
Endoscopic ultrasound-guided fine needle aspiration for smooth benign appearing malignant esophageal stricture: a cross-sectional study
Introduction
Esophageal stricture is a very common clinical manifestation. The most common cause is advanced esophageal cancer, which causes patients to have difficulty swallowing and eating. Most of these diseases can be diagnosed clearly by gastroscopic biopsy. However, few patients present with smooth overlying esophageal mucosa on endoscopy (1,2). In these cases, endoscopic mucosal biopsy results are often negative, making diagnosis difficult. Compared with ordinary endoscopic biopsy, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) biopsy has advantages. Endoscopic biopsy can only bite the inner surface of the esophagus, while EUS-FNA can acquire tissue from mucosal/submucosal tumors, as well as peri-intestinal structures including lymph nodes, pancreas, adrenal gland, gallbladder, bile duct, liver, kidney, and lung. EUS-FNA is a very mature technology, which enables the needle biopsy of the lesions of the digestive tract and its surrounding organs through the digestive tract. Clinically, it is widely used for the diagnosis of pancreatic tumor, lung cancer, and mediastinal lymph node metastasis (3-6). This study sought to examine the use of EUS-FNA in smooth esophageal strictures. Based on cytology, histopathology, and immunohistochemistry results, the cause of smooth esophageal strictures can be identified as a guidance for clinical treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-584/rc).
Methods
Subjects
We collected the case data of patients who underwent EUS-FNA needle biopsies at the Tianjin Medical University Cancer Institute and Hospital. From September 2016 to November 2021, 50 patients were enrolled. All the patients were suspected to have malignant esophageal stricture but had negative biopsies.
All the patients had varying degrees of dysphagia, and computed tomography (CT) revealed a thickening of the esophageal wall, or a mediastinal mass, mediastinal lymph node involvement or the compression of the esophageal wall. The endoscopies revealed esophageal strictures, and the endoscopic biopsies were negative. EUS-FNA was performed for the pathological diagnosis. The clinical manifestations, patient history, imaging examinations, gastroscopic findings, EUS-FNA results, and treatment plans were recorded and analyzed.
EUS-FNA puncture was not accompanied by on-site cytology and obtaining satisfactory tissue strips was the end point (based on the experience of the performer). If 1 of the traditional smears and liquid-based cytology was positive, the cytology was considered positive. When the histology results alone failed to provide a clear diagnosis or reveal the pathological type and tissue source of the lesions, some patients also underwent immunohistochemical examinations. The lesions were considered histologically positive based on histological and immunohistochemical determinations of the nature and pathological type. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (No. bc2022079). Before EUS-FNA, all the patients were fully informed of the relevant risks and signed the informed consent form.
Devices and accessories
A linear-array ultrasound gastroscope (EG 3870UTK; Pentax, Tokyo, Japan) or a bronchoscope (GF UCT 180, Olympus Corp., Tokyo, Japan) was used for the puncture. When necessary, the lesions were assessed using a small probe (20-MHz miniprobe, UM-DG20-31R; Olympus, Tokyo, Japan) before the puncture. The FNA was performed using a 19-G (ECHO 19, Cook Endoscopy) or 22-G (ECHO 3-22, Cook Endoscopy, Winston-Salem, NC, USA) needle. The type of puncture needle used was determined by the operator according to the site, size, and blood supply of a specific lesion.
EUS-FNA
Preoperative routine examinations of electrocardiogram (ECG), coagulation function, and routine blood works were conducted to rule out serious cardiopulmonary diseases and coagulation disorders. Oral anticoagulants, such as aspirin, were stopped for 1 week before surgery. Fasting was prescribed for 4–6 hours before surgery. All patients in the present cohort were operated under conscious sedation and anesthesia, and ECG monitoring was performed during the operation.
