Management of complex benign post-tracheostomy tracheal stenosis with bronchoscopic insertion of silicon tracheal stents, in patients with failed or contraindicated surgical reconstruction of trachea
Introduction
Upper airway stenosis is a potentially life threatening situation. Postintubation tracheal stenosis is caused by regional ischemic necrosis of the tracheal wall. The recognition of the causative mechanisms and improvements in the design and handling of the endotracheal and tracheostomy tubes led to decreased incidence of postintubation stenosis (1). Nevertheless post-intubation and post-tracheostomy benign tracheal stenoses are the most common indications for surgical tracheal reconstruction (1,2). The lesions characteristically occur at the cuff and stomal site, presenting with symptoms and signs of airway obstruction (2). The treatment of choice is surgical resection with primary reconstruction (1,2). Special techniques are required if the subglottic larynx is involved. Best results are achieved at an initial corrective operation (2).
Tracheal stenting for symptomatic cicatricial stenoses is reserved for patients with lesions that are deemed inoperable, due to local or general conditions (3). It provides prompt and durable palliation to patients ineligible for surgical treatment (4). Indications for stenting in benign stenoses include long tracheal strictures, inflammatory disease, post lung transplantation stenoses, and the presence of a disease process and/or comorbidities that preclude surgery (5). Benign stenoses treated with stenting include post-traumatic stenoses (after intubation, tracheostomy), post-anastomotic stenoses (after lung transplantation), and post-inflammatory stenoses (after tuberculosis or Wegener’s granulomatosis). Stenting may also be indicated in tracheobronchomalacia and oesophageal-airway fistulae. Stent insertion may need to be combined with bronchoscopic interventions (such as dilatation and debridement, fulguration, brachytherapy, laser photocoagulation, or combinations of these treatments) (6).
We present two cases of stent insertion in complicated benign post-intubation tracheal stenoses: (I) in a patient after failed previous operation and continuous recurrences after multiple bronchoscopic interventions and (II) in a patient with poor neurological status and a long, inflamed and infectious stenosis involving the subglottic larynx (Video 1).
Case 1
A 39 year old woman treated for acute respiratory failure (repeated haemoptyses due to alveolar bleeding, attributed to seronegative lung vasculitis, treated with cyclophosphamide, corticoids, and plasmaphereses) underwent endotracheal intubation, followed by tracheostomy 5 days later. Her respiratory function gradually improved, the tracheostomy tube was removed 21 days after insertion, and she was discharged home 3 days later. Four days after hospital discharge she was readmitted being dyspnoeic and having an inspiratory stridor. Bronchoscopy revealed tracheal stenosis 2.5 cm peripherally the cricoid cartilage, at the tracheostomy site. She underwent surgery, resection of the stenosed part and end-to-end anastomosis, 12 days after removal of the tracheostomy tube, and received postoperatively cyclophosphamide and corticoids. On the 27th postoperative day, granulation of the anastomotic site was found; histology of the proliferation was negative for leukocytoclastic vasculitis. She received methylprednisolone for prophylaxis of relapse of the main disease. She underwent multiple bronchoscopic interventions (mechanical dilatation, electrofulguration, cryosurgery) that offered only temporal palliation, since symptomatic stenosis always recurred (Figure 1A,B,C, Figure 2). After repeated bougienage (Figure 1D), she underwent tracheal stent insertion, 7 months after the surgical reconstruction. A 12-mm ×4.5-cm, silicone Dumon stent was inserted under general anaesthesia, through rigid bronchoscopy (Figure 3). The airway was immediately re-establish, without complications (tracheal rupture, subcutaneous emphysema, pneumomediastinum, bleeding). The patient was transferred to the ward breathing spontaneously, and was discharged home 2 days after stent placement, on water nebulizations (Figure 4). At 6- and 12-month follow-up, there was no stent migration; luminal patency was maintained without secretion adherence inside the stents, granulation at the ends, or adjacent structure erosion (Figures 5,6). Fifteen months after stent insertion, the patient maintained good respiratory function, being free of dyspnoea, and respiratory infections. She will be evaluated for scheduled stent removal.
