Primary lung carcinoid, a rare cause of paraparesis: report of a case and review of the literature
Introduction
The term carcinoid is used to describe a wide spectrum of tumors derived from various types of neuroendocrine cells, presenting with an unusual and complex disease spectrum (1). Carcinoids can originate from any site in the abdomen and thorax (1), being usually described as originating from the foregut, the midgut, and the hindgut derivatives (2). Carcinoids are relatively rare tumors with absent, obscure, or non-specific symptomatology (1,3,4). Although carcinoids were generally considered to be in their majority benign tumors, with a slow growth, a significant proportion demonstrates aggressive tumour growth (1,3,4).
It appears that the overall incidence of carcinoids has increased over the past 30 years (1,2,5). Improved diagnostic means and disease awareness may have partially accounted to this (1,2,5). According to a large epidemiological study (1) (United States-based databases, 1950-1999, 13,715 carcinoid tumors) the sites of the greatest carcinoid recurrence were the gastrointestinal tract (67.5%) and the bronchopulmonary tree (25.3%). Among the gastrointestinal carcinoids, most tumors occurred in the small intestine (41.8%), the rectum (27.4%), and the stomach (8.7%) (1). The incidence of all site carcinoids was highest in black men (4.48 per 100,000 population per year) (1). Distant metastases were evident at the time of diagnosis in 12.9% of all patients with carcinoid tumors (1). The overall 5-year survival (regardless of site) was 67.2% (1). The highest 5-year survival was noted in patients with rectal (88%), bronchopulmonary (73.5%), and appendiceal (71%) carcinoids; (invasive growth or metastatic spread was exhibited in 3.9%, 27.5%, and 38.8% of the patients, respectively) (1). In a previous large scale epidemiological study (United States-based databases, 1973-1991, 8,305 cases) metastases were evident at diagnosis in 45% of patients, and the overall 5-year survival was 50% (5). Associated non carcinoid tumors were frequently found in conjunction with carcinoids of the gastrointestinal track [small intestinal (29%), gastric (20.5%), colonic (20%), appendicial (18.2%)]. This was attributed to the production by the carcinoid of proliferative peptides that enhance the development or growth of other neoplasia (1). These findings brought into question the relative benignity of carcinoid disease (1). The presence of local invasive growth and/or regional or distant carcinoid metastases are the best indicators of malignancy and prognosis, although proliferation markers and cell nuclear antigen detection have been used (1).
In about 10% of patients, carcinoids secrete bioactive mediators. The ability of carcinoids to cause clinical symptoms by hormone or biogenic amine secretion is best recognised in the carcinoid syndrome (4). Apart from this, Cushing’s syndrome and various paraneoplastic neurological syndromes associated with carcinoids have been reported. We report a rare case of a primary lung carcinoid presented with paraparesis (due to bilateral symmetrical polyneuropathy) that was completely resolved after surgical resection (Video 1).
Magnetic resonance imaging of the lumbar spine showed marked deformities of the bodies of the 12th thoracic, the 1st and the 3rd lumbar vertebrae (attributed to stable bone fractures), with bone protrusion of the deformed vertebral bodies, and a degree of intervertebral disc bulging (mainly L3-L4, and to a lesser extent L4-L5). The resulting spinal stenosis and compression of the meningeal sac were not considered severe. The vertebral arches appeared normal and compression of the nerve roots was not revealed (Figure 2).
Case report
A 69 year old man developed progressively deteriorating bilateral proximal muscular weakness of the legs that within 6 months led to paraparesis, with complete inability to stand up and walk. The patient did not complain for back or leg pain, and had preserved sensation. Electromyography showed motor polyneuropathy.
History, clinical and laboratory data excluded the presence of diabetes mellitus, hypothyroidism, renal or liver failure, cachexia, alcohol abuse, chemotherapy, drug or other toxicity. There was a history of fall and thoracic trauma, 3 months after the first presentation of muscular weakness that was not associated with acute exaggeration or acute change of the symptomatology. Chest radiography was non revealing (Figure 1). Cardiac echocardiography showed mild global hypokinesis and diastolic dysfunction of the left ventricle, with a left ventricular ejection fraction of 50%, and mild insufficiency of the tricuspid and the mitral valve.
