
Qishen Yiqi dropping pills improve isoproterenol-induced cardiomyocyte hypertrophy by regulating X-inactive specific transcript (XIST) expression in rats
Introduction
Despite significant advances in diagnosis and treatment over the last 20 years, including the development of angiotensin receptor blockers (1,2) and left ventricular assist devices (3), chronic heart failure (CHF) remains a leading cause of death (4). Numerous studies have attempted to determine the etiology of and treatment for CHF by analyzing its underlying pathological mechanisms (5-7). The use of isoproterenol (ISO) in rat cardiomyocytes (H9c2) to construct a cardiac hypertrophy model, which can effectively promote cell apoptosis and the levels of cardiac enzymes such as lactate dehydrogenase (LDH) and creatine kinase (CK), and reduce cell growth (8-10), is a common means of studying the molecular mechanisms of CHF in vitro.
Traditional Chinese medicine (TCM) for the treatment of CHF has the advantages of low-toxicity side effects and improved quality of life (11,12). Qishen Yiqi dropping pills (QYDPs) are composed of Astragalus membranaceus, Salvia miltiorrhiza, notoginseng, and Lignum Dalbergiae odoriferae oil, which are widely used in China as drugs to combat cardiovascular diseases (13). However, similar to many other TCMs, such as Panax notoginseng (P. notoginseng) saponins (14,15) and Salvia miltiorrhiza (16), the mechanism of action of QYDPs remains unclear.
The dysregulation of long non-coding RNAs (lncRNAs) is closely related to the progression of CHF (17,18). Li et al. (19) found that overexpression (ov) of the lncRNA, X-inactive specific transcript (XIST), was detrimental to the treatment of hypoxia/reoxygenation-induced rat cardiomyocyte (H9c2) injury. In addition, the expression of XIST is closely related to oxidative stress and inflammation (20,21). Therefore, we speculated that XIST is involved in the process of CHF; however, its role and potential underlying mechanism remain unclear.
New evidence has suggested that oxidative stress (22) and inflammation (23) are closely associated with the development and progression of CHF. QYDPs exert excellent anti-inflammatory (24) and cardiotoxic effects (13). In this study, by regulating the expression of the lncRNA XIST, we explored the molecular mechanism through which QYDP protects H9c2 cells from ISO-induced immunity, lncRNA XIST as a new therapeutic target provided a theoretical basis for the treatment of CHF based on pharmacology and molecular biology. We present the following article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-606/rc).
Methods
Cell culture and transfection
H9c2 rat cardiomyocyte cells from the American Type Culture Collection (Manassas, VA, USA) were stored in Dulbecco’s modified Eagle’s medium [37 °C, 5% carbon dioxide (CO2)] containing 10% fetal bovine serum (FBS). Once the H9c2 cells reached 60–80% confluency, they were transfected with ov or small interfering (si) RNA XIST (100 nM) and their negative controls using Lipofectamine® 2000 (Invitrogen, Thermo Fisher Scientific, Inc., MA, USA) for 4 h. The H9c2 cells were pretreated with different concentrations of QYDPs (0, 10, 50, 100, 250, and 500 µg/mL) and then exposed to ISO (80 µM) for 48 h, as described in a previous study (25). XIST was synthesized by GenePharma Biotechnology Co., Ltd. (Shanghai, China), and the si-XIST sequences are listed in Table 1.
Table 1
| siRNA symbol | Forward oligonucleotide 5'-3' | Reverse oligonucleotide 5'-3' |
|---|---|---|
| si-XIST-1 | GAUUGUUAGUGUGAAUAAUUU | AUUAUUCACACUAACAAUCAU |
| si-XIST-2 | GCACGAUCAAGAAAUGUAAAC | UUACAUUUCUUGAUCGUGCUG |
| si-XIST-3 | GGAGAGAUGUGCCGAAUAAGA | UUAUUCGGCACAUCUCUCCUU |
| si-NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
siRNA, small interfering RNA; XIST, X-inactive specific transcript; NC, negative control.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from H9c2 cells using TRIzol reagent (Invitrogen). After centrifugation (4 °C; 10 min) at 13,000 ×g, the supernatant was adsorbed and discarded, and 10 µL diethyl pyrocarbonate (DEPC; Invitrogen) water was added to the precipitate. Complementary DNA was prepared by reverse transcription using the PrimeScript RT kit (Takara Bio, Japan). The SYBR® Premix Ex TaqTM II kit (Takara) was used for RT-qPCR analysis using the Applied Biosystems® 7500 Real-Time PCR system (California, USA). PCR experiments were performed under the following conditions: 95 °C for 10 min, 55 °C for 2 min, and 72 °C for 2 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 32 s. The primer pair sequences are listed in Table 2. The XIST expression level was normalized to that of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and calculated using the 2−ΔΔCt method (26).