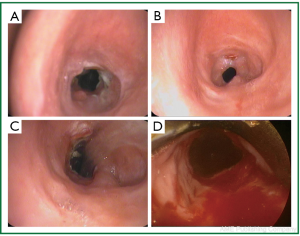 Figure 1. Patient 1. Bronchoscopic pictures before stent insertion. A-C. After mulptiple bronchoscopic interventions, that followed unsuccessful surgical reconstruction (resection of post-tracheostomy stenosis and end-to-end anastomosis); D. Before stent insertion, after bronchoscopic dilatations.
Figure 1. Patient 1. Bronchoscopic pictures before stent insertion. A-C. After mulptiple bronchoscopic interventions, that followed unsuccessful surgical reconstruction (resection of post-tracheostomy stenosis and end-to-end anastomosis); D. Before stent insertion, after bronchoscopic dilatations.
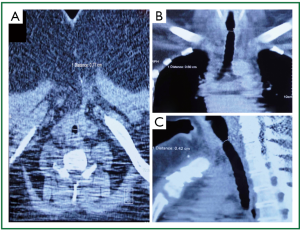 Figure 2. Patient 1. Neck and chest computed tomography 3 months before stent insertion. Tracheal diameter on A. transverse plane 77 mm; B. coronal plane 60 mm; C. sagittal plane 42 mm (sternum fracture and wires from previous tracheal reconstruction).
Figure 2. Patient 1. Neck and chest computed tomography 3 months before stent insertion. Tracheal diameter on A. transverse plane 77 mm; B. coronal plane 60 mm; C. sagittal plane 42 mm (sternum fracture and wires from previous tracheal reconstruction).
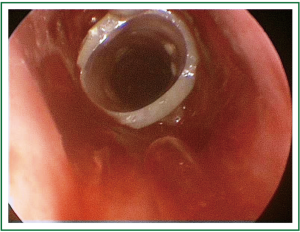 Figure 3. Patient 1. Bronchoscopic picture, immediately after stent insertion (Dumon silicone stent).
Figure 3. Patient 1. Bronchoscopic picture, immediately after stent insertion (Dumon silicone stent).
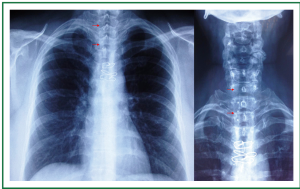 Figure 4. Patient 1. Chest and neck x-rays, 2 weeks after stent insertion. Clear lung fields. Good tracheal patency. Absence of stent migration. Four studs are visible (12-mm × 4.5-cm Dumon stent). Wires from previous surgical tracheal reconstruction (that included resection of post-tracheostomy stenosis, and end-to-end anastomosis).
Figure 4. Patient 1. Chest and neck x-rays, 2 weeks after stent insertion. Clear lung fields. Good tracheal patency. Absence of stent migration. Four studs are visible (12-mm × 4.5-cm Dumon stent). Wires from previous surgical tracheal reconstruction (that included resection of post-tracheostomy stenosis, and end-to-end anastomosis).
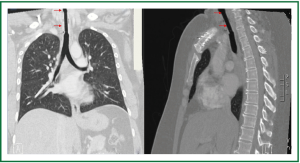 Figure 5. Patient 1. Chest and neck computed tomography, 6 months after stent insertion. Good patency, absence of migration, absence of granulation at the ends of the stent, normal pulmonary parenchyma.
Figure 5. Patient 1. Chest and neck computed tomography, 6 months after stent insertion. Good patency, absence of migration, absence of granulation at the ends of the stent, normal pulmonary parenchyma.
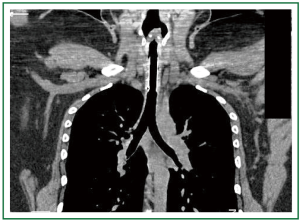 Figure 6. Patient 1. Chest and neck computed tomography, 12 months after stent insertion. Good patency, absence of migration, absence of granulation at the ends of the stent, normal pulmonary parenchyma. (All 4 studs are visible, 12-mm × 4.5-cm Dumon stent).
Figure 6. Patient 1. Chest and neck computed tomography, 12 months after stent insertion. Good patency, absence of migration, absence of granulation at the ends of the stent, normal pulmonary parenchyma. (All 4 studs are visible, 12-mm × 4.5-cm Dumon stent).
