Early hemi-diaphragmatic plication through a video assisted mini-thoracotomy in postcardiotomy phrenic nerve paresis
Introduction
The diaphragm is a major muscle of inspiration. Acquired diaphragmatic paresis or paralysis cause deterioration of ventilatory function and decreased efficiency of respiration, mainly due to less effective or absent caudal movement of the diaphragm during inspiration. Paradoxical motion of the affected hemi-diaphragm (i.e., cephalad movement on inspiration), atelectasis of the ipsilateral lung basis, and contralateral mediastinal shift may be present. Decreased thoracic wall compliance, and decreased basal alveolar expansion, leading to alveolar hypoxia, localized vasoconstriction and decreased perfusion of the ipsilateral lung basis can lead to chronic (and when the underlying cause persists, progressive) dyspnea, which is the principal symptom in most adults (1-3).
Acquired diaphragmatic paresis or paralysis of peripheral origin can result from abnormalities of the neuromuscular axis between the spinal cord and the diaphragm. It may be caused by phrenic nerve trauma, including birth trauma, iatrogenic injury in: cardiothoracic, mediastinal, oesophageal, neck and cervical spine surgery, interscalene nerve block, chiropractic manipulation, cervical spondylosis, infection (such as influenza, herpes zoster, poliomyelitis, measles, typhoid, diphtheria, pulmonary infection, mediastinitis), aortic aneurysm, neoplasms, autoimmune diseases of the phrenic nerve or the diaphragm, and neuromuscular syndromes (including amyotrophic lateral sclerosis in which respiratory failure is a common cause of death) (1-20). Cases in which no aetiology can be found are characterized as idiopathic (it is speculated that they can be attributed to subclinical viral infection) (21-41).
In phrenic nerve paresis/paralysis, the diaphragmatic muscle layer is initially intact but long standing loss of contractility leads to progressive muscular atrophy (2, 3).
The clinical presentation depends on the severity of the lesion (paresis vs. paralysis), the unilateral or bilateral location, the patient’ age, the underlying disease(s) (mainly respiratory and cardiac disease), and other comorbidities (including morbid obesity) (1,2, 3,16,30,42,43).
Phrenic nerve injury in infants usually leads to failure to wean from mechanical ventilation, and, although symptomatic, it is better tolerated by older children (6- 16). In adults, the symptoms vary greatly, from an asymptomatic or mildly symptomatic state diagnosed by diaphragmatic elevation on chest radiography, to a severely disabling clinical problem, and even morbid respiratory failure (1 -5,16- 25). It usually leads to chronic dyspnoea, exertional or at rest (26- 41), worsened in supine posture (orthopnoea, due to cephalad movement of abdominal viscera and further decrease of lung volumes). Hypoxaemia (usually mild) maybe present, leading to hyperventilation and respiratory alkalosis. Pleural effusions and recurrent respiratory infections may occur. Respiratory failure and cor pulmonale can be seen in severe cases. Prolonged mechanical ventilation and failure to wean from mechanical ventilation has also been reported particularly in patients with chronic obstructive pulmonary disease (COPD) or bilateral diaphragmatic paresis. Digestive symptoms have also been reported, particularly in left sided paralysis (1-4, 16-39,42,44,45).
The diagnosis of diaphragm paralysis is suggested by a raised hemi-diaphgram on standard full inspiration posteroanterior and lateral chest radiography. Clinical examination, history and previous roentgenograms facilitate the diagnosis and the differential diagnosis (1-3).
Confirmation of diagnosis can be made by ultrasonography, fluoroscopy (sniff test), pulmonary function tests (PFTs), conventional or spiral thoracic computed tomography (CT), thoracic magnetic resonance imaging (MRI), and most definitively by electromyography (EMG). Fiberoptic bronchoscopy may be indicated to exclude endobronchial pathology, particularly in the presence of atelectasis. CT or MRI may be indicated for differential diagnosis to herniation or diaphragmatic rupture and to exclude existence of malignancies or a subphrenic process. Spirometry usually shows decreased lung volumes with a restricted pattern [decreased forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), functional residual capacity (FRC), and total lung capacity (TLC)], and greater than normal reduction in supine lung volumes in comparison to upright volumes (by 20% to 50%). Changes in the pulmonary function tests are not consistent and do not correlated well with the symptomatic status, mainly being useful in evaluation of progression/improvement in the disease course and/or after surgical treatment. Ultrasound evaluation is a safe, non-invasive method that is considered sensitive, and has largely replaced the EMG and radioscopy (1-3,8,24,31,32,36,46).