Magnetic resonance imaging of the lumbar spine showed marked deformities of the bodies of the 12th thoracic, the 1st and the 3rd lumbar vertebrae (attributed to stable bone fractures), with bone protrusion of the deformed vertebral bodies, and a degree of intervertebral disc bulging (mainly L3-L4, and to a lesser extent L4-L5). The resulting spinal stenosis and compression of the meningeal sac were not considered severe. The vertebral arches appeared normal and compression of the nerve roots was not revealed (Figure 2).
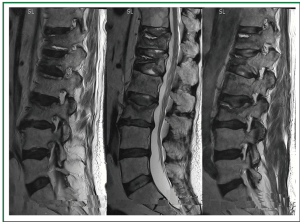 Figure 2. Lumbar spine magnetic resonance imaging (MRI). Marked deformities of the bodies of the 12 thoracic, the 1 and the 3 lumbar vertebrae with bone protrusion of the deformed vertebral bodies (attributed to stable fractures), and a degree of intervertebral disc bulging (mainly L3-L4, and to a lesser extent L4-L5). The resulting spinal stenosis and compression of the meningeal sac were not considered severe. The vertebral arches appeared normal and compression of the nerve roots was not revealed.
Figure 2. Lumbar spine magnetic resonance imaging (MRI). Marked deformities of the bodies of the 12 thoracic, the 1 and the 3 lumbar vertebrae with bone protrusion of the deformed vertebral bodies (attributed to stable fractures), and a degree of intervertebral disc bulging (mainly L3-L4, and to a lesser extent L4-L5). The resulting spinal stenosis and compression of the meningeal sac were not considered severe. The vertebral arches appeared normal and compression of the nerve roots was not revealed.
A paraneoplastic syndrome was suspected and tumor markers were examined. Carcinoembryonic antigen (CEA), α-fetoprotein (AFP), Prostate-specific antigen (PSA), Cancer Antigen 15-3 (CA 15-3) were within normal ranges. The Cancer Antigen 19-9 (CA 19-9) was increased to 118 U/mL (normal range, 0-37 U/mL).
On abdominal computed tomography there was no evidence of abdominal mass or enlarged lymph nodes. Thoracic computed tomography revealed a small peripheral paracardiac right-middle lobe tumor with a maximal diameter of 1.8 cm (Figure 3)
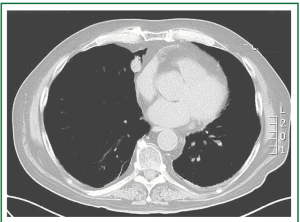 Figure 3. Thoracic computed tomography revealed a small peripheral paracardiac right-middle lobe tumor with a maximal diameter of 1.8 cm.
Figure 3. Thoracic computed tomography revealed a small peripheral paracardiac right-middle lobe tumor with a maximal diameter of 1.8 cm.
A 3-port thoracoscopic wedged resection was performed with an endo-stapler. Histology revealed lung carcinoid of low mitotic index (1-2%) with free of malignancy resection margin. The patient had an uneventful recovery (Figure 4). He was discharged on the 5th postoperative day to a rehabilitation centre, where he remained for 3 months. Progressive motor function improvement was noted and the paraparesis resolved completely within 6 months. At 6-, 12-, and 18-month follow up normal respiratory and neurological status was noted, without evidence of carcinoid recurrence or metastasis (Figure 5).
Discussion
Forty year ago (in 1972), Ross (6) had pointed out the endocrine, metabolic, and neurological manifestations of cancer. He described systemic abnormalities and clinical syndromes caused by cancer. He indicated that the symptoms observed in malignant diseases are not confined to the structural and/or functional impairment of organs and tissues caused by the presence of locally enlarging masses. The several and in many situations inexplicable manifestations may result from the metabolic and endocrine activity of the tumor (6).
Carcinoid syndrome
The endocrine activity of some carcinoids results in carcinoid syndrome. The clinical presentation of the carcinoid syndrome includes flushing (in 94% of patients), diarrhea (in 78%), abdominal cramps (in 51%), cardiac complications (in 37%), telangiectasia (in 25%), bronchoconstriction, cyanosis, and edema (in 17-18%), and proximal myopathy, pigmentation, and arthritis (in about 7%). Hypertension and diabetes were found in 45.9% and 9.7% of patients, respectively (7).
