Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature
Introduction
As catamenial pneumothorax (CP) has been be defined spontaneous, recurrent pneumothorax of women in reproductive age, occurring in temporal relationship with menses (1-17).
Catamenial pneumothorax was generally considered a rare entity (2,8-10,13-15). Its incidence in large epidemiological studies was low, not exceeding 3-6% (2,11,15,18,19). It has been considered though, that CP has been underdiagnosed and the incidence of CP has been often underestimated (1-6,11,15).
In the 2003 prospective study by Alifano et al. the incidence of CP among women with recurrent, spontaneous pneumothoraces referred for surgical treatment was 25% [8 in 32 women, after exclusion of patients with pneumothoraces secondary to a lung disease, mainly chronic obstructive pulmonary disease (COPD)]. In subsequent retrospective studies of referral centres, CP represented up to one third of spontaneous pneumothorax cases among ovulating women that were operated to treat pneumothorax (3,4,5,11).
Catamenial pneumothorax involves the right side in the vast majority of cases (3,11,13,15,17). Right sided CP was reported in 91.7% among 210 reviewed cases [2004] (10). It can be left sided (20) or rarely bilateral (21,22).
Despite increased awareness and interest in the disease, the aetiology of CP remains undetermined and obscure. Catamenial pneumothorax has been associated with thoracic endometriosis (1-17,23-25), which is also considered underdiagnosed, with underestimated incidence, and undetermined aetiology (5,23,24,25). Furthermore, endometriosis in general is considered an enigmatic disease, with unclear aetiology and pathogenesis, difficult diagnosis and treatment (26,27).
Endometriosis is defined as the presence of endometrial tissue outside the uterine cavity. The ectopic (extra-uterine) endometrial tissue is histologically characterized by the presence of endometrial stroma and glands (27).
Endometriosis has been attributed to retrograde menstruation and seeding of endometrial cells to the peritoneum. Nevertheless, since up to 90% of women may have retrograde menstruation, an immune deficiency (or increased tolerance of endometrial cells) may prevent clearance of endometrial cells from the peritoneum in women with endometriosis. Other proposed theories are coelomic metaplasia of undifferentiated peritoneal cells, metastatic spread through lymphatic and/or blood vessels, ectopic endometrial generation by endometrial stem/progenitor cells, iatrogenic implantation, and generation by remnants of the Mŭllerian duct (25,27-31).
The most common form is pelvic endometriosis, a chronic disease related with chronic pelvic pain and infertility (27). Diaphragmatic implants of endometriosis were occasionally found during laparoscopic treatment of pelvic endometriosis (46 cases among 3,008). The implants were multiple (69.5%) or single (30.5%), involving the right hemidiaphragm (86.9%), the left (10.8%) or both hemidiaphragms (2.2%) (32).
Thoracic endometriosis, defined as the presence of ectopic endometrial tissue inside the thoracic cavity, is the most frequent extra-pelvic site of endometriosis (33). It is considered a rare disorder occurring to ovulating women. The prevailing opinion is based on data from small case series studies and case reports (24).
According to a 1996 review, the clinical presentation of thoracic endometriosis in 110 patients was: pneumothorax in 73%, hemothorax in 14%, hemoptysis in 7%, and lung nodules in 6% (23). According to a 2010 review of articles published between 2001 and 2007 (most of which included thoracoscopic findings), the clinical presentation of thoracic endometriosis in 110 patients was: pneumothorax in 72%, haemoptysis in 14%, haemothorax in 12%, and lung nodules in 2% (24).
The right hemithorax was more often affected by thoracic endometriosis (85-90%) The mean age at presentation of thoracic endometriosis was 34-35 years (23,24,34). There was significant association between pelvic and thoracic endometriosis, with the thoracic endometriosis occurring about 5 years later (23,34).
Pneumothorax is the most common manifestation of thoracic endometriosis (23,24,34), but macroscopic evidence of thoracic endometriosis and furthermore histologically proven thoracic endometriosis is not revealed in all cases of catamenial pneumothorax (2-6,11,12,15,35).
Characteristic operative findings associated with catamenial pneumothorax include diaphragmatic defects (described as holes, perforations, fenestrations, pores, porosities, stomata), and/or spots or nodules (usually endometrial implants) on the diaphragm, and/or the visceral and/or the parietal pleura. The lesions may have various sizes and numbers, and the nodules may have various colors. Diaphragmatic abnormalities (defects and/or nodules) are more frequently found than visceral and/or parietal pleura nodules. All these findings can coexist, or only one or more of them may be present (1-17,20,35-38).
These characteristic findings may be absent, and bulla(e) or bleb(s) may be the only pathological findings, or there may be complete absence of identifiable thoracic pathology (9-11,15,16).
Endometriosis tissue may or may not be found in the characteristic lesions. The spots and nodules are usually found to be endometrial implants (1-17), but endometrial tissue has been also found at the edges of the diaphragmatic defects, which may represent cyclical breakdown of endometrial implants (2,3,5,9).
In their 2004 review, Korom et al. (10) reported that among 140 patients with surgically treated CP, diaphragmatic lesions (perforations and/or nodules) were found in 38.6%, visceral pleura endometriosis in 29.6%, bulllae and/or blebs and/or scarring in 23.1%, while there was no apparent lesion in 8.5% of patients. Thoracic endometriosis was diagnosed in a total of 52.1% of patients.
In the 2003 prospective study by Alifano et al. (2), histologically proven endometriosis was found in 7 out of 8 cases of catamenial pneumothorax (87.5%). In their 2007 retrospective study, Alifano et al. (3) reported several cases of pneumothoraces that did not occur in temporal synchrony with menses, but were related with characteristic intraoperative findings of catamenial pneumothorax, and with histological findings of thoracic endometriosis. Diaphragmatic abnormalities (holes and/or nodules) were found in 78.5% (22/28) of women with CP, but also in 24.4% (21/86) of women with non CP. Histologically confirmed endometriosis was found in 64.3% of women with CP and in 12.8% of women with non CP (3). They defined pneumothoraces that occur in the intermenstrual period and are associated with histological findings of thoracic endometriosis as non-catamenial, thoracic endometriosis-related pneumothoraces (3). In the 2011 retrospective study of the same centre (5), among all premenopausal women referred for surgical treatment of spontaneous recurrent pneumothorax [2000-2009, n=156 including all cases of the previous 2 studies (2,3)], 31.4% of patients had catamenial and/or thoracic endometriosis-related pneumothorax. Histologically confirmed thoracic endometriosis was found in 64.86% of patients with CP, and in 10% of women with non CP. Pelvic endometriosis was detected in 51% of women with catamenial and/or thoracic endometriosis-related pneumothorax (5). Lower incidence of histologically proven thoracic endometriosis in cases of CP has been reported in other retrospective studies (9,15).
Methods
We retrospectively reviewed the electronic and paper medical files of all women in reproductive age that were operated for recurrent spontaneous pneumothorax between June 2006 and June 2012. Criteria for patient inclusion in this retrospective study were: operation for recurrent spontaneous pneumothorax in the absence of an underlying lung disease, and presence of at least one of the following: definitive or “probable” preoperative and/or postoperative diagnosis of catamenial and/or endometriosis-related pneumothorax, and/or presence of characteristic intraoperative findings. Yearly follow-up records were reviewed and telephone follow-up was contacted.