Differential diagnosis between acquired diaphragmatic paresis/paralysis and congenital diaphragmatic eventration may be difficult (in the absence of previous chest radiography and/or history of a causative mechanism (trauma, surgery). In both diseases there is diaphragmatic integrity (in the absence of malignancy and systematic disease) and normal diaphragmatic attachments, but in phrenic nerve paresis/paralysis which is usually acquired, there is abnormal phrenic nerve function with presence of diaphragmatic muscular layer (although it may be atrophic in chronic paralysis), while in true eventration, which is congenital, the phrenic nerve is normal but there is absence or paucity of muscle fibers in the affected (part of the) hemi-diaphragm. As far as surgical treatment is concerned, the differential diagnosis bares less significance, since the indications for surgical treatment and the established surgical treatment (diaphragmatic plication) in both situations are common (2).
The diagnosis that dyspnoea is secondary to diaphragmatic paresis is a diagnosis of exclusion. The potentially coexisting causes of dyspnoea (mainly parenchymal lung disease and cardiac failure) should be evaluated and treated. The presentation, the duration, and progression of symptoms, their temporal relationship to trauma (including iatrogenic), infections or systemic diseases should be sought (2,3).
In the postcardiotomy setting, the finding of new hemidiaphragmatic elevation on postoperative chest x-ray and the presence of symptomatic dyspnoea or orthopnoea that is not attributed to other causes (such as cardiac failure), usually suffices for the diagnosis of diaphragmatic paralysis due to intraoperative phrenic nerve injury, and further evaluation is usually not required (2).
Failure to wean from mechanical ventilation is the main indication for diaphragmatic plication in postcardiotomy paresis of infants and young children (<2 years), other indications being acute respiratory failure, persistent or recurrent respiratory distress, persistent postoperative tachypnea and stridor, chronic respiratory failure with symptomatic dyspnoea, and failure to thrive. In paediatric patients postcardiotomy plication is usually performed after 2 weeks on mechanical ventilation (7,9, 12,14,16,42).
In older children (that are less dependent on diaphragmatic respiration) and adults, surgical treatment is justified in the presence of symptoms (malignant cases are usually excluded). Differentiation from other causes of dyspnoea (mainly parenchymal lung disease and chronic heart failure), and careful patient selection for surgical treatment of symptomatic patients with diaphragmatic elevation is required to avoid unnecessary operations (2, 3).
In diaphragmatic paresis/paralysis of peripheral origin surgical treatment (mainly plication) is indicated when there is evidence of respiratory compromise (with major functional effects, such as effort or positional dyspnoea, severe enough to impair simple daily activity or adequate sleep, but also life style limiting dyspnoea) without resolution or improvement despite optimal conservative management (including treatment of pulmonary infection, physical therapy, and body weight control). More rarely digestive symptoms, cardiac symptoms, or pain can be indications for plication (2, 3,36, 42,47).
Relative contraindications to plication are diaphragmatic paresis due to neuromuscular disorders, and the presence of malignancy or morbid obesity. Plication in neuropathies has been rarely reported (35), and patients with neuromuscular disorders should be approached with extreme caution (2). High tetraplegia and central cause hypoventilation have been considered indications for diaphragmatic pacing to wean patients from the ventilator after reconditioning of the diaphragmatic muscle ( 47) (Video 1).