Ross (6) differentiated the metastatic and endocrine activity of carcinoids originating from the mid gut derivatives (such as the small intestine, the caecun and the appendix) to that of carcinoids originating from the fore gut derivatives (such as the broncopulmonary tree) (6). Allthough carcinoids can metastasize to practically any organ, malignant intestinal carcinoids metastasize more commonly to the liver, while malignant bronchopulmonary carcinoids metastasize more commonly to the skin and bone (6). Ross differentiated the typical carcinoid syndrome produced by malignant argentaffin carcinoid tumours originating from the mid gut derivatives to the carcinoid syndrome produced by carcinoids originating from the fore gut derivatives. Intestinal carcinoids with endocrine activity secrete 5-hydroxytryptamine (serotonin) to the portal circulation, causing episodes of abdominal cramps, watery diarrhoea, bronchospasm, face and chest cyanotic flushes, and right-sided stenotic cardiac lesions. (5-hydroxyindoleacetic acid is detected in the urine) (6).
Bronchopulmonary carcinoids with endocrine activity secrete histamine and 5-hydroxytryptophan (lacking the decarboxylase to convert it into 5-hydroxytryptamine) to the systemic circulation, causing episodes of red facial flush/oedema, salivation, lachrymation, and potentially tachycardia, fever, anxiety, tremor, hypotension, and left-sided cardiac lesions. (Histamine, 5-hydroxytryptophan, and 5-hydroxytryptamine are detected in the urine) (6). Similar biochemical findings have also been reported by Hobbs et al. (8).
Cushing’s syndrome produced by ACTH-releasing bronchopulmonary and thymic carcinoids
Extrapituitary adrenocorticotropic hormone (ACTH) producing neoplasias can cause Cushing’s syndrome, the common clinical features of which include proximal myopathy, muscle weakness, and fatigue (9). Bronchopulmonary and thymic carcinoids have been implicated in ectopic ACTH production, leading to the stimulation of the adrenal glands, cortisol hypersecretion and Cushing’s syndrome, an almost universal finding of which is proximal muscle weakness, usually affecting the pelvic girdle muscles (10).
Bronchopulmonary carcinoid tumors are one of the most common causes of ectopic ACTH secretion (11), and the most common tumors implicated in ectopic ACTH-dependent Cushing’s syndrome presentation (12), accounting for approximately 1% of all the Cushing’s syndrome patients (11). The possibility of a lung carcinoid should be considered in patients with Cushing’s syndrome and bilateral adrenal enlargement (13). “Asthenia” was reported in a patient with bronchopulmonary carcinoid related Cushing’s syndrome (11) and complete resolution of the Cushing’s syndrome was reported after surgical resection of bronchopulmonary carcinoids (11,14).
Proximal dominant muscle weakness attributed to steroid myopathy was reported in a patient with an ACTH releasing thymic carcinoid (15). ACTH secretion by a thymic carcinoid and Cushing’s syndrome has been reported in a patient, who succumbed to the malignancy (16). Cushing’s syndrome with bilateral adrenal hyperplasia associated with a carcinoid thymic tumor has also been reported in a patient, who became asymptomatic after surgical resection of the carcinoid (17).
Neurological complications of cancer, and paraneoplastic neurological syndromes (PNS)
In 1972, Ross (6) reported that lung cancer often presents with neurological symptoms that are often disabling. He reported encephalopathies, myelopathies, neuropathies, and myasthenic syndromes (myopathies: polymyositis, myasthenia, dermatomyositis), as neurological complications of cancer. He reported carcinomatous encephalitis or cerebellar degeneration (accompanied or not by dementia) in patients without cerebral metastases. Ross reported that the most frequent neurological complications were mixed peripheral neuropathy, myopathy, or a mixed syndrome, and described a central distribution myopathy, with symmetrical muscle wasting (6). He reported that the cause of carcinomatous encephalopathy and neuromyopathy was then unknown, and being tempted to ascribe it to a humoral cause he reported the presence of anti-brain antibodies detected in cancer patients with sensory neuropathy (6). According to Ross, neoplasms usually involved with neurological complications were bronchus, breast, cervix, ovary and colon neoplasms (6).