The procedure was performed by an experienced endoscopist (who had performed >1,000 EUS-FNAs). Before the puncture, a comprehensive inspection of the lesion was conducted to determine its shape, size and positional relationship in relation to the surrounding organs and blood vessels and to determine the optimal puncture path. The needle was inserted into the lesion under ultrasound monitoring, and the needle core was pulled out and a negative pressure syringe was connected. Negative pressure was maintained at 5–10 mL, and the syringe was lifted and inserted back and forth in the lesion >20 times. A fanning technique was used during the FNA. In patients with severe esophageal stricture for whom EUS scanning was difficult, a small probe was used to scan the lesion to determine the thickness of the esophageal wall and the length of the lesion, and an endobronchial ultrasound (EBUS) was then performed to puncture the lesion through the esophagus.
Sample processing
The first drop of bloody components of the suction material was dropped onto a glass slide, making 2 traditional smears per needle, fixed with 95% alcohol, and sent to the cytology room, where hematoxylin and eosin (H&E) staining was performed for the cytological examination. Normal saline was used to push the inhaled material into the liquid-based cytology bottle through a 10-mL syringe. The strip-forming components were removed and stored in 10% formalin solution, embedded in paraffin, and subjected to H&E staining for the histological examination. The immunohistochemical examination, the centrifugation of the liquid components, preparation, and H&E staining were performed as necessary.
Statistical analysis
IBM SPSS Statistics (v24.0; IBM Corp., United States) were used for data analysis. Continuous variables are presented as mean ± standard deviation and categorical variables as the frequency (n) and percentage (%).
Results
A total of 50 patients (40 male and 10 female) met the criteria during the study period. All the patients had varying degrees of dysphagia. The patients had a mean age of 61.5 years (range, 46–84 years). All patients underwent chest CT examinations, and the CT scans showed esophageal wall thickening in 19 cases, mediastinal masses in 25 cases, and mediastinal lymphadenopathies with or without pulmonary masses in 6 cases. The esophageal strictures were located in the upper esophagus in 13 cases, in the middle in 30 cases, and in the lower esophagus in 7 cases (Table 1). The 19G puncture needle was used in 6 cases, and the 22G puncture needle was used in 44 cases, with 1 to 5 needles (mean: 2.7 needles) punctured. The diagnosis of malignant tumor was confirmed in all patients. The EUS-FNA biopsies were obtained from all patients. Forty-nine (98%) EUS-FNA biopsies were interpreted as malignancy on histological or cytological evaluation. Among them, 4 cases were positive based on the cytology results, 11 cases were positive based on the histology results, 34 cases were positive based on both the cytology and histology results (Table 2). Only 1 (2%) patient who was confirmed to have mediastinal schwannoma by surgery and pathology had a EUS-FNA negative biopsy. In relation to the final diagnoses, there were 16 cases of esophageal squamous cell carcinoma, 2 cases of metastatic esophageal cancer, 1 case of esophageal sarcoma, 15 cases of small cell lung cancer, 2 cases of lung squamous cell carcinoma, 5 cases of lung adenocarcinoma, 1 case of small cell lung cancer with lymph node metastasis, 3 cases of lung squamous cell carcinoma with mediastinal lymph node metastasis, 1 case of giant cell lung cancer with lymph node metastasis, 1 case of mediastinal lymph node metastasis after left thumb squamous cell carcinoma, 2 cases of mediastinal schwannoma, and 1 case of mediastinal malignant mesothelioma. A total of 5 cases were treated with surgery, 28 with chemotherapy, 3 with chemotherapy + surgery, and 12 with radiotherapy and chemotherapy; 2 patients ceased treatment (Table 3).
Table 1
| Characteristics | Values |
|---|---|
| Gender, n (%) | |
| Male | 40 (80) |
| Female | 10 (20) |
| Age, years, mean (range) | 61.5 (46–84) |
| Gastroscopy*, n (%) | 28 (56) |
| CT, n (%) | |
| Thickening of esophageal wall | 19 (38) |
| Mediastinal type lesion | 25 (50) |
| Swelling of mediastinal lymph nodes | 6 (12) |
| Esophageal stricture site, n (%) | |
| Upper esophagus | 13 (26) |
| Middle esophagus | 30 (60) |
| Lower esophagus | 7 (14) |
*, 28 patients underwent gastroscopy and 23 received 1 to 2 biopsies, and all biopsies were negative on pathology. CT, computerized tomography.