Table 2
| Gene symbol | Forward primer 5'-3' | Reverse primer 5'-3' |
|---|---|---|
| XIST | TTGAGCTGGCTCGACAGAATG | GGTTCTTAGACGTCCCAGGC |
| GAPDH | TGGGGCCAAAAGGGTCATCA | GCAGGATGCATTGCTGACAA |
GAPDH was used to normalize XIST expression. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; XIST, X-inactive specific transcript.
Proliferation assay
Cell counting kit-8 (CCK8) reagent (Solarbio, Beijing, China; 10 µL) was added to 96-well plates (containing H9c2 at 4×103 cells) at 0, 24, 48, and 72 h. After 60 min of incubation in the dark at 25 °C, the absorbance was measured at 490 nm using an enzyme-labeled instrument (Thermo Fisher Scientific).
Apoptosis assay
H9c2 cells were washed with phosphate-buffered saline (PBS) and then incubated with 5 µL annexin V-fluorescein isothiocyanate (FITC) for 15 min. Next, 5 µL propidium iodide (PI) was added for another 5 min. Flow cytometry (FCM; BD Biosciences, CA, USA) was used to analyze cell apoptosis. The cells were incubated in the dark at 4 °C.
Reactive oxygen species (ROS) measurement by FCM
H9c2 cells (1×105) were incubated (37 °C) with 1.0 µM 2,7-dichlorodihydrofluorescein diacetate. After 15 min, PRKs were then washed twice with PBS and analyzed by FCM to detect ROS using a 488-nm laser for excitation and a 535-nm laser for detection.
Enzyme-linked immunosorbent assay (ELISA)
The cell supernatant of each subgroup was collected to analyze the levels of LDH, CK, malondialdehyde (MDA), superoxide dismutase (SOD), and the inflammatory factors interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α. The following kits were used according to the manufacturer’s instructions: LDH activity assay kit (Solarbio), CK activity assay kit (Solarbio), micro MDA assay kit (Solarbio), SOD activity detection kit (Solarbio), glutathione (GSH) activity detection kit (Solarbio), IL-1 beta ELISA kit (R&D Systems, Minneapolis, USA), IL-6 Quantikine ELISA kit (R&D Systems), and TNF-α Quantikine ELISA Kit (R&D Systems).
Statistical analysis
Data are presented as the mean ± standard deviation from triplicate evaluations. Statistical analysis was performed using one-way analysis of variance and Bonferroni post hoc tests. The Student’s t-test was used for independent two-group analyses. Differences were considered significant at P<0.05.
Results
QYDP regulates H9c2 proliferation and apoptosis via XIST expression
The CCK8 assay showed that the proliferation of H9c2 cells decreased after pretreatment with 250 and 500 µg/mL QYDPs for 4 h (Figure 1A). Our results indicated that the proliferation of H9C2 cells might be affected by pretreatment with QYDPs at a concentration greater than 250 µg/mL (4 h). To prevent the interference of excessive QYDPs in the experiment, we selected the QYDP concentration of 100 µg/mL (4 h) as the experimental concentration for subsequent analyses of the molecular mechanisms.
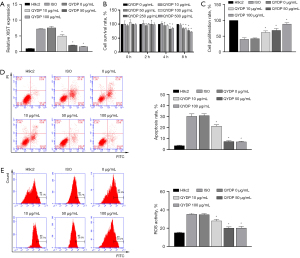
Next, ISO-induced H9c2 cells were used to construct an apoptosis model. RT-qPCR, CCK8 assay, and FCM results showed that ISO induction in H9c2 cells led to upregulated XIST expression, a decreased cell proliferation rate, as well as increased apoptosis and ROS levels (Figure 1B-1E). The effect of ISO induction was reduced after QYDP treatment in a concentration-dependent manner (0, 10, 50, and 100 µg/mL).
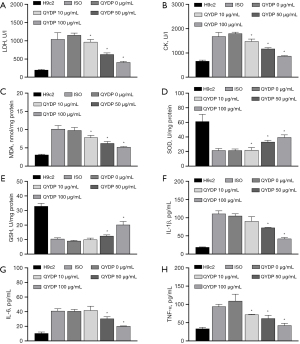
QYDP treatment reduced ISO-induced oxidative stress and inflammation. The ELISA results showed that ISO induced an increase in the levels of myocardial enzymes [increased LDH and CK levels (Figure 2A,2B)], oxidative stress [increased MDA level (Figure 2C), and decreased SOD and GSH levels (Figure 2D,2E)] and inflammatory response [increased IL-1β, IL-6, and TNF-α protein secretion (Figure 2F-2H)] in H9c2 cells, which were inhibited by QYDP treatment.