This search revealed five women, with a mean age of 34+/-9.9 years (median 38 years, range, 19-45 years) referred for surgical treatment of spontaneous recurrent pneumothorax. All pneumothoraces were right sided (all episodes in all patients). The mean number of total episodes of pneumothorax before surgery was 4.4+/-2.5 (median 3, range, 2-8). There was history of pneumothorax occurrence during menstruation in 2 patients, while 2 patients were not certain and one patient reported no synchronicity, although she was not certain about occurrence of pneumothorax in the immediate premenstrual period. Thus, a strictly catamenial character was documented in 40% of patients (2/5). One patient reported menstrual thoracic pain, and all patients denied pelvic pain. There was history of unilateral salpingo-oophorectomy and hormonal treatment for pelvic endometriosis in one patient. All other patients denied gynaecological interventions, including scrapping and laparoscopy. Zero gravity and parity was noted in all patients, but detailed history regarding infertility is not available (Table 1). No patient had symptoms or diagnosis of COPD or other underlying lung disease. On history and routine clinical and laboratory preoperative evaluation there was no evidence of comorbidity.
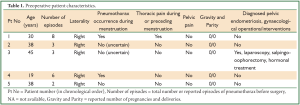
Full table
The symptoms during episodes of pneumothorax included mild to moderate dyspnea and thoracic pain. Chest radiography, and when performed, computed tomography (CT) revealed right-sided pneumothorax in all patients. (Figures 1,2,3,4,5). There was a very rare additional finding on chest x-ray and CT of the 5th patient: multiple “nodules” on the dome of the right hemidiaphragm (Figures 4,5).
 Figure 1. Images of recurrent spontaneous catamenial pneumothoraces with progressively increasing severity in the 1 patient. A. Thoracic CT during the 6th episode (9 months preoperatively): small, apical, right-sided pneumothorax; B. Thoracic CT (supine position) during the 7th episode, 2 months preoperatively: medium-sized, right-sided pneumothorax; C. Posteroanterior chest radiogram (erect position) during the 8th episode, 2 days preoperatively: large, right-sided pneumothorax without mediastinal shift. Basal air-fluid level. (Visceral pleural line - red arrows, air-fluid level - blue arrow).
Figure 1. Images of recurrent spontaneous catamenial pneumothoraces with progressively increasing severity in the 1 patient. A. Thoracic CT during the 6th episode (9 months preoperatively): small, apical, right-sided pneumothorax; B. Thoracic CT (supine position) during the 7th episode, 2 months preoperatively: medium-sized, right-sided pneumothorax; C. Posteroanterior chest radiogram (erect position) during the 8th episode, 2 days preoperatively: large, right-sided pneumothorax without mediastinal shift. Basal air-fluid level. (Visceral pleural line - red arrows, air-fluid level - blue arrow).
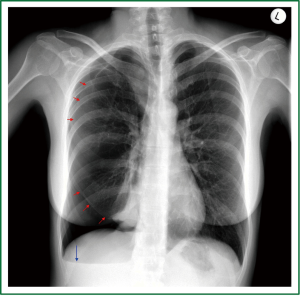 Figure 2. Preoperative posteroanterior chest radiogram (erect position) of the 2nd patient: Medium-sized, apical and subpulmonary, right-sided pneumothorax without mediastinal shift. Basal air-fluid level. (Visceral pleural line - red arrows, air-fluid level - blue arrow).
Figure 2. Preoperative posteroanterior chest radiogram (erect position) of the 2nd patient: Medium-sized, apical and subpulmonary, right-sided pneumothorax without mediastinal shift. Basal air-fluid level. (Visceral pleural line - red arrows, air-fluid level - blue arrow).
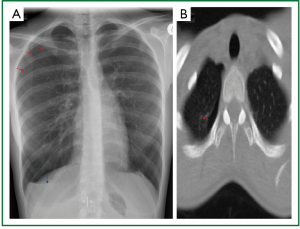 Figure 3. Preoperative imaging of the 4 patient. A. Small apical pneumothorax, basal air-fluid level on erect, posteroanterior, chest radiogram; B. Small apical pneumothorax on transverse plane of (supine) thoracic CT. (Visceral pleural line - red arrows, air-fluid level - blue arrow).
Figure 3. Preoperative imaging of the 4 patient. A. Small apical pneumothorax, basal air-fluid level on erect, posteroanterior, chest radiogram; B. Small apical pneumothorax on transverse plane of (supine) thoracic CT. (Visceral pleural line - red arrows, air-fluid level - blue arrow).
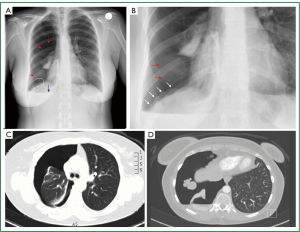 Figure 4. Very rare findings on thoracic imaging of the 5 patient. A. Posteroanterior (erect) chest radiogram: Large, right-sided pneumothorax (red arrows), without mediastinal shift. Basal air-fluid level (blue arrow). “Multinodular appearance” of the contour of the right hemidiaphragm (within oval shaped white line); B. Enlarged image of the right hemidiaphragm: multiple “nodules” extending from the diaphragmatic dome to the costophrenic recess. C. Transverse plane of thoracic contrast CT: large, right-sided pneumothorax; D. Transverse plane of thoracic contrast (supine) CT: large right-sided pneumothorax (posterior air-fluid level, small quantity of pleural fluid), five “nodules” of various sizes and circular contour at the central tendon of the right hemidiaphragm.
Figure 4. Very rare findings on thoracic imaging of the 5 patient. A. Posteroanterior (erect) chest radiogram: Large, right-sided pneumothorax (red arrows), without mediastinal shift. Basal air-fluid level (blue arrow). “Multinodular appearance” of the contour of the right hemidiaphragm (within oval shaped white line); B. Enlarged image of the right hemidiaphragm: multiple “nodules” extending from the diaphragmatic dome to the costophrenic recess. C. Transverse plane of thoracic contrast CT: large, right-sided pneumothorax; D. Transverse plane of thoracic contrast (supine) CT: large right-sided pneumothorax (posterior air-fluid level, small quantity of pleural fluid), five “nodules” of various sizes and circular contour at the central tendon of the right hemidiaphragm.
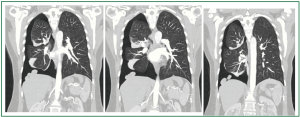 Figure 5. Coronal (frontal) planes of thoracic, contrast CT: large, right-sided pneumothorax, multiple “nodules” on the dome and the lateral surface of the right hemidiaphragm.
Figure 5. Coronal (frontal) planes of thoracic, contrast CT: large, right-sided pneumothorax, multiple “nodules” on the dome and the lateral surface of the right hemidiaphragm.
All patients underwent 3-port video-assisted thoracoscopic surgery (VATS), except for the 5th patient who underwent a video-assisted mini-thoracotomy. Findings characteristic of catamenial and/or endometriosis-related pneumothorax were revealed intraoperatively in 3 out of 5 patients (60%). The first patient had a tiny diaphragmatic hole of less than 2mm at the tendinous centre of the right hemidiaphragm. The 2nd patient had multiple circular or elliptical holes of a few millimetres up to more than 1 cm, adjacent to red spots of the same or smaller size, located at the periphery of the right leaflet of the central tendon (Figure 6). The 5th patient had multiple confluent diaphragmatic defects on the central tendon, resulting in a huge central defect, partitioned by several thin and long connective tissue bridges containing blood vessels. There was intrathoracic liver herniation through this huge multipartitioned defect. At the periphery of the central defect there were smaller defects, an elongated with a maximal length of about 2 cm and a smaller circular hole (Figure 7).
