMed]
- Dupuy DE, Mayo-Smith WW, Abbott GF, et al. Clinical applications of radio-frequency tumor ablation in the thorax. Radiographics 2002;22:S259-69. [Crossref] [PubMed]