Table 2
| EUS-FNA | Patients (n=50) |
|---|---|
| Needle, n [%] | |
| 19-G | 6 [12] |
| 22-G | 44 [88] |
| Pass, mean [range] | 2.7 [1–5] |
| Smear, mean [range] | 5.4 [2–10] |
| Pathology, n [%] | |
| Cytology only (+) | 4 [8] |
| Histology only (+) | 11 [22] |
| Both (+) | 34 [68] |
| Both (−) | 1 [2] |
EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; +, the presentation of cancer cell; −, the absence of cancer cell.
Table 3
| Sources and pathologic types | Treatment | Total | ||||
|---|---|---|---|---|---|---|
| Surgery | Chemotherapy | Chemotherapy + surgery | Radiochemotherapy | Giving up treatment | ||
| Esophageal tumor | ||||||
| Esophageal squamous carcinoma | 2 | 4 | 3 | 7 | 16 | |
| Metastatic esophageal cancer* | 2 | 2 | ||||
| Esophageal sarcoma | 1 | 1 | ||||
| Lung cancer | ||||||
| Small cell lung cancer | 12 | 2 | 1 | 15 | ||
| Squamous cell carcinoma of the lung | 1 | 1 | 2 | |||
| Lung adenocarcinoma | 4 | 1 | 5 | |||
| Mediastinal lymph nodes | ||||||
| Small cell lung cancer | 1 | 1 | ||||
| Squamous cell carcinoma of the lung | 2 | 1 | 3 | |||
| Giant-cell lung cancer | 1 | 1 | ||||
| Other# | 1 | 1 | ||||
| Mediastinal tumor | ||||||
| Nerve sheath tumors | 2 | 2 | ||||
| Malignant mesothelioma | 1 | 1 | ||||
| Total | 5 | 28 | 3 | 12 | 2 | 50 |
*, esophageal metastasis from adenocarcinoma of the lung (n=1) and esophageal metastasis from breast cancer (n=1); #, a case of a patient who had received surgery for squamous carcinoma of the left thumb, with multiple lymph node metastases in the lungs and mediastinum as well as esophageal involvement.
No obvious complications, such as bleeding and mediastinal infection, were observed. Two patients experienced mild pain (numeric rating scale, 1–3) after EUS-FNA.
Discussion
There are many clinical causes of esophageal stricture, which may be accompanied by dysphagia, vomiting, an inability to eat, and may result in malnutrition. Esophageal cancer is a common cause of esophageal stricture, and most esophageal cancers can be pathologically diagnosed by endoscopic biopsy. However, it is not uncommon for esophageal cancer to present with smooth strictures of the esophagus (7). Gastroscope biopsies are difficult in such cases. Some investigators attempt dilation before biopsy (8). However, dilation carries the risk of perforation (9-11), especially in patients with advanced esophageal cancer. Molina et al. (12) reported that the dilation of advanced esophageal cancer caused perforation in up to 10.6% (6/55) of patients. Transnasal ultrafine gastroscope is another option (13), as it can pass through some esophageal strictures that cannot be passed through by ordinary gastroscopes, but the biopsy hole is small and the tissue obtained is limited (7,10). EUS is currently the most reliable method for the staging of esophageal cancer (14), but EUS-FNA has not been fully applied in the diagnosis of esophageal cancer.
Currently, only a limited number of studies have examined the role of EUS-FNA in these patients (12,15). Canadian scholars reported on the use of EUS-FNA in 2 cases of esophageal cancer, in which EUS-FNA was performed after dilation (15). Dahale et al. (1) reported 11 cases of esophageal cancer leading to smooth esophagus stenosis, performed EUS-FNA without dilating the stenosis, and obtained a pathological diagnosis for all 11 patients. In the current study, a total of 17 cases of primary esophageal malignant tumors were diagnosed by EUS-FNA, including 16 cases of esophageal squamous cell carcinoma and 1 case of esophageal sarcoma. CT before EUS-FNA showed the thickening of the esophageal wall, and a gastroscopic examination was performed at our hospital or other hospitals. As no valid tissue was obtained from the biopsy specimens, the diagnosis could not be confirmed. Cytological and histological specimens were obtained from 17 patients by EUS-FNA, and some patients also underwent immunohistochemical examinations. Based on the cytopathology, histology, and immunohistochemistry results, definite diagnoses were obtained for all patients, yielding a diagnosis rate of 100%. To date, this cohort comprises the largest sample size of patients with smooth esophagus stenosis caused by a primary malignant tumor of the esophagus; the pathologically negative gastroscopic biopsy was confirmed by EUS-FNA.