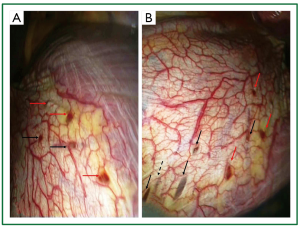 Figure 6. Characteristic diaphragmatic lesions on intraoperative photographs of the 2 patient. A and B. Multiple diaphragmatic red spots (red arrows) and holes (black arrows) at the periphery of the right leaflet of the central tendon, most of them less than 1cm in their maximal dimesion; B. the liver can be seen underneath the perforations (black arrows). The black, dashed arrow shows a spot of thin transparent diaphragmatic central tendon (as if a partial-thickness implant has undergone apoptosis, without resultant perforation).
Figure 6. Characteristic diaphragmatic lesions on intraoperative photographs of the 2 patient. A and B. Multiple diaphragmatic red spots (red arrows) and holes (black arrows) at the periphery of the right leaflet of the central tendon, most of them less than 1cm in their maximal dimesion; B. the liver can be seen underneath the perforations (black arrows). The black, dashed arrow shows a spot of thin transparent diaphragmatic central tendon (as if a partial-thickness implant has undergone apoptosis, without resultant perforation).
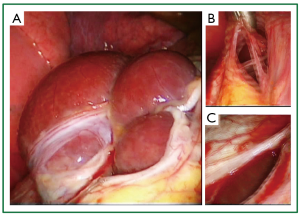 Figure 7. Very rare intraoperative findings on intraoperative photographs of the 5th patient. A. Liver protrusion through a large, central, multipartitioned defect of the tendinous part of the left hemidiaphragm, consisted of multiple confluent diaphragmatic fenestrations of various sizes; B. Thin, elongated, connective tissue bridges between the confluent diaphragmatic defects; C. Smaller, oval shaped perforation at the periphery of the large multipartitioned central defect. Although very rare, these intraoperative findings share common characteristics with at least 2 individually reported cases of catamenial pneumothorax, and furthermore they bare characteristic similarity with a third individually reported case of catamenial pneumothorax. Thus, in our opinion, despite the rareness, these findings can be considered characteristic of catamenial and/or endometriosis related pneumothorax.
Figure 7. Very rare intraoperative findings on intraoperative photographs of the 5th patient. A. Liver protrusion through a large, central, multipartitioned defect of the tendinous part of the left hemidiaphragm, consisted of multiple confluent diaphragmatic fenestrations of various sizes; B. Thin, elongated, connective tissue bridges between the confluent diaphragmatic defects; C. Smaller, oval shaped perforation at the periphery of the large multipartitioned central defect. Although very rare, these intraoperative findings share common characteristics with at least 2 individually reported cases of catamenial pneumothorax, and furthermore they bare characteristic similarity with a third individually reported case of catamenial pneumothorax. Thus, in our opinion, despite the rareness, these findings can be considered characteristic of catamenial and/or endometriosis related pneumothorax.
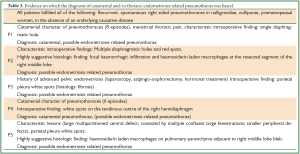
The catamenial character of spontaneous pneumothorax was documented in only 1 out of the 3 patients with characteristic findings. Diaphragmatic resection was not performed and diaphragmatic tissue was not examined histologically in any of these patients (Tables 1,2,3)

Full table
Full table
No characteristic lesions (apart from white spots consisted of fibrous tissue on the parietal pleura or the diaphragm) were found in the other 2 patients, and the diagnosis was made on clinical grounds: catamenial character (4th patient), and history of advanced pelvic endometriosis (3rd patient) (Tables 1,2,3).
All patients underwent apical and/or atypical right middle lobe wedge resection(s) of lung parenchyma containing blebs or having abnormal appearance. Abrasion and talc pleurodesis was performed in 4 patients, and pleurectomy in one. Partial diaphragmatic plication and suturing of isolated holes was performed in the 2nd patient, and diaphragmatic plication plus bovine pericardial patch diaphragmatic coverage was applied in the 5th patient (Table 2).
Histology of the resected lung parenchyma showed the presence of hemosiderin-laden macrophages in 2 patients with characteristic diaphragmatic lesions (2nd and 5th). Fibrosis and rare chronic inflammation cells were found on histology of white parietal pleural or diaphragmatic spots. Lung or pleural endometriosis was not revealed. No tissue samples were taken from the perforated diaphragms (Tables 2,3).
Results
All patients had a satisfactory recovery. Apart from a persistent air leak in the 3rd patient associated with small subpulmonary loculated pneumothorax, there was no postoperative morbidity. The patients were discharged home at a mean of 7+/-4 days (median 6 days, range, 4-14 days). Two patients received gonadotropin releasing hormone (GnRH) analogue (triptorelin) for 6 months postoperatively. At a mean follow-up of 34+/-12.98 months (median 39 months, range, 16-46 months) there was recurrence in one patient. The recurrence was noted in the first patient in whom a tiny diaphragmatic hole (<2 mm) was left untreated, in the belief that pleurodesis would suffice. Furthermore, the patient did not receive hormonal treatment in the immediate postoperative period. She had recurrence of right sided pneumothorax 6.5 months postoperatively that was successfully treated with triptorelin (Table 2).
Discussion
Terms and definitions
The spectrum of recurrent spontaneous pneumothoraces occurring in women of reproductive age, in the absence of an underlying aetiologic lung disease, includes catamenial pneumothorax (occurring in temporal relationship with menses, with or without characteristic findings, with or without histologic evidence of endometriosis) (1-17), but also non catamenial, thoracic endometriosis-related pneumothoraces (intermestrual with macroscopic and histologic evidence of thoracic endometriosis) (3-6).
Although not highlighted in the literature, in pneumothoraces classified as catamenial there was absence of a known underlying lung disease. Pneumothorax cases considered secondary to a known lung disease have been excluded from the prospective and the retrospective studies by Alifano et al. (2,3). The relationship between catamenial pneumothorax and pneumothoraces considered secondary to a significant lung disease (including COPD, acute severe asthma, emphysema, bullus disease, cystic fibrosis, interstitial lung disease, or connective tissue disease) has not been investigated.
The term recurrent does not define the exact number of episodes. Although the mean number of episodes is usually higher (5 recurrences in the 2004 review), at least 2 episodes in total are required (3).
The exact temporal relationship with menses has not been uniformly defined. Pneumothorax “associated” (10), “correlated” (15), or “timed with” (11) menses, “in synchrony with the menstrual cycle” (14), “during (or preceding) menstruation” (8,16), and “close to menstrual periods” (12) are some of the usual expressions.
Alifano et al. defined as CP, spontaneous recurrent pneumothorax occurring within 72 hours after the onset of menstruation [2003] (2) or within 24 hours before and 72 hours after the onset of menstruation [2007, 2011] (3,6). Peikert et al. (12) and Leong et al. (13) defined as CP, pneumothorax occurring within 72 hours before or after the onset of menstruation.
Alifano et al. [2007] (3) and Rousset Jablonski et al. [2011] (5) (same referral centre) defined thoracic endometriosis as histologically “proven” when both endometrial stroma and glands were found at histology of the lesions, and as “probable” when only stroma was found.
Signs of pulmonary parenchymal focal hemorrhages and/or haemosiderin containing macrophages, suggesting previous nearby bleeding, have been found around the endometrial tissue of lung nodules (22). Haemosiderin containing macrophages have been found at pericardial “implants” (35) and lung “nodules” without histologic evidence of endometriosis (2,3).
Clinical manifestations- diagnosis
The typical clinical presentation of catamenial pneumothorax involves spontaneous pneumothorax during or preceding menses, usually manifested with pain, dyspnoea and cough. Thoracic pain preceding or during menstruation, history of previous spontaneous pneumothorax(ces) with or without history of previous intervention(s), symptoms of pelvic endometriosis (dysmenorrhea, dyspareunia), history of primary or secondary infertility, with or without diagnosis of previous pelvic endometriosis, history of previous gynaecologic procedure (2-7,9), and rarely history of catamenial haemoptysis (5,9) may be present.