Case 2
A 20-year old man treated for severe head trauma after a car accident, underwent tracheostomy and developed a long tracheal stricture that was initially treated with a silicon Montgomery T-tube. Being in an organic psychosyndrome, he experienced near suffocation when he displaced (by pulling) his T-tube, which was urgently replaced by a curved fenestrated tracheostomy tube. Chest x-ray showed large diffuse opacities of the right upper and lower lung zones (collapse, consolidation) (Figure 7). The stomal tissues were inflamed; on microbiological cultures of stomal swabs methicillin resistant staphylococcus aureus was detected. Computed tomography showed a 4 cm long stricture involving the posterior sublottic larynx and the upper trachea (Figure 8). After debridement and bougienage he underwent tracheal stent insertion. A 14-mm ×7-cm silicone Dumon stent was introduced under general anaesthesia and rigid bronchoscopy (Figures 9,10). The airway was immediately established, without complications (tracheal rupture, subcutaneous emphysema, pneumomediastinum, bleeding). The patient was transferred to the intensive care unit breathing spontaneously (Figure 11). The right lung re-expanded and the chest radiogram was soon normalized. The patient was discharged home 3 days after stent placement, on water nebulizations (Figures 12,13). The tracheostomy tissue inflammation resolved and the stoma was nicely healed. New infection was not noted. At 6-month follow-up there was no stent migration; luminal patency was maintained without secretion adherence inside the stents, granulation at the ends, or adjacent structure erosion (Figure 14). Ten months after stent insertion the patient maintained good respiratory function, being free of dyspnoea and respiratory infections. He will be evaluated for scheduled stent removal.
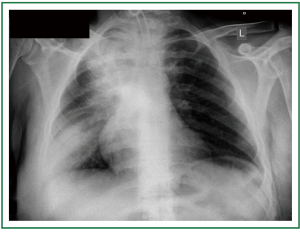 Figure 7. Patient 2. Chest x-ray before stent insertion. Curved fenestrated tracheostomy tube in place. Large opacifications at the upper and lower lung fields.
Figure 7. Patient 2. Chest x-ray before stent insertion. Curved fenestrated tracheostomy tube in place. Large opacifications at the upper and lower lung fields.
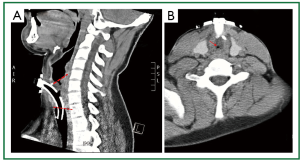 Figure 8. Patient 2. Neck computed tomography before stent insertion. A. (Median) sagittal plane. Long upper tracheal stenosis involving the lower posterior subglottic larynx (a curved tracheostomy tube was urgently placed after removal by the patient of a Montgomery T-tube); B. Transverse plane (above the entrance of the tracheostomy tube to the trachea). Subtotal obstruction of the upper trachea.
Figure 8. Patient 2. Neck computed tomography before stent insertion. A. (Median) sagittal plane. Long upper tracheal stenosis involving the lower posterior subglottic larynx (a curved tracheostomy tube was urgently placed after removal by the patient of a Montgomery T-tube); B. Transverse plane (above the entrance of the tracheostomy tube to the trachea). Subtotal obstruction of the upper trachea.
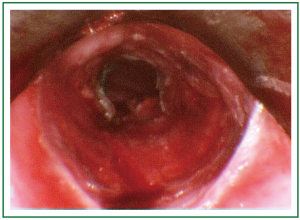 Figure 9. Patient 2. Bronchoscopic picture before stent insertion, after debridement and dilatation.
Figure 9. Patient 2. Bronchoscopic picture before stent insertion, after debridement and dilatation.
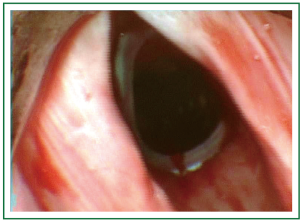 Figure 10. Patient 2. Bronchoscopic picture after stent insertion. The stent can be seen inferiorly to the vocal cords (Dumon silicone stent).
Figure 10. Patient 2. Bronchoscopic picture after stent insertion. The stent can be seen inferiorly to the vocal cords (Dumon silicone stent).
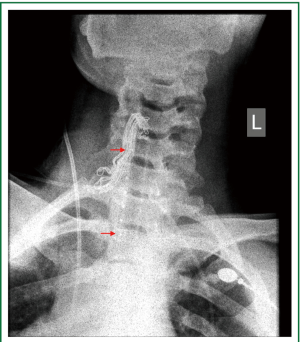 Figure 11. Patient 2. Neck x-ray, immediately after stent insertion. The patient is breathing spontaneously. Good tracheal patency. Partial re-expansion of the left lung. Seven studs are visible (14-mm × 7-cm Dumon stent).