Metastatic esophageal cancer is very rare clinically. Since the first case of metastatic esophageal cancer from the prostate was reported by Gross et al. (16) in 1942, various metastatic esophageal cancers in different organs have been reported, including the breast, lungs, ovary, liver, colon, pancreas, bladder, kidneys, and stomach (17-25). The most common primary sites for metastatic esophageal cancer are the breast and lungs (17,18). The incidence of metastatic esophageal cancer found by autopsies is about 0.3–6.1% (26-28), most of which are micrometastases (67.8%) (28), which are not easy to find clinically. In symptomatic metastatic esophageal cancer, dysphagia is the most common first presentation (28).
The CT manifestations of metastatic esophageal cancer include the circumferential thickening of the esophageal wall, while the endoscopic manifestations include esophageal circumferential stenosis, and smooth surface mucosa. Biopsies often fail to obtain pathological diagnosis, which creates clinical diagnosis challenges. Breast cancer esophageal metastases may appear 20 years after breast cancer surgery (29), and are often considered a primary esophageal tumor. Sunada et al. (30) reported a case of esophageal metastasis from breast cancer diagnosed by endoscopic mucosal resection (EMR). However, surgical pathology and autopsy results showed that most metastatic esophageal cancers were located in the submucosa and muscular layer of the esophagus, where the surface mucosa was intact and EMR only removed the surface mucosa. The issue of whether a pathological diagnosis can be obtained by EMR is a concern. Conversely, EUS-FNA can even make a clear pathological diagnosis of submucosal tumors (6,31,32).
EUS-FNA is a method of choice when a valid specimen of clinically suspected metastatic esophageal cancer cannot be obtained by endoscopic biopsy (2,33). Suzuki et al. (2) reported a case of breast cancer with esophageal metastasis diagnosed by EUS-FNA. In our current cohort, 1 patient with lung cancer with mediastinal lymph node metastasis developed dysphagia after half a year of chemotherapy. CT showed circumferential thickening of the mid-section wall, accompanied by mediastinal lymph node enlargement. EUS showed that the 5-layer structure of the esophageal wall disappeared, and the esophageal wall was thickened. After EUS-FNA puncture, based on the histology and immunohistochemistry results, a diagnosis of esophageal metastasis of lung adenocarcinoma was considered. The other case was a post-operative breast cancer patient who had undergone total right mastectomy for breast cancer 14 years before. Some 3 months ago, the patient experienced difficulty swallowing, which became progressively aggravated. The upper gastrointestinal angiography at our hospital showed stricture of the middle and lower esophagus, and the surface mucosa was smooth. A gastroscopy showed a circumferential stenosis in the middle esophagus with smooth surface mucosa, and no clear tumor cells were found in the biopsy pathology results. Due to the severe esophageal stricture, EUS could not comprehensively scan the lesions, and puncture was difficult. A scan of the lesion with a small-diameter ultrasound probe showed that the esophageal wall at the stricture site was unclear and had a circumferential thickening. The lesions were punctured through the esophagus by EBUS instead, and satisfactory cytological and histological specimens were obtained. Malignant cells were found by traditional smear and liquid-based cytology, and the histology and immunohistochemistry results confirmed the presence of breast cancer esophageal metastasis (Figures 1-10). These 2 cases of esophageal metastatic carcinoma in our series were both diagnosed by EUS or EBUS. Thus, for patients with malignant tumors in other sites (especially the breast or lungs), suffering from dysphagia, with a thickened esophagus wall on CT, and with a smooth and narrowed esophagus under gastroscopy, EUS or EBUS punctures can be performed to confirm if there is a metastatic esophageal carcinoma.