Rousset-Jablonski C et al. (5) reported that menstrual scapular and/or thoracic pain preceded the occurrence of pneumothorax in more than 25% of 49 retrospectively studied patients with CP and/or endometriosis related pneumothorax, being highly specific for the diagnosis of thoracic endometriosis. Infertility and a history of a uterine surgical procedure and/or uterine scraping were strongly associated with catamenial and/or thoracic endometriosis-related pneumothorax.
The previously described symptoms and medical history should be systematically evaluated for earlier diagnosis (5). While their presence should increase suspicion for catamenial and/or endometriosis related pneumothorax, their absence should not preclude its diagnosis. Presentation in the intermenstrual period should not preclude the diagnosis of endometriosis related pneumothorax (3,39), even in the absence of diagnosis and/or absence of symptoms of pelvic endometriosis. “Catamenial” pulmonary endometriosis-related pneumothorax has been reported in a pregnant woman (8 weeks) (40).
Catamenial and/or endometriosis related pneumothoraces are usually mild to moderate, but they can rarely be life-threatening (as reported in a case with widespread thoracic endometriosis, adhesions, and ruptured bulla after previous surgeries) (41).
There is a case report of catamenial pneumoperitoneum mimicking acute abdomen in a woman with multiple recurrences of catamenial, thoracic endometriosis-related pneumothorax (42). Spontaneous diaphragmatic rupture with right pneumothorax and pneumoperitoneum has been reported in a patient with findings of diaphragmatic endometriosis (43).
The usually reported mean age at operation of patients with CP and/or thoracic endometriosis-related pneumothorax is 34-37 years (3,5,9,10).
There are generally no specific diagnostic imaging criteria. Rare findings included pneumoperitoneum on chest x-ray and/or computed tomography (CT) co-existing with right pneumothorax (44,45), small diaphragmatic defects (“air bubbles”) on chest X-ray corresponding to small diaphragmatic perforations (36), a diaphragmatic opacity on chest X-ray corresponding to a large diaphragmatic laceration with intrathoracic liver protrusion (38), nodular appearance of the diaphragm on chest X-ray and CT corresponding to partial intrathoracic liver herniation (Pryshchepau et al. 2010) (46), a diaphragmatic nodule on CT proven a relatively large diaphragmatic endometrial implant (15), and pleural based masses on magnetic resonance imaging (MRI) attributed to endometrial implants (47).
Our own findings on preoperative chest X-ray and CT of the 5th patient (Figures 4,5) multinodular contour of the right hemidiaphragm due to partial intrathoracic liver herniation through a multipartitioned central tendinous defect) bare characteristic resemblance with the above mentioned findings of Pryshchepau et al. [2010] (46).
Although lacking specificity (34), increased levels of cancer antigen 125 (CA 125) have been considered a helpful adjunct to early diagnosis of endometriosis-related pneumothorax (33).
While medical history of recurrent pneumothoraces in synchronicity with menses is adequate for the diagnosis of catamenial pneumothorax, the definitive diagnosis of thoracic endometriosis-related pneumothorax is made with surgical treatment based on visual inspection and subsequent histological examination of the characteristic lesions.
Characteristic lesions (intraoperative findings)
Characteristic lesions of the disease spectrum under the term catamenial and/or thoracic endometriosis-related pneumothorax include diaphragmatic perforations, and/or diaphragmatic nodules, and/or visceral pleural nodules, and/or parietal pleural nodules. These characteristic lesions are not found in all cases of catamenial pneumothorax (occurring during or close to menstruation) (9,11,15,16). Endometriosis tissue may or may not be found in the characteristic lesions (2-6,8,9). Characteristic lesions with or without histologic evidence of thoracic endometriosis have been found in cases of non-catamenial (intermenstrual) pneumothoraces (3).
The spots or nodules can be single or multiple, described as red, purple, violet, blueberry, blue, brown(1-17), but also black, white (48), greyish, and greyish-purple (43). They are found mainly on the diaphragm (usually at the central tendon and rarely on the muscular portion), but also at the visceral, and/or the parietal pleura. Histology usually reveals endometriosis (1-17,20,35-37).
We found white spots on the diaphragm or the parietal pleura in 3 out of 5 patients. Histology showed fibrosis without endometrial tissue. White spots may represent “healed” endometriosis implants with fibrous tissue generation after sloughing.
The diaphragmatic defect(s) can be single or multiple, usually located at the central tendon of the diaphragm, often adjacent to co-existing implants. They can be tiny holes (20) measuring 1-3 milimeters (10), or larger measuring up to 10 mm (2,8), and rarely more than 10 mm (13). Larger fenestrations (4-10 cm, or complete diaphragmatic ruptured) have been reported in rare cases (38,43,46,49).
Bobbio et al. [2007] (38) revealed a 4 cm laceration at the tendinous part of the right hemidiaphragm with partial intrathoracic liver herniation in a patient with recurrent catamenial pneumothraces (at least 2 consequent episodes during menstruation). Although they did not perform biopsy of the defect edges, they assumed that the catamenial pneumothorax was endometriosis-related, based on clinical history (dysmenorrhea, pelvic adhesions, intestinal obstruction) and increased CA 125 levels.
Makhija et al. [2009] (49) reported a case of right catamenial pneumothorax with multiple large diaphragmatic fenestrations, the larger of which had a maximum dimension of 4 inches (10.16 cm).
Pryshchepau et al. [2010] (46) reported a case of right pneumothorax and “tumoral” or “nodular aspect” of the right hemidiaphragm on chest X-ray and CT. At thoracoscopy the nodular appearance of the right hemidiaphragm was proved to be liver protrusion through a huge partitioned diaphragmatic defect. No information was given about the temporal relationship with menses or about histological findings; nevertheless, the authors stated that “catamenial” pneumothorax was discovered at thoracoscopy.
Triponez et al. [2010] (43) reported spontaneous right diaphragmatic rupture with intrathoracic herniation of the liver, the gall bladder and the colon, along with right pneumothorax and pneumoperitoneum in a patient without known history of endometriosis, but with history of premenstrual periscapular pain. A nodule characteristic of endometrial implants was found at the edge of the huge diaphragmatic defect. Histology did not reveal endometrial stroma or glands but showed “hemosiderin-loaded macrophages compatible with endometriosis”. The authors considered this case as endometriosis-related, although it did not fulfil the histologic criteria set by their team in other studies [proven endometriosis: glands and stroma, probable endometriosis: stroma only (3,5)]. Triponez et al. (43) referring to the cases reported by Bobbio (38) and Pryshchepau (46) (described above), considered them as limited diaphragmatic rupture (with partial liver herniation) “probably caused by endometriosis”.
Despite the definitions, it seems that in the literature pneumothoracies are diagnosed as “catamenial” without strict relationship with menses [even when occurring during pregnancy (40)], and as “endometriosis-related” without histologic evidence of endometrial stroma or glands (38,43,46).
The terms catamenial pneumothorax and/or endometriosis related pneumothorax have been “liberally” used to describe the spectrum of spontaneous recurrent pneumothoraces of premenopausal women without an underlying causative disease, in the presence of characteristic intraoperative findings, even without proven temporal relationship with menses and in the absence of histologic evidence of endometrial stroma or glands. The main criterion for the diagnosis is the presence of characteristic lesions, which is usually supported by the presence of clinical history and/or presentation implying a relationship with endometriosis. In our opinion this is justified, since the characteristic lesions have not been described associated with any other pathologic entity, syndrome or disease. Furthermore it is clinically meaningful since the risk of underdiagnosis exceeds the risk of overdiagnosis.