Figure 11. Patient 2. Neck x-ray, immediately after stent insertion. The patient is breathing spontaneously. Good tracheal patency. Partial re-expansion of the left lung. Seven studs are visible (14-mm × 7-cm Dumon stent).
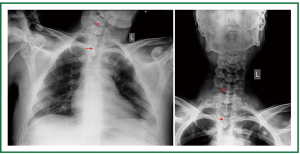 Figure 12. Patient 2. Chest and neck x-rays, on the first day after stent insertion. Good tracheal patency, absence of stent migration, reasonably clear lung fields. Seven studs are visible (14-mm × 7-cm Dumon stent).
Figure 12. Patient 2. Chest and neck x-rays, on the first day after stent insertion. Good tracheal patency, absence of stent migration, reasonably clear lung fields. Seven studs are visible (14-mm × 7-cm Dumon stent).
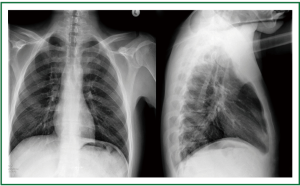 Figure 13. Patient 2. Posteroanterior and lateral chest x-ray 1 week after stent insertion. Normal lung translucency. Good tracheal patency, absence of stent migration.
Figure 13. Patient 2. Posteroanterior and lateral chest x-ray 1 week after stent insertion. Normal lung translucency. Good tracheal patency, absence of stent migration.
 Figure 14. Patient 2. Neck and chest computed tomography [coronal (frontal) and (median) sagittal plane], 6 months after stent insertion. Good patency, absence of migration, absence of granulation at the ends of the stent.
Figure 14. Patient 2. Neck and chest computed tomography [coronal (frontal) and (median) sagittal plane], 6 months after stent insertion. Good patency, absence of migration, absence of granulation at the ends of the stent.
Discussion
Postintubation tracheal stenosis results from pressure ulceration and subsequent healing, starting with granulation, progressing to cicatrisation, and finally scar contraction (7). The use of low pressure high volume cuffs led to decreased incidence of tracheal stenosis post endotracheal intubation (2), but the early application of tracheostomy to patients treated in intensive care units led to increased overall occurrence (8). Treatment by dilatation, mechanical debridement, cryosurgery, laser and steroids generally fails (7).
Surgical reconstruction is the gold standard in the management of benign postintubation tracheal stenosis.
From 1965 through 1992, Hermes Grillo performed more than 500 tracheal reconstructions for postintubation stenosis (including subglottic stenoses, and reoperations) with good or satisfactory results in 93.7% of patients, failure in 3.9%, and a mortality rate of 2.4%. The most common complication was suture line granulation (1,2,9).
Grillo refused operation to very few patients due to systemic reasons, while a more common contraindication to surgical repair of benign postintubation stenosis was the presence of a very lengthy stenosis (7) (having performed resections with lengths of 1.0 to 7.5 cm, and most commonly between 2 and 4 cm) (9).
Among 80 patients with laryngotracheal resection and reconstruction for subglottic laryngeal and upper tracheal stenosis, excellent, good or satisfactory long term results were achieved in 92.5% of patients, failure was noted in 2.5%, and the mortality rate was 2.5% (10). Among 75 patients with reoperative tracheal resection and reconstruction, after unsuccessful repair of postintubation stenosis, complications occurred in 39% of patients, the overall outcome was good or satisfactory in 91,9% of patients, unsuccessful in 5.3%, and the mortality rate was 2.6% (11).
A retrospective review of 901 patients who underwent tracheal resection for benign or malignant stenoses, by the Grillo team (including 589 patients with postintubation tracheal stenosis) was conducted, to identify risk factors for anastomotic complications. Stepwise multivariable analysis revealed that reoperation, diabetes mellitus, lengthy resections (>/=4 cm), laryngotracheal resections, age </=17 years, and need for tracheostomy before operation were risk factors for anastomotic complications (12).