Role and potential mechanism of XIST in the H9c2 cell apoptosis model
To further explore the mechanism of action of XIST, we constructed an ov-XIST plasmid and three siRNAs targeting XIST and then transfected them into H9c2 cells. RT-qPCR results showed that XIST expression was increased in the ov-XIST transfection group and decreased in the three si-XIST transfection groups, indicating the successful construction of ov-XIST and si-XIST (Figure 3A). Notably, si-XIST-1 showed the best inhibition efficiency and was thus selected as the antagonist of XIST (si-XIST) in subsequent experiments.
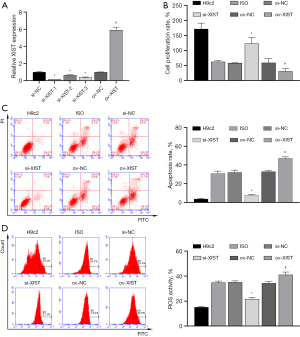
We transfected ov-XIST and si-XIST into ISO-induced H9c2 cells. The results of CCK8, FCM, and ELISA showed that ISO-induced inhibition of the proliferation rate of H9c2 cells (Figure 3B) and promotion of apoptosis (Figure 3C), ROS levels (Figure 3D), myocardial enzyme levels [increased LDH and CK levels (Figure 4A,4B)], oxidative stress [increased MDA level (Figure 4C), decreased SOD and GSH level (Figure 4D,4E)], and inflammatory reactions [increased secretion of IL-1β, IL-6, and TNF-α proteins (Figure 4F-4H)] were all reversed by si-XIST. However, the effect of QYDPs on the improvement of ISO induction in H9c2 cells was reversed by XIST ov.
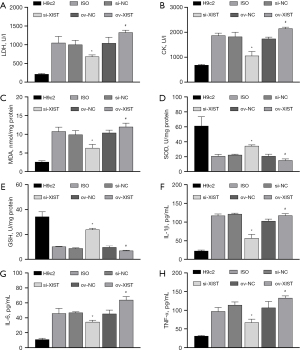
To investigate the association between XIST expression and QYDPs, we added QYDP (100 µg/mL) to the overexpressed XIST transfection group. The CCK8, FCM, and ELISA results showed that QYDP treatment improved the inhibition of ISO-induced proliferation, apoptosis, myocardial enzymes, oxidative stress, and promotion of inflammation. This effect was reversed by the ov of XIST (Figure 5A-5K).
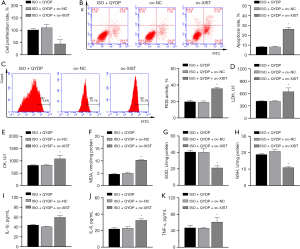
Discussion
Globally, the mortality rate of patients with CHF is very high (27,28), and its underlying pathological mechanisms remain unknown, which makes studying CHF crucial. Numerous studies have employed TCM or TCM extracts to effectively treat various diseases, such as chronic kidney disease (29) and colitis (30). Therefore, we used TCM as the starting point to analyze the role and potential mechanism of TCM in CHF. Wang et al. (31) used a variety of TCMs (such as Danshen, P. notoginseng, Angelica, and Astragalus) to screen for a more suitable treatment for CHF. They found that P. notoginseng exerts anti-apoptotic effects, Danshen possesses anti-oxidative stress properties, and Astragalus exerts anti-inflammatory effects. The main components of QYDPs are P. notoginseng, Danshen, and Astragalus; therefore, QYDPs are a reasonable choice for the treatment of CHF.
An important finding of this study was that ISO induced an increase in the expression of XIST, reduced the proliferation rate of H9c2 cells, and increased apoptosis and myocardial enzyme levels. These results are similar to those reported by Xiao et al. (32). In addition, QYDPs alleviated ISO-induced effects and partially restored the expression of XIST. These results suggest that XIST is closely related to the damage process in H9c2 cells. In addition, we found that QYDPs improved the oxidative stress and inflammation induced and promoted by ISO, which is consistent with the results of Cinar et al. (33). These results suggest that the role of QYDPs in protecting H9c2 cells involves oxidative stress and inflammation, but the mechanism of action of XIST remains unclear.