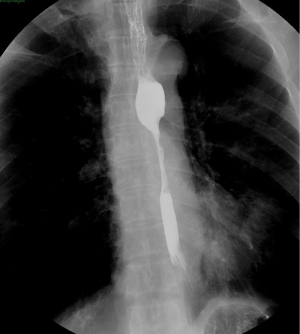
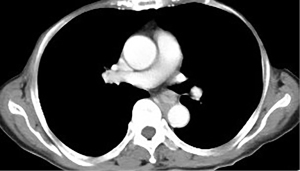
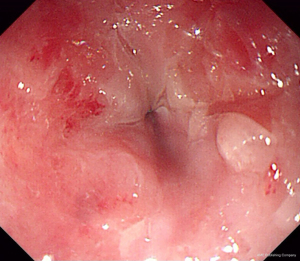
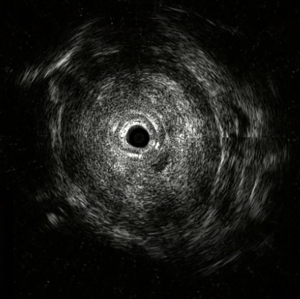
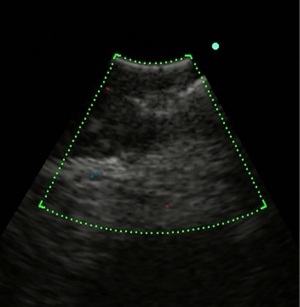
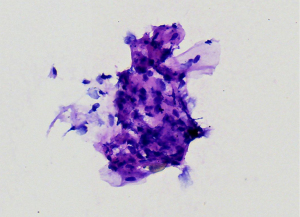
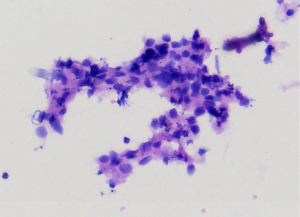
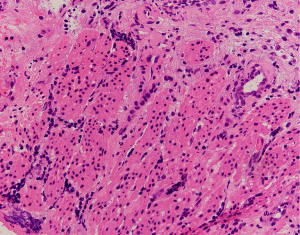
Due to the anatomical location, mediastinal lung cancer and mediastinal masses or enlarged lymph nodes involving the esophagus and compressing the esophagus may be another cause of smooth esophageal stricture. Dysphagia has been reported in 6–7% of lung cancer patients throughout the course of the disease (34). Direct tumor involvement or compression, the compression of mediastinal lymph nodes, and esophageal stricture caused by radiotherapy are the 3 main mechanisms of dysphagia caused by lung cancer. Studies have confirmed that lung cancer accompanied by dysphagia is significantly associated with a lower survival rate (35,36). Obtaining diseased tissue before treatment and making a clear diagnosis are crucial for treatment.
Lung cancer, mediastinal masses, and lymph nodes that cause smooth esophagus stenosis are located in the posterior mediastinum, and it is difficult to make a pathological diagnosis through other means. EUS can easily scan such lesions through the esophagus and allows needle biopsies to be performed. It is the preferred diagnosis and treatment method for such patients. In the present cohort, there were 31 cases of smooth esophageal stricture caused by lung cancer, mediastinal lymph node metastasis, or mediastinal tumor, of which 30 cases were diagnosed by EUS-FNA (30/31); only 1 case was negative for mediastinal mass cytology and tissue. That patient ultimately underwent surgery, and a mediastinal schwannoma was confirmed.
Cell and tissue specimens were obtained for all subjects by EUS-FNA. Based on the cytology, histology, and immunohistochemistry results, 49 patients obtained a clear pathological diagnosis, with a positive rate of 98%. No obvious procedure-related complications were observed. Thus, EUS-FNA can provide a pathological diagnosis for patients with smooth esophageal stricture and guide clinical treatment.
However, our current study was limited by its retrospective design and small sample size. Notably, there were only 2 patients with metastatic esophageal tumors in our analysis. The diagnosis of such cases needs to be further verified by multicenter studies with larger sample sizes.