Thus, absolute contraindication to surgical reconstruction of benign postintubation tracheal stenoses is the presence of an excessively long lesion (7) (roughly 50% or more of the whole tracheal length) (3). Other definitive or temporary local conditions that have been considered contraindications to surgical repair include involvement of the proximal subglottic larynx, florid inflammation at the stenotic site, and incompetent glottis with previous aspiration pneumonia. General conditions that may be contraindications to surgery include respiratory failure, sequelae of head injury or neurosurgery (that are usually related to the disease that caused the prolonged intubation), cardiovascular, or other disabling disease (3,8). In these situations the preferred initial treatment is tracheal stenting, usually after bronchoscopic interventions (3-6,8).
Airway stenting was proved valuable in the management of airway stenosis, both malignant and benign (6), being indicated for symptomatic relief of severe dyspnea (13). Airway stenting has been mainly applied in malignant stenoses [for palliation of inoperable patients with unresectable malignant disease (14)], and to a lesser extent in benign stenoses causing airway compromise (5).
Grillo recognized that “airway stents are a useful concept” highlighting their sensible use, that involves not only patient selection but also stent selection (7).
An ideal stent should be easy to insert and re-establish the airway promptly with minimal morbidity and mortality. It should be thin and durable, and maintain patency without causing pressure ischaemia, erosion, or granulation. It should not migrate, but it should be easily removable. It should not interfere with the mucociliary mechanisms and it should fit into tortuous stenosis (5). Although a variety of stents are available, the ideal stent does not exist (5,15). Stents are constructed of silicone, metal mesh or a combination of these. Different stent characteristics define their indicated use (5).
Advantages of uncovered self expandable metallic stents include: insertion with flexible bronchoscopy, higher internal diameter due to decreased width of the wire mesh, less migration, less interruption of the mucosa, less interference with the ciliary mechanism, and ability to fit into tortuous stenoses. Disadvantages include granulation (and/or tumor) ingrowth through the interstices and restenosis, very difficult removal or reposition, risk of mucosa necrosis and fistula formation, risk of collapse under high external pressure (5,16).
Advantages of the silicone stents are that they are removable and exchangeable, they provoke no or minimal granulation, they resist external pressure, they are inexpensive and can be used for stenoses with carinal involvement (Y shaped). Disadvantages include need for general anaesthesia and rigid bronchoscopy for insertion, higher risk of migration, interference with the mucociliary mechanisms, risk of mucous adherence and patency compromise, decreased inner diameter due to increased wall width, and inability to conform to tortuous deformities (5).
Covered self expanding metal stents have “hybrid” intermediate characteristics (5).
Uncovered metal stents are mainly used in malignant airway obstruction (13). Uncovered self expandable metallic stents provide a permanent source of structural support. Within a period of a few weeks the stent wires are gradually covered with mucosa and the stent becomes incorporated within the tracheal wall. Therefore uncovered metallic stents should be considered permanent and nonremovable. When removal is essential, uncovered metallic stents should not be inserted, in favour of silicone stents. Furthermore, uncovered metallic stents predispose to granulation, particularly at the points of maximal contact pressure. Granulation formation seems inevitable, through the very same mechanism that led to the initial lesion (7). In the presence of inflammation a silicone prosthesis is preferable (16).
Uncovered self-expanding metal stents inserted in benign inflammatory airway stenoses (including postintubation tracheal stenosis) may cause lengthening of the initial damage by stricture and granulation formation within previously normal airway, subglottic strictures, and bronchoesophageal fistula (7,17). The lesions can be severe and occur fast after stenting (17). The ensuing tracheal stenosis is difficult to manage (even with palliative laser), bronchoscopic stent removal is often impossible, and surgical removal through a linear tracheal incision is often difficult (7). This elongation and deterioration of the lesion may preclude definite surgical treatment or require more extensive resection. Thus, this generation of uncovered metal stents should be avoided in benign tracheal stenosis (3,7,17).
A silicone T-tube has been used for temporary or permanent tracheal stenting (18,19) and silicone tracheal stents evolved from this (7). Temporary tracheal stenting may allow a safer curative resection at a later stage; after resolution of the local inflammation, improvement in clinical status, and weaning from steroids (3). Thus silicone tracheal stenting may be used as a bridge to operation.
Bridging to operation or other therapies with uncovered metallic tracheal stents is not recommended, due to the difficult or risky removal (3,7,20). Dutau et al. (20) commented that one of the reasons of the popularity of the metal stents (21,22) is that they can be inserted without rigid bronchoscopy, but in his opinion stent placement and removal through rigid bronchoscopy is safer and faster, irrespective of the type of the stent.