XIST may be involved in oxidative stress and inflammation to regulate cell proliferation and apoptosis (34,35). In this study, we investigated the effect of XIST on oxidative stress and inflammation, as well as its potential mechanism, by regulating the expression of XIST. In subsequent experiments, we observed that XIST could regulate the ISO-induced effects on cell proliferation, apoptosis, myocardial enzyme levels, oxidative stress, and inflammation in H9c2 cells. This provides new evidence that XIST induces cell damage by promoting oxidative stress and inflammation. Finally, we observed that the effect of QYDPs on the improvement of ISO induction in H9c2 cells was reversed by XIST ov. Thus, QYDP can improve the effect of ISO induction through XIST. Therefore, our study shows that QYDP may have a positive effect on the treatment and prognosis of CHF.
This study had several limitations that should be noted. Firstly, we did not analyze the effects of QYDPs on oxidative stress and inflammation in an animal model. Second, we did not conduct an in-depth analysis of the mechanism through which XIST acts as an endogenous competitive RNA to adsorb microRNAs to coregulate cell proliferation and apoptosis. Moreover, the process of lncRNA entering the clinic is hindered by issues related to specificity (off-target effects), delivery mode, and immunogenicity, which limit the clinical development of lncRNA therapeutics.
In summary, QYDPs protected H9c2 cells from damage caused by ISO-induced oxidative stress and inflammation by suppressing the expression of XIST. Therefore, this study confirmed that QYDP, as a new therapeutic drug for CHF, has a solid pharmacological and molecular biological basis for the treatment of CHF.
Acknowledgments
Funding: This work was supported by the Hainan Provincial Key Research and Development Program (grant No. ZDYF2019121).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-606/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-606/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-606/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Minà C, Ajello L, Gesaro GD, et al. Hyperkalemia in heart failure: current treatment and new therapeutic perspectives. Rev Cardiovasc Med 2020;21:241-52. [Crossref] [PubMed]
- Martin N, Manoharan K, Davies C, et al. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev 2021;5:CD012721. [PubMed]
- Capriotti T, Micari M. Chronic Heart Failure Treatment With the Left Ventricular Assist Device. Home Healthc Now 2019;37:190-7. [Crossref] [PubMed]
- Tereshchenko SN, Zhirov IV. Chronic cardiac failure in the XXI century. Ter Arkh 2011;83:60-6. [PubMed]
- Packer M. Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: implications for understanding the effects of current and future treatments for heart failure. Eur Heart J 2020;41:3856-61. [Crossref] [PubMed]
- Shao Z, Schuster A, Borowski AG, et al. Soluble angiotensin converting enzyme 2 levels in chronic heart failure is associated with decreased exercise capacity and increased oxidative stress-mediated endothelial dysfunction. Transl Res 2019;212:80-8. [Crossref] [PubMed]
- Doimo S, Pavan D. Novelties in Therapy of Chronic Heart Failure. Heart Fail Clin 2021;17:255-62. [Crossref] [PubMed]
- Han JW, Kang C, Kim Y, et al. Isoproterenol-induced hypertrophy of neonatal cardiac myocytes and H9c2 cell is dependent on TRPC3-regulated CaV1.2 expression. Cell Calcium 2020;92:102305. [Crossref] [PubMed]
- Tang G, Shen Y, Gao P, et al. Klotho attenuates isoproterenol-induced hypertrophic response in H9C2 cells by activating Na+/K+-ATPase and inhibiting the reverse mode of Na+/Ca2+-exchanger. In Vitro Cell Dev Biol Anim 2018;54:250-6. [Crossref] [PubMed]
- Ma D, Zhang J, Zhang Y, et al. Inhibition of myocardial hypertrophy by magnesium isoglycyrrhizinate through the TLR4/NF-κB signaling pathway in mice. Int Immunopharmacol 2018;55:237-44. [Crossref] [PubMed]
- Lu Y, Wang D, Yuan X, et al. Protective effect of Qishen Yiqi dropping pills on the myocardium of rats with chronic heart failure. Exp Ther Med 2019;17:378-82. [PubMed]
- Lin SS, Liu CX, Zhang JH, et al. Efficacy and Safety of Oral Chinese Patent Medicine Combined with Conventional Therapy for Heart Failure: An Overview of Systematic Reviews. Evid Based Complement Alternat Med 2020;2020:8620186.