Case report
A 72 year old woman, with infective endocarditis, on triple antibiotic regiment, and increasing in size vegetations of a bioprosthetic mitral valve (obstructive vegetation of 1 cm in diameter), and the native aortic valve (elongated vegetation of 1.1 cm in length moving freely at the free edge of the non coronary leaflet), and acute renal failure, underwent urgent redo mitral valve replacement and aortic valve replacement (predicted mortality Euroscore II: 23.86%). Resternotomy, and blunt dissection of total cardiac adhesions by scissors and electrocautery was performed. After ascending aorta and bicaval cannulation, cardiopulmonary by pass (CPB) was instituted at mild hypothermia (32° Celsius). Retrograde warm blood cardioplegia was administered. Bioprosthetic mitral valve “endocarditis”, with a large vegetation, thick, fused and shortened leaflets, and native aortic valve endocarditis with several vegetations, the largest of which was more than 1 cm long (revealed at preoperative echocardiogram) was confirmed intraoperatively. The bioprosthetic mitral valve and the native aortic valve were resected and replaced by mechanical valves [mitral valve prosthesis Carbomedics Standard (Sorin) 25 mm, and aortic valve prosthesis Carbomedics Standard (Sorin) 21 mm, respectively]. The patient was weaned from CBP on atrioventricular pacing, and was transferred to the intensive care unit being haemodynamically stable on minimal inotropic support, after routine closure of the sternotomy. Her haemodynamic status remained stable, her renal function was normalized and she was soon weaned from inotropic support. She was extubated on the 3 rd postoperative day, and the next day right hemidiaphragm elevation was noted, and right phrenic nerve paresis was suspected. She required intermittent non invasive positive pressure ventilation and underwent physiotherapy. She underwent implantation of a permanent atrioventricular pacemaker on the 10 th postoperative day, due to postoperative third degree atrioventricular block, and was transferred to the ward, on intermittent Boussignac continuous positive airway pressure (CPAP) System. Despite good valve and cardiac function, good renal function, infection control, and weaning from mechanical ventilation, the patient had persistent respiratory distress (dyspnoea, tachypnoea, orthopnoea, hypoxaemia, need for continuous oxygen supplementation), having a transcutaneous oxygen saturation of 80% when breathing room air. The chest radiography showed persistent right hemi-diaphragm elevation, with severe progressing lung atelectasis and right-sided pleural effusion that recurred after tube thoracostomy and needle aspiration (Figure 1A,B). Thoracic computed tomography was performed and confirmed the presence of an elevated right hemidiaphragm, extended lung basis atelectasis and pleural effusion (Figure 1C,D, Figure 2). The echocardiogram showed good cardiac function, with mild concentric left ventricular hypertrophy, a left ventricular ejection fraction of 60%, good function of both prosthetic valves, and absence of vegetations or pericardial fluid. The patient had no signs of sepsis (normal sensorium, normal haemodynamics, normal renal/liver function, negative blood, 3-lumen catheter, and urine cultures) and remained apyrexial on antibiotic scheme, but she was dyspnoeic, having difficulty to eat, walk and sleep, despite medical treatment (including CPAP mask, aggressive physiotherapy, mucolytic drugs and bronchodilators). Since no progress was observed surgical treatment was decided. Right hemi-diaphragm plication was performed through a video assisted right mini-thoracotomy (8 cm) on the 25 th postoperative day. Teflon felt buttressed, interrupted, non absorbable U stitches were placed, starting at the dome of the right hemidiaphragm and progressing anteromedially towards the cardiophrenic angle and posterolaterally towards the costophrenic recess (resulting in an oblique plication line parallel to the long axis of the right leaflet of the central tendon). The patient was extubated on the first postoperative day after plication of the right hemidiaphragm. Adequate respiratory function was soon established, chest radiography was dramatically improved (normal right hemi-diaphragm position, right lung re-expansion) (Figure 3). After mobilization and rehabilitation the patient was discharged on the 8 th day after plication (Figure 4).
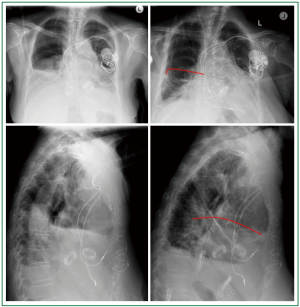
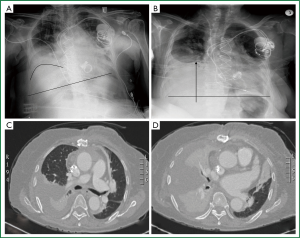 Figure 1. A, B. Frontal upright chest x-rays. Right hemidiaphragm elevation. Basal atelectasis, pleural
effusion; C, D. Thoracic CT, transverse plane. Confirmation of the radiography findings.
Figure 1. A, B. Frontal upright chest x-rays. Right hemidiaphragm elevation. Basal atelectasis, pleural
effusion; C, D. Thoracic CT, transverse plane. Confirmation of the radiography findings.
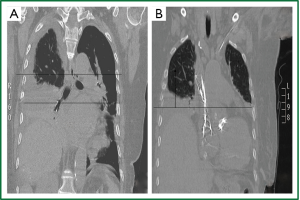 Figure 2. Thoracic CT (coronal plane, supine position). A,B. Elevation of the right henidiaphragm (8 cm
above the the left, 2-2.5 intercostal spaces). Lower and middle lobe atelectasis. Pleural
effusion (A).
Figure 2. Thoracic CT (coronal plane, supine position). A,B. Elevation of the right henidiaphragm (8 cm
above the the left, 2-2.5 intercostal spaces). Lower and middle lobe atelectasis. Pleural
effusion (A).