The Food and Drug Administration (FDA) recommendation in 2005 stated that use of metallic stents to treat benign tracheal stenoses should be considered only after thorough exploration of all other treatment options, such as surgical treatment or insertion of silicone stents (20).
In patients with significant postintubation tracheal stenosis for whom surgery is not initially feasible the treatment of choice is bronchscopic insertion of silicone stents, usually after dilatation (laser, ballooning or bougienage) (3,8,20,23).
Apart from bridging to operation (or other therapeutic modalities), the silicone tracheal stents can be used for curative tracheal reconstruction (bridge to recovery), and as a definitive treatment (long term palliative treatment) (3,8,20,24).
Martinez-Ballarin et al. (24) reported tracheal stenting with silicone Dumon stents in 64 patients with fibrous or fibroinflammatory benign tracheal stenosis, mainly postintubation or post-tracheostomy. The stents were well tolerated. Complications included migration (17.5%), granulation (6.3%), and airway obstruction due to mucostasis (6.3%). One patient died during urgent tracheostomy due to mucous obstruction. Eleven patients who underwent permanent palliative silicone stenting were follow-up for 486+/-260 days. Among 48 patients in whom tracheal silicone stents were inserted for curative restoration, stent removal was accomplished in 43.75%. Among them, therapy was successful in 81%, while restenosis after stent removal occurred in 19%. Thus successful long term curative restoration was achieved in 35.4% of patients in whom the stent was inserted with curative intention (17/48), and in 26.5% of all patients (17/64) (24).
Puma et al. (3) reported tracheal silicone stenting (Montgomery T-tube, Dumon stent, Dynamic Y stent) in 45 patients with cicatricial stenoses (mild, with residual lumen >50%, moderate, with residual lumen 30-50%; and sever with residual lumen <30%, and a length of 2 to 6 cm). The stents were well tolerated. Nine patients (20%) were bridged to surgery at a median interval of 9 months (4-67 months). Among 37 patients in whom the stent was inserted as a “definitive” treatment (for palliation), the stent was removed in 14 patients (37.83%) (since the authors found, to their surprise, that long-term stent removal was possible). Among patients in whom the stent was removed, in 4 patients a new stent was positioned, within 6 weeks, due to restenosis, while stent removal was successful in 10 patients, in whom the T-tube (n=7) or the Dumon stent (n=3) were permanently removed, after a median time of 32 months (range, 8-70 months). Thus, curative restoration of trachea was achieved with silicone stenting in 22% of all patients, and in 27% of patients in whom the stent was inserted as “definitive” treatment. Although totally normal tracheal lumen was not achieved, the residual stricture was well tolerated, due to its limited degree (6/10) or to the limited patients’ physical activity (4/10) (3).
Park et al. (23) reported insertion of a silicon stent (Natural stent) in 32 patients with postitubation tracheal stenosis. Sixteen per cent of patients were bridged to surgery after initial stabilization. The stent was successfully removed without restenosis in 38% of patients, after a median time of 7 months. The stent could not be removed or required reinsertion in 31% of all patients. Late complications were restenosis (40%), granulation (38%), migration (34%), and mucostasis (31%), at a median follow up of 22 months (23).
Lim et al. (8) reported silicon stent insertion (Natural stent) in 55 patients with postintubation tracheal stenosis [including 22 patients that were also included in the study by Park et al. (23)]. The stent was removed in 93% of patients (in all patients except those who were lost to follow up). Among them, successful stent removal without restenosis was achieved in 40% (22/55) after a median time of 12 months. Restenosis occurred in the remaining 60% (33/55), treated with permanent stent insertion (23/33) or surgery (10/33). Thus, among all patients for whom long term data is available, successful removal (curative reconstruction) was achieved in 40%, bridging to surgery in 18%, and definitive stenting in 42%. Multivariate logistic regression analysis showed that absence of cardiovascular disease and early stenting (within 6 months postintubation) were independent predictors of successful stent removal.