Paraneoplastic neurological syndromes can be defined as the remote effects of cancer, not caused directly by the tumor itself or its metastases, or by infection, ischemia or metabolic disruptions (18). Paraneoplastic neurological syndromes (PNS) are rare (affecting <1/10,000 patients with cancer (18), imperfectly understood, and frequently underdiagnosed entities (19,20). PNS are caused by autoimmune processes that are triggered by the cancer and are directed against antigens that can be found to both cancer and the nervous system (18). Thus PNS can be roughly described “as caused by an immune response to an underlying malignanc” (19) that although is produced as a reaction to cancer antigens, attacks the nervous system through a cross-reaction to self-antigens (auto-antigens) (21). These common antigens expressed into the tumor and in the patient’s normal neurons are defined as onconeural antigens (18,20). Antibodies to onconeural antigens, defined as onconeural antibodies (22) or anti-onconeural antibodies (18), are found in the serum of many patients with PNS, being highly specific markers of the paraneoplastic aetiology (20,22). The onconeural antibodies, produced as an immune response to cancer, act as autoantibodies targeting intracellular antigens, in contrast with autoantibodies non associated with cancer that are targeting the cellular membrane antigens (22-24).
There are various tumors associated with PNS, in particular small cell lung cancer, breast cancer, ovarian cancer, and thymoma (20).
PNS include a highly heterogeneous group of neurological disorders affecting any part of the central nervous system, the peripheral nervous system, the neuromuscular junction, or the muscles (18), while they can often affect several areas of the nervous system (20). The most common PNS are: Lambert-Eaton myasthenic syndrome (occurring in about 1% of small cell lung cancer patients), subacute cerebellar ataxia, cerebellar degeneration, limbic encephalitis, encephalomyelitis, opsoclonus-myoclonus, retinopathies, stiff-person syndrome, chronic gastrointestinal pseudoobstruction, (subacute) sensory neuronopathy, and dermatomyositis (18-20). PNS are usually severely disabling (18).
Definite diagnosis of a neurological syndrome as paraneoplastic is made by identification in the patient’s serum of one of the well-characterized onconeural antibodies (anti-Hu, Yo, Ri, CV2, CRMP5, amphiphysin, Ma2 and Tr) (18,22). These onconeural antibodies, characterizing a restricted range of malignancies, can guide the search for occult cancer (18). About 30% of patients do not have detectable onconeural antibodies, while 5-10% have an atypical (not well-characterized) antibody (18). Absence of detectable onconeural antibodies does not rule out the PNS diagnosis (19,22).
Paraneoplastic neurological syndromes often precede the diagnosis of cancer, and PNS clinical recognition should lead to immediate search for occult cancer, even in the absence of detectable antibodies (18-20).
Paraneoplastic peripheral neuropathies have been classified as “definite” or “possible” according to the presence of “classical” paraneoplastic neuropathic disorders, the detection of onconeural antibodies, the time-interval between tumor and neuropathy occurrence, and the improvement after tumor treatment” (24).
Subacute sensory neuronopathy (resulting from inflammatory damage of the cell bodies of the sensory neurons in the dorsal root ganglia) is frequently associated with onconeuronal antibodies and is considered a classical paraneoplastic disorder (25,26). Encephalomyelitis or motor nerve involvement (of unclear mechanism) may also be found (26). Other heterogenous neuropathies having variable clinical features and no association to onconeuronal antibodies have been considered as non classical paraneoplastic neurological syndromes (25) or possible paraneoplastic neuropathies (24).
The best treatment of PNS is the treatment of the underlying cancer as soon as possible (18). Immunosuppressive treatment may be considered. The prognosis varies, depending on the degree of the neuronal lesions (20).
Paraneoplastic syndromes and neuropathies associated with carcinoids
Paraneoplastic neurological syndromes are remote effects of cancer (mainly small cell lung cancer, gynaecological cancer, and lymphomas). In a few studies the association of carcinoid tumors to neurological syndromes is reported, but it mainly involves serotonin-related myopathy (27). Tschernatsch et al. (27) reported 4 cases of paraneoplastic neurological syndromes associated with carcinoid. The carcinoid-related neurological syndromes were sensory neuropathy, myelopathy, limbic encephalitis, and brain stem encephalitis. Antineuronal autoantibodies were found in 2 patients (one anti-Hu, one anti-Yo), antinuclear antibodies were found in 1, while in 1 patient no autoantibodies were detected. Expression of HuD in the tumour was demonstrated in 1 patient. This study demonstrated that carcinoids can be associated with the classical antineuronal antibody-associated paraneoplastic neurological syndromes (PNS) (27).
A carcinoid-like well-differentiated breast carcinoma (with metastases to the liver spleen and lung), systemic amyloidosis (involving the liver, spleen, heart and kidneys), axonal neuropathy and neurogenic peripheral muscular atrophy (without amyloid deposition) were discovered at autopsy of a patient (who died in septic shock after a urinary infection) who had presented neurological findings of progressive axonal sensorimotor polyneuropathy (weakness and paraesthesias in both legs) (28).