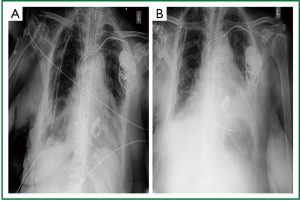 Figure 3. Post plication anteroposterior chest x-ray. Good anatomical posistion of the right hemidiaphragm.
Partial lung re-expansion. A. Immediately postoperatively; B. on the 5
postoperative day after plication.
Figure 3. Post plication anteroposterior chest x-ray. Good anatomical posistion of the right hemidiaphragm.
Partial lung re-expansion. A. Immediately postoperatively; B. on the 5
postoperative day after plication.
Discussion
Diaphragmatic plication is the indicated treatment for symptomatic diaphragmatic paresis. There is consensus that in infants and young children with postcardiotomy diaphragmatic paresis early plication is indicated, while there is variability in the timing of surgery in adults. The incidence of postcardiotomy diaphragmatic paresis has decreased after abandonment of topical cooling. Currently, internal thoracic artery harvesting has been mainly related with postcardiotomy phrenic nerve paresis in adults, while the reported incidence after valve surgery is low.
Incidence and causes of postcardiotomy diaphragmatic paresis/paralysis
The reported incidence of phrenic nerve injury after cardiac surgery varies greatly among studies depending on the definition, the era of the study, the study design, the diagnostic means, and the diligence with which it is sought (4,7- 10,13,14,17,20-22,27,33).
Criteria for diagnosis of diaphragmatic paresis/paralysis postcardiotomy were considered elevation of the right hemidiaphragm by 2 or more intercostal spaces higher than the left and elevation of the left hemidiaphragm by 1 or more intercostal space higher than the right (22, 33). When the evaluation method was phrenic electromyography, prolonged phrenic nerve latencies and/or increased diaphragmatic action potential amplitudes were used as criteria (10, 48).
Incidence of diaphragmatic paresis/paralysis after paediatric cardiac surgery
The reported incidence of phrenic nerve injury and/or diaphragmatic paresis/paralysis after paediatric cardiac surgery varied among retrospective studies ( 7-9,12,14) being always lower than the reported incidence in relevant prospective studies (10,48).
In a retrospective study, Shoemaker et al. [1981] (7) reported sustained phrenic nerve injury resulting in diaphragmatic paralysis requiring operation in 0.46% among 1500 operated infants and children. In other retrospective studies, diaphragmatic paralysis in infants and children postcardiotomy (requiring or not surgery) was reported in 3% [Hamiltonet al., 1990, n=960 (9)], 1.5% [Tönzet al., 1996, n=1,656 (12)], 1.6% [de Leeuwet al., 1999, n=168 ( 13)], and 5.4% [Joho-Arreola, 2005, n=43 (14)] of operated patients (40% or more of whom required plication). In a prospective study of 100 infants and children, Mok et al. (10) reported a 10% incidence of phrenic nerve injury, diagnosed by direct percutaneous phrenic nerve stimulation. In a prospective study of 59 paediatric cardiac patients, Yemisci et al. (48) reported electrophysiologic detection of phrenic nerve dysfunction early postcardiotomy in up to 44% (evidenced by abnormal phrenic nerve latencies and/or abnormal diaphragmatic action potential amplitudes mainly unilateral but also bilateral). Permanent palsy was found in only 1.69%.
Incidence of diaphragmatic paresis/paralysis after adult cardiac surgery
In adult cardiac surgery cold induced phrenic nerve injury was very common in the era of topical myocardial cooling (intended as an adjunct to myocardial protection). Topical cooling predisposes to diaphragmatic paresis/paralysis and the incidence is further increased when the internal thoracic artery is harvested (22). In the absence of cold induced injury, internal thoracic artery harvesting has been mainly related to diaphragmatic paresis/paralysis. Mechanical injury during internal mammary harvesting, including partial or total resection, thermal (electrocautery) and/or stretch (traction) injury during harversting, ischaemic injury after harvesting (due to vascular compromise after internal thoracic artery branch division and ligation, particularly after ligation of the pericardiophrenic artery) or combinations of the above have been related with permanent or transient phrenic nerve dysfunction. Postcardiotomy phrenic nerve dysfunction in the adults ranges from minimal dysfunction lasting less than 1 week to permanent phrenic nerve paralysis (3,21 -24,26-28,30,49).