Thus, not all postintubation stenoses are amenable to curative stenting treatment. Furthermore the time required for tracheal recovery, and the criteria for stent removal have not been defined. In 22% to 40% of patients stent removal was successful (without restenosis) after a mean period of stenting of 7 to 32 months (3,8,23,24). According to Puma et al. (3) superficial mucosal injuries with granulation are more likely to improve by stenting with a highly tolerated material (such as silicone), while recovery is less likely to occur in full-thickness lesions with destruction of tracheal cartilages. It has been recommend to keep the stents in place for at least 6-12, or 18 months. The required stenting period probably depends on the degree of the tracheal lesion; full thickness lesions require longer periods for stabilization, if this can be achieved. Puma et al. (3) believe that in severe circumferential stenoses with tracheomalacia at least 2 years of stenting is required. Decision making can be based on clinical and endoscopic criteria. The performance status should be considered. A residual tracheal stricture may be tolerated by patients with decreased physical activity. Epithelialization, residual stenosis and tracheomalacia should be carefully evaluated. Examination with a fiberoptic bronchoscope after stent removal is essential for evaluating residual tracheomalacia and the degree of residual stenosis. Initial patency should not be considered definitive, since the stricture may gradually relapse (3,7). Scheduled follow-up and monitoring with sequential video recorded endoscopy is essential. Long term restenting (for 1-2 years) after initial restenosis following stent removal has been attempted with curative results (3).
Both our patients were heavily symptomatic. The first patient suffered from severe debilitating dyspnoea with inspiratory stridor after the initial surgical correction (resection of post-intubation stenosis and end-to-end anastomosis), being only moderately and temporally relieved by multiple bronchoscopic interventions. Florid granulation was noted at the anastomotic site early postoperatively and after all bronchoscopic interventions. The inflammatory nature of the initial disease that required prolonged intubation, and formation of a large cheloid at the surgical wound were taken into consideration. Re-operation has been refused in the centre where the initial operation was performed and elsewhere due to the presence of fibroinflammatory lesion. Reoperation was identified as a strong predictor of postoperative anastomotic complications [odds ratio (OR): 3.03, 95% confidence interval (CI): 1.69-5.43, P=0.002] (12).
The second patient suffered respiratory arrest on displacement of a T-tube (treated with urgent insertion of a tracheostomy tube), had a long (4 cm) and tight stenosis involving the lower subglottic larynx, infection of the tracheostomy site, and a poor neurological status. A poor neurological status is a common general contraindication to initial operation (3,8). Lengthy (> or =4 cm) resections, laryngotracheal resection, and need for tracheostomy before operation have been identified as predictors of postoperative anastomotic complications, [with odds ratios (OR) and confidence intervals (CI) as follows (respectively): OR 2.01, 95% CI, 1.21-3.35, P=0.007, OR 1.80, 95% CI 1.07-3.01, P=0.03, and OR 1.79, 95% CI, 1.03-3.14, P=0.04].
According to literature review and expert opinion, placement of silicone stents is the treatment of choice in benign tracheal stenoses non initially amenable to surgery, particularly in the presence of granulation and inflammation, and when removal is desirable. Among various silicone stents, the Dumon stent is the most widely used (13) being considered as the gold standard (20).
In both our patients the airway was immediately restored and the patency is maintained 15 and 10 months after insertion, without complications, such as stent migration, mucostasis, granulation at the ends of the stents, or infection. The stents are well tolerated. Proper stent sizing may have accounted for the good results. Insertion of a small diameter prosthesis may predispose to migration, while insertion of a large diameter prosthesis may predispose to granulation due to pressure injury. Mucostasis was avoided due to good clinical status (absence of pulmonary disease, adequate coughing, absence of cachexia, young age) facilitated by water nebulizations.
Thus, patient selection and stent selection are deemed justified. Good palliation has been provided. The challenge that still remains is stent removal without long term restenosis (bridge to recovery). Nevertheless if stent removal is not associated with a curative restoration, bridging to surgery, to re-stenting, or to future treatments are remaining options.