Visceral neuropathy of the myenteric plexus has been reported in patients with bronchopulmonary carcinoid. Paraneoplastic visceral neuropathy (including achalasia, gastroparesis, functional stenosis of the sphincter of Oddi [obstructive jaundice), intestinal pseudoobstruction (subileus), constipation] was reported in a patient with metastasised atypical bronchial carcinoid. After surgical treatment, histology revealed lymphocytic infiltration of the myenteric plexus, with loss of neurones. The presence of positive rheumatoid factor, and circulating immune complexes and antibodies suggested a possible autoimmune pathogenesis (29). Severe gastrointestinal motor dysfunction due to visceral neuropathy of the myenteric plexus was reported as a paraneoplastic effect in 6 patients with small cell carcinoma and 1 patient with pulmonary carcinoid. In addition to intestinal pseudoobstruction and obstipation/constipation, 2 patients had peripheral neuropathy, autonomic insufficiency, and neurogenic bladders. Post-mortem or surgical sample examinations showed neuron and axon degeneration and dropout, decreased neuron numbers, lymphoplasmacytic infiltration, and glial cell proliferation in the myenteric plexus (30).
Paraneoplastic vasculitic neuropathy and ischaemic neuropathies have been reported in patients with carcinoid tumors (31). Bilateral lower extremity ischemic neuropathy, (severe pain and hyperesthesias in the legs, dermal fibrosis, and digital gangrene of the feet) associated with vasospasm (partially relieved by nifedipine and lumbar sympathectomy) was reported in a patient with carcinoid syndrome. It was postulated that serotonin and other vasoconstrictors secreted from the tumor might had been responsible for the vasospasm and the ischemic neuropathy (32). Recurrent bilateral facial palsy, followed by nonarteritic ischemic optic neuropathy was reported in a patient with thymic carcinoid metastasized to the mediastinum (33).
Development of nephrotic syndrome in conjunction with a malignancy-related neuropathy was described in a patient with metastatic bronchial carcinoid tumor. Both syndromes were considered paraneoplastic (34).
Neuropathies have been reported in patients with recurrent and metastatic carcinoids who received potentially neurotoxic treatment. Axonal length dependent polyneuropathy of both motor and sensory fibers was found on clinical and electrophysiological examination of a patient (with feet numbness and tingling, and negative anti-Hu antibody) during epothilone B treatment for recurrent carcinoid. The patient had been enrolled in a dose escalation trial, the polyneuropathy was attributed to epothilone neurotoxicity (35). Epothilones are used for the treatment of resistant cancer and are known to cause toxic peripheral neuropathy. The incidence of epothilone-induced peripheral neuropathy is considered related to dose and pre-existing neuropathy (36). Peripheral sensory neuropathy has been reported as a common adverse event of bortezomib treatment in patients with metastatic neuroendocrine tumors (carcinoid and islet cell). (Bortezomib is a proteasome inhibitor used in the treatment of multiple myeloma and other cancers) The association of the autonomic neuropathy with bortezomib has not been clarified (37).
Mononeuropathies due to compression from metastatic carcinoid or due to intranerval carcinoid metastasis have been reported. Left optic neuropathy that improved after partial decompression of the left orbit was reported in a patient with primary lung carcinoid metastasized to the left orbit and the inferior occipital lobe (38).
There is a case report of carcinoid metastasis to the muscolocutaneous nerve causing biceps brachii weakness and arm pain 10 years after treatment for primary carcinoid, followed by intrafascicular carcinoid metastasis in the ulnar nerve causing numbness and pain several months later. Surgical resection of metastases was performed accordingly. This report showed that multiple mononeuropathies (resembling polyneuropathy) may be caused by intranerval carcinoid metastasis (39).
Paraparesis of acute -onset was reported in a patient 40 years after thoracotomy for a carcinoid adenoma. No neoplastic origin could be found. Paraparesis was attributed to the inflammatory contents of the pneumonectomy cavity (40).