Our own intraoperative findings in the 5th patient (Figure 7) share common characteristics with the intraoperative findings of Bobbio et al. [2007] (38) (that to the best of the authors’ knowledge were the first in the literature), and the findings of Makhija et al. [2009] (49). Furthermore our intraoperative findings (Figure 7A), as well as the findings on preoperative chest x-ray and CT (Figure 4A,B, Figure5) are practically similar to the relevant findings by Pryshchepau et al. [2010] (46). Thus, we can probably assume that these findings, although rarely reported, can be considered as characteristic findings of catamenial and/or endometriosis related pneumothorax.
Histology of the resected lung parenchyma in 2 of our patients with characteristic diaphragmatic lesions (2nd, 5th) showed haemosiderin-laden macrophages, which is considered compatible and even suggestive of endometriosis (2,3,22,35,43).
Unrecognized cases
In a retrospective study [Alifano et al. 2011 (6)] of all consecutive premenopausal women that underwent repeat surgery for recurrent spontaneous pneumothorax, among 35 reoperated women, evidence of thoracic endometriosis was found at re-operation in 5 cases that were initially considered as idiopathic and in 8 cases that were initially considered as catamenial but not endometriosis-related. Thus, in 13 patients evidence of endometriosis was discovered only at reoperation. Three cases initially diagnosed as endometriosis-related non-catamenial were diagnosed as endometriosis-related catamenial. Thus the catamenial character was not present or was missed before the first operation.
Korom et al. (10) revealed characteristic diaphragmatic lesions and endometrial implants at video-assisted thoracoscopic surgery (VATS) in 3 patients with recurrent pneumothoraces after various unsuccessful thoracoscopic procedures, during which lesions had not been identified.
Bobbio et al. [2007] (38) revealed in a patient with multiple recurrent spontaneous pneumothoraces a large diaphragmatic defect (4 cm), that had not been reported after previous unsuccessful video-assisted thoracoscopic (Argon beam) apical pleurodesis. The last 2 episodes of pneumothorax occurred during menses, while the previous temporal relationship with menses had not been determined, despite the presence of dysmenorrhea and recurrent pelvic pain plus history of two laparotomies (after an appendectomy) that showed pelvic adhesions and did not require intestinal resection.
It is reasonable to assume that the eventually discovered pathology existed at the time of the first intervention but remained elusive, was not treated adequately, and caused recurrent pneumothorax. It appears that both the temporal relationship with menses and the characteristic intraoperative findings can be easily missed (6,10,38).
Absence of characteristic lesions simply means that at inspection during thoracotomy or thoracoscopy no macroscopic evidence was found. This depends on the awareness of the disease, on meticulous inspection of the thoracic cavity including the diaphragm (surgeon related), but also on the morphology, the size, and the number of the characteristic lesions, thus on the stage of the disease, the catamenial variations (during the phases of each menstrual cycle), and the longer-term variations in the time course of the disease, since exaggerations or remissions may occur (disease related).
Tiny diaphragmatic holes or small red or brown spots can be very easily missed or misinterpreted. Slasky et al. (50) proved the presence of occult diaphragmatic defects in a patient with catamenial pneumothorax only after diagnostic pneumoperitoneum. For the fear of leaving untreated occult diaphragmatic defects, Bagan et al. (9) suggested systematic coverage of the diaphragm with a mesh (including diaphragms with normal appearance). The histological confirmation of endometriosis depends on appropriate tissue sampling and appropriate histologic examinations.
Reoperations in patients with catamenial and/or endometriosis related pneumothorax can be attributed not only to underdiagnosis but also to the persistence of the disease, that particularly if left untreated or incompletely treated relapses and progresses in severity.
Morcos et al. (41) reported successful surgical treatment of a life-threatening recurrent catamenial pneumothorax due to widespread thoracic endometriosis in a patient that had previously undergone 2 thoracotomies, the second of which included partial diaphragmatic resection due to the presence of endometriosis implants and perforations. Endometriosis-related pneumothorax has been revealed even (6 years) after hysterectomy performed for pelvic endometriosis (1,22).
Aetiopathogenetic theories of catamenial pneumothorax
Although various theories or proposed mechanisms have been suggested, the aetiopathology of catamenial and/or endometriosis related pneumothorax remains obscure.
The physiologic hypothesis: high levels of circulating prostaglandin F2 during menstruation may cause vasoconstriction and bronchospasm, with subsequent alveolar rupture, and pneumothorax. Blebs or bullae may also be more susceptible to rupture during hormonal changes(2,9,10,13,21,25,35).
The migration theory: endometrial diaphragmatic implants may result from migration of endometrial tissue from uterus to pelvis, and through the peritoneal flow to the subdiaphragmatic area (preferentially the right, through the right paracolic gutter, due to clockwise peritoneal circulation, and the “piston” action of the liver). Cyclical necrosis of diaphragmatic endometrial implants can produce diaphragmatic defect(s). Endometrial tissue has been found on the edges of such defects in many cases of CP (9). Endometrial cells can pass through the created diaphragmatic defect(s) and spread into the thoracic cavity, including the visceral pleura. Cyclical necrosis, or sloughing and apoptosis of the visceral pleura endometrial implants may result in rupture of the underlying alveolus and pneumothorax (2,9,10,13,21,23,25).
The metastatic or lymphovascular microembolization theory: metastatic spread (or microembolization) of endometrial cells to the lungs through the venous system or the transdiaphragmatic lymphatics, and cyclical necrosis of pulmonary parenchymal foci close to the pleura may cause air leaks. Centrally located lesions may result in haemoptysis (2,7,9,10,13,23,25).
The transgenital-transdiaphragmatic passage of air theory: passage of air through congenital or acquired (secondary to endometriosis) diaphragmatic defects has also been proposed. This involves entrance of atmospheric air from the vagina, into the cervix (due to absence of cervical mucous during menstruation), passage to the uterus, the fallopian tubes, the peritoneal cavity, and finally the pleural space through the diaphragmatic defects (2,9,10,13,21,25,35). Catamenial pneumothorax with diaphragmatic defects but without signs of diaphragmatic or thoracic endometriosis has been related to porous diaphragmatic syndrome (2,51-54).
In the presence of diaphragmatic defects, pneumoperitoneum can result in pneumothorax. There are very rare case reports of “spontaneous” or “idiopathic” or “non surgical” pneumoperitoneum in women, postpartum, after knee to chest postpartum exercise, postcoitus (even in hysterectomized women, attributed to the presence of a fistulous track in one case), after oral-genital insufflation, vaginal douching or pelvic examination (25,55-57). These rare cases may support the transgenital-transdiaphragmatic passage of air theory and/or the pore theory.
A very rare radiologic finding of 2 adjacent diaphragmatic small “bubbles” associated with homolateral catamenial pneumothorax has offered support to the transgenital-transdiaphragmatic passage of air theory (36). This theory has also been supported by rare cases of concurrent pneumoperitoneum and catamenial pneumothoraces. Downey et al. (44) reporting such a case noted that the abdominal pressure exceeds the thoracic pressure by 20-30 cmH20, and thus, considered that pneumoperitoneum preceded the pneumothorax. Jablonski et al. (45) reported 3 cases of coexistence of catamenial pneumothorax and pneumoperitoneum, suggesting transdiaphragmatic passage of air through perforations secondary to endometrial implants. Pneumoperitoneum was considered an intermediate step of passage of air from the genitalia to the thorax. Grunewald and Wiggins (42) reported a case that, having a history of multiple recurrent catamenial right pneumothoraces, presented with symptoms of acute abdomen and pneumoperitoneum (small amount of air under the right hemidiaphragm) during menses, without evidence of pneumothorax. The patient underwent laparotomy, which did not reveal hollow viscus perforation or pelvic endometriosis (the diaphragm was not inspected). During the next menstrual period the patient had a recurrent right pneumothorax. Open lung biopsy showed endometriosis (without histologic confirmation). Menstruation suppression prevented further episodes. The catamenial pneumoperitoneum without pneumothorax supports the transgenital-transdiaphragmatic passage of air theory, associated with endometriosis.