Mazzoni et al. (29) reported abolished contaction of phrenic nerve (on intraoperative monitoring of compound diaphragmatic action potentials through transcutaneous phrenic nerve stimulation) after ice slush heart submersion, with or without recovery on rewarming.
In retrospective studies, the reported incidence of diaphragmatic paresis and/or phrenic nerve injury (mainly diagnosed as new hemidiaphragmatic elevation on chest radiography early postcardiotomy) among patients who received local cooling ranged between 2.5% and 73% (related at least partly with the degree and method of cooling). Dajee et al. (17) reported a 9.3% (16/172) incidence of phrenic nerve injury among patients who survived after cardiac surgery with the use of topical hypothermia. Rousou et al. (20) reported left phrenic nerve paresis in 24% of patients who underwent CABG with iced slush topical hypothermia, in 12.5% of patients in whom a cardiac cooling jacket and cold saline was used, and 22.9% of patients in whom both the cooling jacket and iced slush were used. Esposito et al. (21) reported diaphragmatic elevation on chest radiography in 73% of patients with direct contact of ice slush to the phrenic nerve, and in 17% of patients in whom an insulation pericardial pad was used (P<0.001). Curtis et al. (22) reported elevated diaphragms on discharge radiograms in 23.7% (109/460) among patients in whom topical ice slush was used, and in 2.5% (7/285) among patients in whom topical cooling with 4 °C normal saline solution was used (P<0.001). Diaphragmatic elevation involved the left hemidiaphragm in 95% of cases. Among patients who received topical cooling, the incidence of diaphragmatic elevation was 10.6% in patients who underwent valvular operations, 27.2% in patients who underwent CABG, and 20.9% in patients who underwent complex procedures that included CABG (P=0.013) When topical ice slush was applied, elevated hemidiaphragms were found in 26% of patients (72/280) without internal thoracic artery harvesting versus 39.4% of patients (13/33) with internal thoracic artery harvesting (P=0.047) (22). Allen et al. (26) reported increased incidence of diaphragmatic elevation on chest radiography postcardiotomy in patients who received topical hypothermia (10/50 ice slush topical cooling vs. 8/50 saline topical cooling vs. 5/50 no cooling). Nikas et al. (28) reported increased incidence of diaphragmatic paralysis in patients who received topical ice slush hypothermia in comparison to those who did not (25% on the 1 st postoperative day, 18% on the 15 th postoperative day, and 8% at 6 months vs. 2%, 1%, and 1% respectively (P<0.001).
Efthimiou et al. (27) conducted a prospective randomized study of 100 patients who received open heart surgery with or without topical ice slush hypothermia (56/44), and reported that unilateral (mainly left sided) diaphragmatic paralysis (with unrecoverable phrenic nerve conduction time) 1 week postoperatively developed in 36% of patients who received local cooling and in none of the patients who did not. The paralysis was present in 34% of patients at 1 month, in 9% at 1 year, and in 2% at 2 years.
Abandonment of topical cooling resulted in decreased incidence of postcardiotomy diaphragmatic paralysis, and, in the current era, phrenic nerve injury is more commonly related with internal thoracic artery harvesting (3).
Deng et al. [2003] (33) reported a 4% incidence (31/783, confidence interval: 2.6-5.3%) of right phrenic nerve injury (diagnosed by right hemidiaphragm elevation on chest radiography by >/=2 intercostal spaces higher than the left or by intraoperative visualization of transection) associated with high right internal thoracic artery harvesting. Deng et al. [2006] (34) reported an overall 2.5% (53/2,084) incidence of phrenic nerve injury postcardiotomy, with absence of phrenic nerve injury in non coronary surgery or in coronary surgery without internal thoracic artery harvesting. Logistic analysis showed that among 28 preoperative and operation-related variables the independent risk factors for phrenic nerve injury were internal thoracic artery harvesting (odds ratio 14.5) and the presence of COPD (odds ratio 2.9).
Cohen et al. (30) reported a 43% (29/67) incidence of sustained phrenic nerve injury among patients with major COPD who underwent CABG.
Diabetes and older age have been considered associated with higher risk of postcardiotomy phrenic nerve paralysis (3,23).
A meta-analysis of 2 randomized and 33 non randomized studies comparing conventional open mitral valve surgery to minimally invasive mitral valve surgery, showed phrenic nerve palsy in 3% of patients who underwent minimally invasive surgery vs. 0% in patients who underwent open surgery (50).