Conclusions
Proximal myopathy is included in the features of carcinoid syndrome occurring in about 7% of patients with it. Proximal myopathy is an almost universal finding in Cushing’s syndrome, and bronchopulmonary carcinoids represent the commonest site of ectopic ACTH production, being the cause of Cushing’s syndrome in 1% of patients presenting this syndrome (7,9,10,41). Myopathy has been reported in patients with ACTH producing bronchopulmonary or thymic carcinoids. Paraneoplastic neurological syndromes including sensory neuropathy, sensorimotor polyneuropathy, and splanchnic neuropathies have been rarely reported in patients with carcinoids. Sensory or sensorimotor neuropathies have been reported in patients with metastatic carcinoid treated with drugs known to cause toxic neuropathies. There are case reports of mononeuropathies due to external nerve compression or intranerval growth of metastatic carcinoid.
In the case we report hereby, the diagnosis of the paraneoplastic neurological syndrome preceded the diagnosis of carcinoid. Progressively deteriorating leg muscular weakness was the only symptom. Sensory function was preserved and electromyography showed motor polyneuropathy. There were no clinical features of carcinoid syndrome or Cushing’s syndrome. We have not searched for onconeural antibodies. Thoracic CT showed the presence of a small tumor (maximal diameter of 1.8 cm) that was resected as soon as it was revealed. Histology showed carcinoid tumor. The gradual and complete resolution of the paraparesis after resection of the pulmonary carcinoid suggests paraneoplastic subacute motor polyneuropathy associated with primary bronchopulmonary carcinoid that to the best of our knowledge has not been previously reported.
Acknowledgements
Disclosure: The authors declare no conflict of interest
References
- Modlin IM, Lye KD, Kidd M, A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;934-59. [PubMed ]
- Zuetenhorst JM, Taal BG, Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;123-31. [PubMed ]
- Caplin ME, Buscombe JR, Hilson AJ, Carcinoid tumour. Lancet. 1998;799-805. [PubMed ]
- McStay MK, Caplin ME, Carcinoid tumour. Minerva Med. 2002;389-401. [PubMed ]
- Modlin IM, Sandor A, An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;813-29. [PubMed ]
- Ross EJ, Endocrine and metabolic manifestations of cancer. Br Med J. 1972;293-4. [PubMed ]
- Klibanski A, Melmed S, Clemmons DR, The endocrine tumor summit 2008: appraising therapeutic approaches for acromegaly and carcinoid syndrome. Pituitary. 2010;266-86. [PubMed ]
- Hobbs CB, Miller AL, Review of endocrine syndromes associated with tumours of non-endocrine origin. J Clin Pathol. 1966;119-27. [PubMed ]
- Guaraldi F, Salvatori R., Cushing syndrome: maybe not so uncommon of an endocrine disease. J Am Board Fam Med. 2012;199-208. [PubMed ]
- Kendall-Taylor P, Turnbull DM, Endocrine myopathies. Br Med J (Clin Res Ed). 1983;705-8. [PubMed ]
- Scanagatta P, Montresor E, Pergher S, Cushing’s syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases. Chir Ital. 2004;63-70. [PubMed ]
- Sutton BJ, Parks GE, Manavi CK, Cushing’s syndrome and nocardiosis associated with a pulmonary carcinoid tumor: report of a case and review of the literature. Diagn Cytopathol. 2011;359-62. [PubMed ]
- Amer KM, Ibrahim NB, Forrester-Wood CP, Lung carcinoid related Cushing’s syndrome: report of three cases and review of the literature. Postgrad Med J. 2001;464-7. [PubMed ]
- de Matos LL, Trufelli DC, Cushing’s syndrome secondary to bronchopulmonary carcinoid tumor: report of two cases and literature review. Lung Cancer. 2006;381-6 , das Neves-Pereira JC, et al. [PubMed ]
- Idezuka J, Nakano R, Kuwabara T, A patient with an ACTH-releasing thymic carcinoid tumor presenting with proximal muscle weakness. No To Shinkei. 1998;380-2. [PubMed ]
- Muñoz Ruiz AI, Calvo Elipe A, Llorente Herrero E, An ACTH-secreting carcinoid tumor of the thymus. A report of a new case. An Med Interna. 1995;189-91. [PubMed ]