Absence of recurrence of catamenial pneumothorax after tubal ligation (50) has also been used to support the transgenital-transdiaphragmatic passage of air theory (57). Nevertheless, it can also support the migration theory, since ligation of the fallopian tubes not only prevents entrance of atmospheric air to the peritoneal cavity, but also stops endometrial retrograde seeding and thus, diaphragmatic and thoracic spread.
“Cure” but also recurrence of catamenial pneumothorax has been reported after hysterectomy (1,22,57,58). Absence of recurrence after hysterectomy can support 3 pathogenetic theories: the transgenital-transdiaphragmatic passage of air, the migration, and the microembolization theories.
Thus, absence of recurrence of pneumothorax after ligation of the fallopian tubes or after hysterectomy does not prove the transgenital- transdiaphragmatic passage of air theory. On the contrary, recurrences of pneumothorax after hysterectomy and after fallopian tube ligation, provide strong evidence that the transgenital-transdiaphragmatic passage of air theory cannot explain all the cases of catamenial pneumothorax (1,10,22,57,58).
Alifano et al. (6) reported recurrent pneumothorax after surgery that included diaphragmatic resection, and in some cases total adhesion of the lung basis to the diaphragm. They commented that the pathogenetic mechanism was not the “classically” described transgenital-transdiaphragmatic passage of air, suggesting alveolar rupture due to microscopic visceral pleura endometriosis implants (migration theory), or vasospasm and bronchospasm due to circulating prostaglandins (physiologic theory).
Andrade-Alegre and González (59) reported a case with co-existence of diaphragmatic adjacent red endometrial implants, brown sloughed endometrial implants, and perforations. In our opinion this case illustrates a part of the pathologic process of the migration theory: diaphragmatic endometrial tissue implantation, shoughing, and perforation after apoptosis of a full thickness implant, which can easily happen at the thin tendinous centre of the diaphragm.
Suzuki et al. (20) reported disrupted elastic fibers of the visceral pleura close to endometrial tissue, assuming cyclic erosion resulting in pulmonary air leak. Yoshioka et al. (40) found endometrial stroma on the wall of pulmonary cystic lesions associated with pneumothorax (during pregnancy). Morcos et al. (41) found endometrial tissue on the wall of a ruptured bulla responsible for a life-threatening pneumothorax.
According to Joseph et al. the migration and the microembolization theories provide the most plausible explanation (23). Korom et al. (10) hypothesized that diaphragmatic and consequent subpleural erosions by endometrial implants represent the basic mechanism of CP (migration theory). Funatsu (57) advocated the theory of pulmonary air leaks caused by breakdown of endometrial visceral pleural implants that entered the thorax through the diaphragmatic defects (migration theory).
In our opinion most cases can be explained by the migration theory. Thoracic endometriosis in the presence of an “intact” diaphragm can be explained not only by other pathways of spread, but also by passage through a diaphragmatic perforation that remains elusive (undiagnosed), or by the earlier presence of a perforation that after allowing passage of endometriosis tissue to the thorax was healed (closed by connective tissue, leaving a small white scar).
Surgical treatment
Joseph et al. (23) in their 1996 review on thoracic endometriosis concluded that surgical treatment had better results than hormonal treatment, mainly due to less long term recurrences.
In 2004, Korom et al. (10) reviewing 229 cases of CP published in the literature, reported that among 195 patients (for whom adequate information was given), 154 patients (78.9%) were treated surgically. In 81.7% of the cases pleurodesis was performed, with or without diaphragmatic repair or lung wedged resection. Diaphragmatic repair was performed in 38.8% of patients and lung wedged resection in 20.1%. Partial diaphragmatic resection and/or resection of visceral pleural implants, and pleurodesis, are nowadays frequently performed (4).
Video-assisted thoracoscopic surgery (VATS) has been considered the treatment of choice and has been mainly applied since 2000. A video-assisted mini-thoracotomy or a muscle sparing thoracotomy may be preferable when extensive diaphragmatic repair is required. A thoracotomy may be occasionally necessary, particularly at reoperations (2-7,9-11,13-17,20).
Careful and meticulous inspection of the diaphragm, the lung, the parietal pleura, and the pericardium for characteristic lesions is of paramount importance. Bullae, blebs, air leaks, should be sought and treated. Magnification provided by VATS may facilitate recognition and identification of the lesions (2-6,9-11,13-17). Video-recording can prove valuable (48). Tissue sampling for histologic confirmation facilitates the diagnosis of thoracic endometriosis (4).
Marshall et al. (11) suggested that all pathological findings should be specifically addressed at surgery, and performed diaphragmatic resection, excision of blebs, and pleurodesis or pleurectomy. Ciriaco et al. (15) performed pleurodesis along with apical resection and apical pleurectomy, and, in the presence of defects, diaphragmatic plication. Korom et al. (10) applied plication of the involved diaphragmatic area (with or without resection), pleurodesis, and bulla excision or apical wedged resection when required. Attaran et al. (17) performed VATS pleurectomy/abrasion and diaphragmatic repair, with a PTFE mesh coverage in the presence of diaphragmatic defects. Bagan et al. (9) suggested surgery during menstruation for better visualization of the endometriosis lesions, pleurodesis and systematic diaphragmatic coverage by polyglactin mesh, (even of diaphragms with normal appearance) to avoid leaving untreated small occult defects, to reinforce the diaphragm, and induce adhesion to the lung, aiming to avoid recurrences. Leong et al. (13) also suggested mechanical pleurodesis and diaphragmatic coverage with an artificial (polyglactin or polypropylene) mesh.
Alifano et al. (2) suggested resection of all visible lesions (when technically feasible, for optimal treatment and prevention of further intrathoracic spread), including partial diaphragmatic resection, resection of endometriosis pulmonary lesions, bleb(s) or bulla(e), with limited wedged resection of the diseased area when indicated, (with endostapler if feasible), and limited parietal pleurectomy to remove pleural lesions. After treating and studying 37 cases of CP and/or endometriosis-related pneumothoraces that recurred after initial surgery and required reoperation, Alifano et al. (6) suggested that diaphragmatic resection along with pleurodesis and possibly hormonal treatment deals satisfactorily with the local disease. They suggested diaphragmatic resection instead of simple suturing or plication to avoid recurrences and provide tissue for histologic confirmation(6), since diaphragmatic plication alone leaves behind endometrial tissue that continues to undergo cyclical changes, increasing the risk of diaphragmatic disruption and recurrence (39). Nevertheless, recurrences occurred even after diaphragmatic resection (6).
Medical treatment
There is consensus that hormonal treatment is a helpful adjunct to prevent recurrences, being indicated particularly in high risk patients. Hormonal treatment immediately after surgery is now suggested for all patients with proven catamenial and/or endometriosis-related pneumothorax. Multimodality management and gonadotrophin-releasing hormone (GnRH) analogue therapy for 6-12 months is suggested. GnRH analogues induce hypogonadotropic hypogonadism and amenorrhea, aiming to ovarian rest and suppression of ectopic endometrium activity (3,4,9,10,11,13,15,17,34). Alifano et al. (39) stressed the importance of producing amenorrhea immediately postoperatively, because effective pleural adhesions require some time to occur and cyclic hormonal changes before accomplishment of effective pleurodesis may result to recurrence.