- Wang L, Wang L, Zhou X, et al. Qishen Yiqi Dropping Pills Ameliorates Doxorubicin-induced Cardiotoxicity in Mice via Enhancement of Cardiac Angiogenesis. Med Sci Monit 2019;25:2435-44. [Crossref] [PubMed]
- Xie W, Meng X, Zhai Y, et al. Panax Notoginseng Saponins: A Review of Its Mechanisms of Antidepressant or Anxiolytic Effects and Network Analysis on Phytochemistry and Pharmacology. Molecules 2018;23:940. [Crossref] [PubMed]
- Wang D, Lv L, Xu Y, et al. Cardioprotection of Panax Notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed Pharmacother 2021;136:111287. [Crossref] [PubMed]
- Piao CL, Luo JL. Utilizing network pharmacology to explore the underlying mechanism of Radix Salviae in diabetic retinopathy. Chin Med 2019;14:58. [Crossref] [PubMed]
- Piccoli MT, Gupta SK, Viereck J, et al. Inhibition of the Cardiac Fibroblast-Enriched lncRNA Meg3 Prevents Cardiac Fibrosis and Diastolic Dysfunction. Circ Res 2017;121:575-83. [Crossref] [PubMed]
- Deng H, Ouyang W, Zhang L, et al. LncRNA GASL1 is downregulated in chronic heart failure and regulates cardiomyocyte apoptosis. Cell Mol Biol Lett 2019;24:41. [Crossref] [PubMed]
- Li Z, Zhang Y, Ding N, et al. Inhibition of lncRNA XIST Improves Myocardial I/R Injury by Targeting miR-133a through Inhibition of Autophagy and Regulation of SOCS2. Mol Ther Nucleic Acids 2019;18:764-73. [Crossref] [PubMed]
- Wen JF, Jiang YQ, Li C, et al. LncRNA-XIST promotes the oxidative stress-induced migration, invasion, and epithelial-to-mesenchymal transition of osteosarcoma cancer cells through miR-153-SNAI1 axis. Cell Biol Int 2020;44:1991-2001. [Crossref] [PubMed]
- Zhang Y, Zhu Y, Gao G, et al. Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and inflammation injury via targeting miR-370-3p/TLR4 in acute pneumonia. Cell Biochem Funct 2019;37:348-58. [Crossref] [PubMed]
- Toyoda S, Haruyama A, Inami S, et al. Effects of carvedilol vs bisoprolol on inflammation and oxidative stress in patients with chronic heart failure. J Cardiol 2020;75:140-7. [Crossref] [PubMed]
- Wu Z, Zhao X, Miyamoto A, et al. Effects of steroidal saponins extract from Ophiopogon japonicus root ameliorates doxorubicin-induced chronic heart failure by inhibiting oxidative stress and inflammatory response. Pharm Biol 2019;57:176-83. [Crossref] [PubMed]
- Yang Q, Cao Y. Study on mechanisms and myocardial protective effect of Qishen Yiqi dropping pills on rats with myocardial infarction. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017;29:501-5. [PubMed]
- Fan C, Tang X, Ye M, et al. Qi-Li-Qiang-Xin Alleviates Isoproterenol-Induced Myocardial Injury by Inhibiting Excessive Autophagy via Activating AKT/mTOR Pathway. Front Pharmacol 2019;10:1329. [Crossref] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Brennan EJ. Chronic heart failure nursing: integrated multidisciplinary care. Br J Nurs 2018;27:681-8. [Crossref] [PubMed]
- Authors/Task Force Members. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4-131. [Crossref] [PubMed]
- Chen Y, Cai G, Sun X, et al. Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae). Clin Exp Pharmacol Physiol 2016;43:145-8. [Crossref] [PubMed]
- Zhang C, Jiang M, Lu A. Considerations of traditional Chinese medicine as adjunct therapy in the management of ulcerative colitis. Clin Rev Allergy Immunol 2013;44:274-83. [Crossref] [PubMed]
- Wang Y, Wang Q, Li C, et al. A Review of Chinese Herbal Medicine for the Treatment of Chronic Heart Failure. Curr Pharm Des 2017;23:5115-24. [PubMed]
- Xiao L, Gu Y, Sun Y, et al. The long noncoding RNA XIST regulates cardiac hypertrophy by targeting miR-101. J Cell Physiol 2019;234:13680-92. [Crossref] [PubMed]
- Cinar I, Yayla M, Tavaci T, et al. In Vivo and In Vitro Cardioprotective Effect of Gossypin Against Isoproterenol-Induced Myocardial Infarction Injury. Cardiovasc Toxicol 2022;22:52-62. [Crossref] [PubMed]
- Shen C, Li J. LncRNA XIST silencing protects against sepsis-induced acute liver injury via inhibition of BRD4 expression. Inflammation 2021;44:194-205. [Crossref] [PubMed]
- Zhao J, Cui L, Sun J, et al. Notoginsenoside R1 alleviates oxidized low-density lipoprotein-induced apoptosis, inflammatory response, and oxidative stress in HUVECS through modulation of XIST/miR-221-3p/TRAF6 axis. Cell Signal 2020;76:109781. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