Conclusions
For smooth esophagus stenosis caused by various etiologies, satisfactory pathological specimens can be obtained by EUS-FNA. Based on cytology, histology, and immunohistochemistry results, definite diagnoses can be made for most patients. EUS-FNA is the preferred diagnostic tool.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-584/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-584/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-584/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (No. bc2022079). Before EUS-FNA, all the patients were fully informed of the relevant risks and signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dahale AS, Srivastava S, Sonika U, et al. Role of linear endosonography in the diagnosis of biopsy-negative malignant esophageal strictures: Exploring the unexplored. JGH Open 2020;4:113-6. [Crossref] [PubMed]
- Suzuki R, Singh H, Ramireddy S, et al. Endoscopic ultrasound-guided fine needle aspiration for smooth benign appearing esophageal stricture due to metastatic breast cancer. Endosc Ultrasound 2013;2:35-7. [Crossref] [PubMed]
- Clementsen PF, Bodtger U, Konge L, et al. Diagnosis and staging of lung cancer with the use of one single echoendoscope in both the trachea and the esophagus: A practical guide. Endosc Ultrasound 2021;10:325-34. [Crossref] [PubMed]
- Masoumi-Moghaddam S, Lundy J, Gao H, et al. The EUS molecular evaluation of pancreatic cancer: A prospective multicenter cohort trial. Endosc Ultrasound 2021;10:335-43. [Crossref] [PubMed]
- Solonitsyn EG, Danilov IN, Poddymova AV, et al. EUS-FNA biopsy of parathyroid gland. Endosc Ultrasound 2021;10:315-6. [Crossref] [PubMed]
- Kamata K, Kurita A, Yasukawa S, et al. Utility of a 20G needle with a core trap in EUS-guided fine-needle biopsy for gastric submucosal tumors: A multicentric prospective trial. Endosc Ultrasound 2021;10:134-40. [Crossref] [PubMed]
- Faigel DO, Deveney C, Phillips D, et al. Biopsy-negative malignant esophageal stricture: diagnosis by endoscopic ultrasound. Am J Gastroenterol 1998;93:2257-60. [Crossref] [PubMed]
- Standards of Practice Committee. Esophageal dilation. Gastrointest Endosc 2006;63:755-60. [Crossref] [PubMed]
- Hagel AF, Naegel A, Dauth W, et al. Perforation during esophageal dilatation: a 10-year experience. J Gastrointestin Liver Dis 2013;22:385-9. [PubMed]
- Benites Goñi HE, Arcana López R, Bustamante Robles KY, et al. Factors associated with complications during endoscopic esophageal dilation. Rev Esp Enferm Dig 2018;110:440-5. [Crossref] [PubMed]
- Tucker LE. Esophageal Dilation for Strictures: A 36-Year Prospective Experience in Private Practice Setting. Mo Med 2020;117:555-8. [PubMed]
- Molina JC, Goudie E, Pollock C, et al. Balloon Dilation for Endosonographic Staging in Esophageal Cancer: A Phase 1 Clinical Trial. Ann Thorac Surg 2021;111:1150-5. [Crossref] [PubMed]
- Aydinli M, Koruk I, Dag MS, et al. Ultrathin endoscopy for gastrointestinal strictures. Dig Endosc 2012;24:150-3. [Crossref] [PubMed]
- DaVee T, Ajani JA, Lee JH. Is endoscopic ultrasound examination necessary in the management of esophageal cancer? World J Gastroenterol 2017;23:751-62. [Crossref] [PubMed]
- Adrián-de-Ganzo Z, Gimeno-García AZ, Elwassief A, et al. Linitis-like squamous esophageal cancer diagnosed by endoscopic ultrasonography-guided fine-needle aspiration cytology: report of two cases. Eur J Gastroenterol Hepatol 2013;25:1488-91. [Crossref] [PubMed]
- Gross P, Freedman LJ. Obstructive secondary carcinoma of the esophagus as a cause of dysphagia. Arch Pathol 1942;38:361-4.