Hormonal treatment was administered to most patients that required reoperation for catamenial and/or endometriosis-related pneumothorax for a longer period (median 17.5 months) than usual. Patients excluded were those for whom hormonal treatment was proven ineffective or caused significant side effects (6).
Results of treatment
Operations for catamenial pneumothoraces and/or endometriosis related pneumothoraces have practically zero mortality and no significant morbidity. The most common complication is recurrence. High recurrence rates have been reported, exceeding those of idiopathic pneumothorax (3,4,6,9,13,15).
In their 2004 review, Korom et al. (10) reported that the median recurrence-free interval was 61 months among 28 patients who underwent pleurodesis, and 23.6 months among 15 who underwent diaphragmatic excision (with or without pleurodesis). Alifano et al. (3) reported that among 114 patients operated between 2000-2006 for recurrent spontaneous pneumothorax, the recurrence rates were 32% for catamenial pneumothorax, 27% for non-catamenial but endometriosis-related and 5.3% for non-catamenial, non-endometriosis-related pneumothoraces, at a mean follow-up of 32.7 months. Marshall et al. (11) reported 27.5% (3/8) recurrence rate at a mean follow-up of 48 months. Ciriaco et al. (15) reported 40% (4/10) recurrence rate at a mean follow-up of 52 months, positively affected by postoperative hormonal treatment. Attaran et al. reported 8.3% recurrence rate (1/12) at a mean follow-up of 45.8 months, by application of abrasion/pleurectomy, PTFE mesh diaphragmatic repair and routine hormonal treatment. The recurrence occurred before initiation of hormonal treatment in one patient.
In our patients there was a recurrence rate of 20% (1/5) at a mean follow-up of 34 months. The recurrence occurred to a patient in whom a tiny diaphragmatic defect was underestimated and left untreated, in the belief that pleurodesis would suffice. Furthermore the patient did not receive hormonal treatment in the immediate postoperative period.
Conclusions
Early diagnosis, and early treatment dealing with all thoracic pathology including diaphragmatic repair, plus multidisciplinary approach and hormonal treatment dealing with the main chronic disease may reduce the recurrence rate of catamenial and/or endometriosis related pneumothorax (2-6,9-11,15-17,23).
Catamenial and/or thoracic endometriosis-related pneumothorax represents a complex puzzle, and finding some pieces of the puzzle should suffice for high suspicion on its diagnosis. We speculate that all cases classified under this definition represent one sole entity, which furthermore includes, in our opinion, unclassified cases with characteristic findings of catamenial and/or thoracic endometriosis-related pneumothorax in the absence of catamenial character and in the absence of histologically proven endometriosis. Characteristic findings, mainly diaphragmatic holes and/or nodules, are highly diagnostic and, in our opinion, sufficient for the diagnosis. We agree with Peikert et al. (12) and Leong et al. (13) that pneumothoraces occurring in the late secretory phase should also be consider as catamenial.
The diagnosis of endometriosis related pneumothorax is highly dependent on the intraoperative findings that merely represent a small scene in a long play of a continuously changing disease, undergoing cyclical hormonal changes and long term remissions and exaggerations. If there is not a high degree of suspicion and meticulous complete search, the characteristic lesions may remain undisclosed, both after open surgery or thoracoscopy. The catamenial character of pneumothoraces can also be missed (particularly when occurring in the premenstrual period).
We assume that all described aetiopathogenetic mechanisms may account for a percentage of pneumothoraces of women in reproductive age, in the absence of another underlying causative lung disease. Nevertheless, we believe that the majority of cases of catamenial and/or endometriosis-related pneumothorax can be explained by the migration theory.
The surgical treatment should be early, should aim to address all lesions (if feasible) and take into account occult spread, as well as the presence of a chronic disease (i.e., endometriosis). Diaphragmatic coverage seems reasonable, particularly in the presence of lesions. Hormonal treatment, aiming to amenorrhea immediately after surgery, should be administered in all cases of proven catamenial and/or endometriosis related pneumothoraces, unless there are significant contra-indications, and in our opinion in non proven but highly “probable” cases. Asymptomatic pelvic endometriosis may coexist. Early diagnosis and treatment of pelvic endometriosis is essential for the prevention of thoracic spread and the recurrences of thoracic endometriosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Alifano M, Vénissac N, Mouroux J., Recurrent pneumothorax associated with thoracic endometriosis. Surg Endosc. 2000;680. [PubMed ]
- Alifano M, Roth T, Broët SC, Catamenial pneumothorax: a prospective study. Chest. 2003;1004-8. [PubMed ]
- Alifano M, Jablonski C, Kadiri H, Catamenial and noncatamenial, endometriosis-related or nonendometriosis-related pneumothorax referred for surgery. Am J Respir Crit Care Med. 2007;1048-53. [PubMed ]
- Alifano M., Catamenial pneumothorax. Curr Opin Pulm Med. 2010;381-6. [PubMed ]
- Rousset-Jablonski C, Alifano M, Plu-Bureau G, Catamenial pneumothorax and endometriosis-related pneumothorax: clinical features and risk factors. Hum Reprod. 2011;2322-9. [PubMed ]
- Alifano M, Legras A, Rousset-Jablonski C, Pneumothorax recurrence after surgery in women: clinicopathologic characteristics and management. Ann Thorac Surg. 2011;322-6. [PubMed ]
- Van Schil PE, Vercauteren SR, Vermeire PA, Catamenial pneumothorax caused by thoracic endometriosis. Ann Thorac Surg. 1996;585-6. [PubMed ]
- Cowl CT, Dunn WF, Deschamps C, Visualization of diaphragmatic fenestration associated with catamenial pneumothorax. Ann Thorac Surg. 1999;1413-4. [PubMed ]
- Bagan P, Le Pimpec Barthes F, Assouad J, et al. Catamenial pneumothorax: retrospective study of surgical treatment. Ann Thorac Surg 2003;75:378-81; discusssion 381.