- Claret C, Chillarón JJ, Flores JA, Carcinoid tumor of the thymus associated with Cushing’s syndrome and dysgeusia: case report and review of the literature. Endocrine. 2010;1-5. [PubMed ]
- Honnorat J, Antoine JC, Paraneoplastic neurological syndromes. Orphanet J Rare Dis. 2007;22-. [PubMed ]
- Braik T, Evans AT, Telfer M, Paraneoplastic neurological syndromes: unusual presentations of cancer. A practical review. Am J Med Sci. 2010;301-8. [PubMed ]
- Storstein A, Vedeler CA, Paraneoplastic neurological syndromes and onconeural antibodies: clinical and immunological aspects. Adv Clin Chem. 2007;143-85. [PubMed ]
- Inuzuka T, Hayashi Y, Kimura A., Pareneoplastic neurological syndrome --update. Rinsho Shinkeigaku. 2011;834-7. [PubMed ]
- Raspotnig M, Vedeler CA, Storstein A, Onconeural antibodies in patients with neurological symptoms: detection and clinical significance. Acta Neurol Scand Suppl. 2011;191:83-8. [PubMed ]
- Honnorat J, Viaccoz A., New concepts in paraneoplastic neurological syndromes. Rev Neurol (Paris). 2011;729-36. [PubMed ]
- Antoine JC, Paraneoplastic peripheral neuropathies. Rev Neurol (Paris). 2008;1068-72. [PubMed ]
- Mitsui Y, Kusunoki S., Neuropathy associated with paraneoplastic neurological syndrome. Brain Nerve. 2010;387-93. [PubMed ]
- Gazic B, Pisem J, Dolenc-Groselj L, Paraneoplastic encephalomyelitis/sensory motor peripheral neuropathy - an autopsy case study. Folia Neuropathol. 113-7. [PubMed ]
- Tschernatsch M, Dierkes C, Gerriets T, Paraneoplastic neurological syndromes in patients with carcinoid. Eur J Neurol. 2008;1390-4. [PubMed ]
- Krüger S, Kreft B, Heide W, Sensorimotor polyneuropathy and systemic amyloidosis as paraneoplastic symptoms of a carcinoid-like well differentiated carcinoma of the breast. Dtsch Med Wochenschr. 1998;179-84. [PubMed ]
- Gerl A, Storck M, Schalhorn A, Paraneoplastic chronic intestinal pseudoobstruction as a rare complication of bronchial carcinoid. Gut. 1992;1000-3. [PubMed ]
- Chinn JS, Schuffler MD, Paraneoplastic visceral neuropathy as a cause of severe gastrointestinal motor dysfunction. Gastroenterology. 1988;1279-86. [PubMed ]
- Ashok Muley S, Brown K, Parry GJ, Paraneoplastic vasculitic neuropathy related to carcinoid tumor. J Neurol. 2008;1085-7. [PubMed ]
- Kucuk O, Noskin G, Petersen K, Lower extremity vasospasm associated with ischemic neuropathy, dermal fibrosis, and digital gangrene in a patient with carcinoid syndrome. Cancer. 1988;1026-9. [PubMed ]
- Skibell BC, Hesse RJ, Recurrent facial palsy occurring with metastatic thymic carcinoid and nonarteritic ischemic optic neuropathy. Ophthal Plast Reconstr Surg. 2003;164-5. [PubMed ]
- Becker BN, Goldin G, Santos R, Carcinoid tumor and the nephrotic syndrome: a novel association between neoplasia and glomerular disease. South Med J. 1996;240-2. [PubMed ]
- Grewal RP, Axonal neuropathy caused by epothilone B. Neurol India. 2007;178-9. [PubMed ]
- Argyriou AA, Marmiroli P, Cavaletti G, Epothilone-induced peripheral neuropathy: a review of current knowledge. J Pain Symptom Manage. 2011;931-40. [PubMed ]
- Shah MH, Young D, Kindler HL, Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004;6111-8. [PubMed ]
- Wein FB, Perry JD, Miller NR, Pulmonary carcinoid tumor presenting with simultaneous orbital and intracranial metastases: value of transnasal endoscopic orbital biopsy and decompression. Orbit. 1999;267-72. [PubMed ]
- Grisold W, Piza-Katzer H, Jahn R, Intraneural nerve metastasis with multiple mononeuropathies. J Peripher Nerv Syst. 2000;163-7. [PubMed ]
- Heth JA, Howard MA, Rossi N, Paraparesis induced by inflammatory contents of a pneumonectomy cavity. Case report. J Neurosurg. 2000;1065-8. [PubMed ]
- Porpodis K, Karanikas M, Zarogoulidis P, A case of typical pulmonary carcinoid tumor treated with bronchoscopic therapy followed by lobectomy. J Multidiscip Healthc. 2012;47-51. [PubMed ]