The technology of stents is continuously evolving. Covered self expandable metallic stents are under clinical evaluation for benign indications. The absence of a metallic wire in direct contact with the tracheal wall decreases the local pressure (and thus the potential ischaemic injury, decreasing the risk of granulation and restenosis) and allows easier removal. This could be the future of metallic stents (20). After experimental evaluation, biodegradable stents have been recently clinically applied in a pilot study in children (15,25). Until new technology becomes available to the clinical armamentarium, the use of silicone stents is a good treatment for initially not amenable to surgery benign tracheal lesions, as a curative treatment, a bridge to surgery, a palliation, a bridge to restenting, and furthermore a bridge to future treatments.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Wain JC, Postintubation tracheal stenosis. Chest Surg Clin N Am. 2003;231-46. [PubMed ]
- Grillo HC, Donahue DM, Post intubation tracheal stenosis. Semin Thorac Cardiovasc Surg. 1996;370-80. [PubMed ]
- Puma F, Ragusa M, Avenia N, The role of silicone stents in the treatment of cicatricial tracheal stenoses. J Thorac Cardiovasc Surg. 2000;1064-9. [PubMed ]
- Wood DE, Liu YH, Vallières E, Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg. 2003;167-72. [PubMed ]
- Chin CS, Litle V, Yun J, Airway stents. Ann Thorac Surg. 2008;S792-6. [PubMed ]
- Phillips MJ, Stenting therapy for stenosing airway diseases. Respirology. 1998;215-9. [PubMed ]
- Grillo HC, Stents and sense. Ann Thorac Surg. 2000;1142. [PubMed ]
- Lim SY, Kim H, Jeon K, Prognostic factors for endotracheal silicone stenting in the management of inoperable post-intubation tracheal stenosis. Yonsei Med J. 2012;565-70. [PubMed ]
- Grillo HC, Donahue DM, Mathisen DJ, Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg. 1995;486-92. [PubMed ]
- Grillo HC, Mathisen DJ, Wain JC, Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg. 1992;54-63. [PubMed ]
- Donahue DM, Grillo HC, Wain JC, Reoperative tracheal resection and reconstruction for unsuccessful repair of postintubation stenosis. J Thorac Cardiovasc Surg. 1997;934-8. [PubMed ]
- Wright CD, Grillo HC, Wain JC, Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg. 2004;731-9. [PubMed ]
- Kim H., Stenting therapy for stenosing airway disease. Respirology. 1998;221-8. [PubMed ]
- McGrath EE, Warriner D, Anderson P, The insertion of self expanding metal stents with flexible bronchoscopy under sedation for malignant tracheobronchial stenosis: a single-center retrospective analysis. Arch Bronconeumol. 2012;43-8. [PubMed ]
- Rafanan AL, Mehta AC, Stenting of the tracheobronchial tree. Radiol Clin North Am. 2000;395-408. [PubMed ]
- Nesbitt JC, Carrasco H, Expandable stents. Chest Surg Clin N Am. 1996;305-28. [PubMed ]
- Gaissert HA, Grillo HC, Wright CD, Complication of benign tracheobronchial strictures by self-expanding metal stents. J Thorac Cardiovasc Surg. 2003;744-7. [PubMed ]
- Cooper JD, Pearson FG, Patterson GA, Use of silicone stents in the management of airway problems. Ann Thorac Surg. 1989;371-8. [PubMed ]
- Gaissert HA, Grillo HC, Mathisen DJ, Temporary and permanent restoration of airway continuity with the tracheal T-tube. J Thorac Cardiovasc Surg. 1994;600-6. [PubMed ]
- Dutau H., Airway stenting for benign tracheal stenosis: what is really behind the choice of the stent?. Eur J Cardiothorac Surg. 2011;924-5. [PubMed ]
- Charokopos N, Foroulis CN, Rouska E, The management of post-intubation tracheal stenoses with self-expandable stents: early and long-term results in 11 cases. Eur J Cardiothorac Surg. 2011;919-24. [PubMed ]
- Eller RL, Livingston WJ, Morgan CE, Expandable tracheal stenting for benign disease: worth the complications?. Ann Otol Rhinol Laryngol. 2006;247-52. [PubMed ]
- Park HY, Kim H, Koh WJ, Natural stent in the management of post-intubation tracheal stenosis. Respirology. 2009;583-8. [PubMed ]
- Martinez-Ballarin JI, Diaz-Jimenez JP, Castro MJ, Silicone stents in the management of benign tracheobronchial stenoses. Tolerance and early results in 63 patients. Chest. 1996;626-9. [PubMed ]
- Vondrys D, Elliott MJ, McLaren CA, First experience with biodegradable airway stents in children. Ann Thorac Surg. 2011;1870-4. [PubMed ]