- Korom S, Canyurt H, Missbach A, Catamenial pneumothorax revisited: clinical approach and systematic review of the literature. J Thorac Cardiovasc Surg. 2004;502-8. [PubMed ]
- Marshall MB, Ahmed Z, Kucharczuk JC, Catamenial pneumothorax: optimal hormonal and surgical management. Eur J Cardiothorac Surg. 2005;662-6. [PubMed ]
- Peikert T, Gillespie DJ, Cassivi SD, Catamenial pneumothorax. Mayo Clin Proc. 2005;677-80. [PubMed ]
- Leong AC, Coonar AS, Lang-Lazdunski L, Catamenial pneumothorax: surgical repair of the diaphragm and hormone treatment. Ann R Coll Surg Engl. 2006;547-9. [PubMed ]
- Mikroulis DA, Didilis VN, Konstantinou F, Catamenial pneumothorax. Thorac Cardiovasc Surg. 2008;374-5. [PubMed ]
- Ciriaco P, Negri G, Libretti L, Surgical treatment of catamenial pneumothorax: a single centre experience. Interact Cardiovasc Thorac Surg. 2009;349-52. [PubMed ]
- Majak P, Langebrekke A, Hagen OM, Catamenial pneumothorax, clinical manifestations--a multidisciplinary challenge. Pneumonol Alergol Pol. 2011;347-50. [PubMed ]
- Attaran S, Bille A, Karenovics W, Videothoracoscopic repair of diaphragm and pleurectomy/abrasion in patients with catamenial pneumothorax: a 9-year experience. Chest. 2012; . . [PubMed ]
- Nakamura H, Konishiike J, Sugamura A, Epidemiology of spontaneous pneumothorax in women. Chest. 1986;378-82. [PubMed ]
- Shearin RP, Hepper NG, Payne WS, Recurrent spontaneous pneumothorax concurrent with menses. Mayo Clin Proc. 1974;98-101. [PubMed ]
- Suzuki S, Yasuda K, Matsumura Y, Left-side catamenial pneumothorax with endometrial tissue on the visceral pleura. Jpn J Thorac Cardiovasc Surg. 2006;225-7. [PubMed ]
- Laws HL, Fox LS, Younger JB, Bilateral catamenial pneumothorax. Arch Surg. 1977;627-8. [PubMed ]
- Nezhat C, King LP, Paka C, Bilateral thoracic endometriosis affecting the lung and diaphragm. JSLS. 2012;140-2. [PubMed ]
- Joseph J, Sahn SA, Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. Am J Med. 1996;164-70. [PubMed ]
- Channabasavaiah AD, Joseph JV, Thoracic endometriosis: revisiting the association between clinical presentation and thoracic pathology based on thoracoscopic findings in 110 patients. Medicine (Baltimore). 2010;183-8. [PubMed ]
- Alifano M, Trisolini R, Cancellieri A, Thoracic endometriosis: current knowledge. Ann Thorac Surg. 2006;761-9. [PubMed ]
- Szamatowicz M., Endometriosis--still an enigmatic disease. What are the causes, how to diagnose it and how to treat successfully?. Gynecol Endocrinol. 2008;535-6. [PubMed ]
- Attaran M, Falcone T, Goldberg J., Endometriosis: still tough to diagnose and treat. Cleve Clin J Med. 2002;647-53. [PubMed ]
- Bontis JN, Vavilis DT, Etiopathology of endometriosis. Ann N Y Acad Sci. 1997;305-9. [PubMed ]
- Vinatier D, Orazi G, Cosson M, Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2001;21-34. [PubMed ]
- Maruyama T, Yoshimura Y., Stem cell theory for the pathogenesis of endometriosis. Front Biosci. 2012;2854-63 ;:-. [PubMed ]
- Jiang QY, Wu RJ, Growth mechanisms of endometriotic cells in implanted places: a review. Gynecol Endocrinol. 2012;562-7. [PubMed ]
- Ceccaroni M, Roviglione G, Giampaolino P, Laparoscopic surgical treatment of diaphragmatic endometriosis: a 7-year single-institution retrospective review. Surg Endosc. 2012; . . [PubMed ]
- Bagan P, Berna P, Assouad J, Value of cancer antigen 125 for diagnosis of pleural endometriosis in females with recurrent pneumothorax. Eur Respir J. 2008;140-2. [PubMed ]
- Hagneré P, Deswarte S, Leleu O., Thoracic endometriosis: A difficult diagnosis. Rev Mal Respir. 2011;908-12. [PubMed ]
- Fonseca P., Catamenial pneumothorax: a multifactorial etiology. J Thorac Cardiovasc Surg. 1998;872-3. [PubMed ]
- Roth T, Alifano M, Schussler O, Catamenial pneumothorax: chest X-ray sign and thoracoscopic treatment. Ann Thorac Surg. 2002;563-5. [PubMed ]
- Kronauer CM, Images in clinical medicine. Catamenial pneumothorax. N Engl J Med. 2006;e9. [PubMed ]
- Bobbio A, Carbognani P, Ampollini L, Diaphragmatic laceration, partial liver herniation and catamenial pneumothorax. Asian Cardiovasc Thorac Ann. 2007;249-51. [PubMed ]
- Alifano M, Magdeleinat P, Regnard JF, Catamenial pneumothorax: some commentaries. J Thorac Cardiovasc Surg. 2005;1199-author reply 1199-200 . ;:; . [PubMed ]
- Yoshioka H, Fukui T, Mori S, Catamenial pneumothorax in a pregnant patient. Jpn J Thorac Cardiovasc Surg. 2005;280-2. [PubMed ]
- Morcos M, Alifano M, Gompel A, Life-threatening endometriosis-related hemopneumothorax. Ann Thorac Surg. 2006;726-9. [PubMed ]
- Grunewald RA, Wiggins J, Pulmonary endometriosis mimicking an acute abdomen. Postgrad Med J. 1988;865-6. [PubMed ]
- Triponez F, Alifano M, Bobbio A, Endometriosis-related spontaneous diaphragmatic rupture. Interact Cardiovasc Thorac Surg. 2010;485-7. [PubMed ]
- Downey DB, Towers MJ, Poon PY, Pneumoperitoneum with catamenial pneumothorax. AJR Am J Roentgenol. 1990;29-30. [PubMed ]
- Jablonski C, Alifano M, Regnard JF, Pneumoperitoneum associated with catamenial pneumothorax in women with thoracic endometriosis. Fertil Steril. 2009;930. [PubMed ]
- Pryshchepau M, Gossot D, Magdeleinat P., Unusual presentation of catamenial pneumothorax. Eur J Cardiothorac Surg. 2010;1221. [PubMed ]
- Picozzi G, Beccani D, Innocenti F, MRI features of pleural endometriosis after catamenial haemothorax. Thorax. 2007;744. [PubMed ]
- Kumakiri J, Kumakiri Y, Miyamoto H, Gynecologic evaluation of catamenial pneumothorax associated with endometriosis. J Minim Invasive Gynecol. 2010;593-9. [PubMed ]
- Makhija Z, Marrinan M., A case of catamenial pneumothorax with diaphragmatic fenestrations. J Emerg Med. 2012;e1-3. [PubMed ]
- Slasky BS, Siewers RD, Lecky JW, Catamenial pneumothorax: the roles of diaphragmatic defects and endometriosis. AJR Am J Roentgenol. 1982;639-43. [PubMed ]
- Kirschner PA, Porous diaphragm syndromes. Chest Surg Clin N Am. 1998;449-72. [PubMed ]
- Cerfolio RJ, Bryant AS, Efficacy of video-assisted thoracoscopic surgery with talc pleurodesis for porous diaphragm syndrome in patients with refractory hepatic hydrothorax. Ann Thorac Surg. 2006;457-9. [PubMed ]
- Saito F, Tashiro H, Honda R, Twisted ovarian tumor causing progressive hemothorax: a case report of porous diaphragm syndrome. Gynecol Obstet Invest. 2008;134-7. [PubMed ]
- Kocaman O, Sipahi M, Cubukçu A, Porous diaphragm syndrome after ERCP in a patient with bile duct stricture. Turk J Gastroenterol. 2009;157-8. [PubMed ]
- Lozman H, Newman AJ, Spontaneous pneumoperitoneum occurring during postpartum exercises in the knee-chest position. Am J Obstet Gynecol. 1956;903-5. [PubMed ]
- Williams NM, Watkin DF, Spontaneous pneumoperitoneum and other nonsurgical causes of intraperitoneal free gas. Postgrad Med J. 1997;531-7. [PubMed ]
- Funatsu K., Catamenial pneumothorax: can all cases be explained by the pore hypothesis?. Chest. 2003;766-author reply 766. [PubMed ]
- Soderberg CH, Dahlquist EH, Catamenial pneumothorax. Surgery. 1976;236-9. [PubMed ]
- Andrade-Alegre R, González W., Catamenial pneumothorax. J Am Coll Surg. 2007;724. [PubMed ]