- Miyake M, Yamada A, Miyake K, et al. Esophageal metastasis of breast cancer during endocrine therapy for pleural dissemination 21 years after breast surgery: a case report. Surg Case Rep 2019;5:22. [Crossref] [PubMed]
- Forti E, Bonato G, Dioscoridi L, et al. A Smooth Esophageal Stricture Causing Dysphagia. Dysphagia 2018;33:399-402. [Crossref] [PubMed]
- Weissman S, Mehta TI, Berry R, et al. A Path Less Traveled: Metastatic Esophageal Carcinoma from a Primary Ovarian Malignancy. J Gastrointest Cancer 2020;51:329-31. [Crossref] [PubMed]
- Tsubouchi E, Hirasaki S, Kataoka J, et al. Unusual metastasis of hepatocellular carcinoma to the esophagus. Intern Med 2005;44:444-7. [Crossref] [PubMed]
- Watanabe S, Takashima A, Taniguchi H, et al. Esophageal Metastasis from Rectal Cancer Successfully Treated with Fluorouracil-Based Chemotherapy with Bevacizumab: A Case Report and Review of the Literature. Case Rep Oncol 2017;10:407-15. [Crossref] [PubMed]
- Rosati LM, Kummerlowe MN, Poling J, et al. A rare case of esophageal metastasis from pancreatic ductal adenocarcinoma: a case report and literature review. Oncotarget 2017;8:100942-50. [Crossref] [PubMed]
- Jung JL, Abouelfadel Z, Prevot-Maupoix M, et al. Esophageal metastasis of cancer of the bladder. Ann Urol (Paris) 1997;31:205-6. [PubMed]
- Ohnita K, Higashi S, Hirai S, et al. Esophageal metastasis of renal cell carcinoma resected by endoscopic submucosal dissection: a case report. BMC Gastroenterol 2021;21:348. [Crossref] [PubMed]
- Káposztás Z, Cseke L, Horváth OP. Resectable esophageal metastasis of stomach cancer. Magy Seb 2003;56:207-8. [PubMed]
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74-85. [Crossref] [PubMed]
- Telerman A, Gerard B, Van den Heule B, et al. Gastrointestinal metastases from extra-abdominal tumors. Endoscopy 1985;17:99-101. [Crossref] [PubMed]
- Mizobuchi S, Tachimori Y, Kato H, et al. Metastatic esophageal tumors from distant primary lesions: report of three esophagectomies and study of 1835 autopsy cases. Jpn J Clin Oncol 1997;27:410-4. [Crossref] [PubMed]
- Rampado S, Ruol A, Guido M, et al. Mediastinal carcinosis involving the esophagus in breast cancer: the "breast-esophagus" syndrome: report on 25 cases and guidelines for diagnosis and treatment. Ann Surg 2007;246:316-22. [Crossref] [PubMed]
- Sunada F, Yamamoto H, Kita H, et al. A case of esophageal stricture due to metastatic breast cancer diagnosed by endoscopic mucosal resection. Jpn J Clin Oncol 2005;35:483-6. [Crossref] [PubMed]
- Cazacu IM, Singh BS, Luzuriaga Chavez AA, et al. EUS and EUS-guided FNA/core biopsies in the evaluation of subepithelial lesions of the lower gastrointestinal tract: 10-year experience. Endosc Ultrasound 2020;9:329-36. [Crossref] [PubMed]
- Nagai K, Sofuni A, Tsuchiya T, et al. Efficacy of the Franseen needle for diagnosing gastrointestinal submucosal lesions including small tumors. Endosc Ultrasound 2021;10:424-30. [Crossref] [PubMed]
- Liu A, Feng Y, Chen B, et al. A case report of metastatic breast cancer initially presenting with esophageal dysphagia. Medicine (Baltimore) 2018;97:e13184. [Crossref] [PubMed]
- Hyde L, Hyde CI. Clinical manifestations of lung cancer. Chest 1974;65:299-306. [Crossref] [PubMed]
- Marmor S, Cohen S, Fujioka N, et al. Dysphagia prevalence and associated survival differences in older patients with lung cancer: A SEER-Medicare population-based study. J Geriatr Oncol 2020;11:1115-7. [Crossref] [PubMed]
- Yang R, Cheung MC, Pedroso FE, et al. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res 2011;170:e75-83. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