Recovery of phrenic nerve function and timing of plication in infants and young children
de Vries et al. (6) reported spontaneous recovery of obstetric (birth) phrenic nerve injury within 1 month in 27.8% of patients (5/18), while plication was performed in 72.2% of patients (13/18) after an mean observation period of 100 days. They suggested waiting for spontaneous recovery before plication for up to 1 month, but immediate plication (within the first month of life) in case of clinical deterioration.
de Leeuw et al. (13) reported that early spontaneous recovery of diaphragmatic paresis in children postcardiotomy is rare and suggested aggressive surgical treatment.
In children with postcardiotomy diaphragmatic paresis early plication is usually performed, more commonly due to failure to wean from mechanical ventilation (7 -16). Joho-Arreola et al. (14) reported a median time from cardiac surgery to plication of 21 days. de Leeuw et al. (13) reported a median time from cardiac surgery to investigation for diaphragmatic paresis of 5 days (with 57% of patients ventilated at the time of diagnosis) and a median time from investigation to plication of 15 days. Baker et al. (15) reported diagnosis of postcardiotomy phrenic nerve injury at a median time of 8 days postoperatively and surgical plication at a median time from diagnosis of 2 days. Yemisci et al. (48) reported that dysfunctional diaphragms (evaluated by electromyography in a prospective study) were found in 44% of paediatric patients in the first postoperative week after cardiac surgery, but persistent dysfunction was found in only 1.67% 2 weeks postoperatively, and suggested that surgical treatment should be considered after the 1 st postoperative week.
It appears that early plication does not preclude subsequent phrenic nerve recovery. Stone et al. (8) reported return to normal diaphragmatic function in 6 of 6 patients studied at 1 to 7 years after plication performed for postcardiotomy paralysis. Baker et al. (15) reported return of diaphragmatic function after early postcardiotomy plication in 77% of 17 children investigated at a median time from plication of 16.4 months. A trend for improvement overtime was also noted.
Recovery of phrenic nerve function in adults
Variable recovery rates at variable time periods after the diagnosis of diaphragmatic paresis have been reported in adults; recovery may be prolonged and prediction of recovery is difficult. Evidence is provided by mainly retrospective small scale studies, and is often inconsistent.
Electrophysiologic studies (5) and ultrasound monitoring have been suggested to predict the possibility of recovery and evaluate the rate of recovery of the phrenic nerve (24,46).
Wilcox et al. (24) monitored 5 patients with phrenic nerve paralysis (n=4) or paresis (n=1) after CABG, by pulmonary function tests, fluoroscopy and electromyography. Partial (n=4) or complete (n=1) recovery was shown, but it was delayed and continued for more than 1 year, in keeping with the rate of peripheral nerve regeneration.
Summerhill et al. (46) monitored the recovery of paralysed diaphragms of various aetiology, including cardiac surgery (aortic valve replacement in 1 patient), by serial ultrasound evaluation (two-dimensional, B-mode measurements) of diaphragmatic thickening during inspiration, and reported spontaneous functional recovery of paralysed diaphragms in 69% of patients (11/16). There was positive correlation between improvements in diaphragmatic thickening and pulmonary function tests. There was absence of thickening in patients who did not recover. The mean time for recovery was 14.9 months (ranging between 5 and 25 months).
Gayan-Ramirez et al. (51) performed a retrospective study in 23 patients with uni- or bilateral diaphragm paralysis of various aetiology to evaluate whether functional respiratory recovery (defined as increase in FVC>400 mL) can be predicted from the diaphragmatic compound motor action potential at the time of diagnosis. They reported functional recovery in 43% of patients (10/23) after 1 year, and in 52% (12/23) after 2 years. The aetiology of paralysis did not influence the recovery. The initial diaphragmatic compound motor action potential and the baseline pulmonary function were not predictive of recovery. Respiratory muscle training was not associated with a higher percentage of functional respiratory recovery. Relapse after initial improvement was observed in 26% of patients.
It appears that in the vast majority of cases with cold induced phrenic nerve injury, recovery appears within 2 years (22, 27,28). An experimental animal study showed that crashed ice contact for 30-60 min caused reversible phrenic nerve paralysis with responsiveness return within 2 months. Cold injury consisted of myelin sheath injury with axon preservation (52). In clinical studies, the time required for functional recovery of cold induced phrenic nerve injury postcardiotomy varied greatly between 1 month and 2 years (22). Nikas et al. (28) reported that among patients with elevated hemidiaphragms on the first postoperative day recovery was observed in 68% at 6 months. Dajee et al. (17) reported recovery in 75% of patients (12/16) at 1 year. Curtis et al. (22) reported recovery of the affected hemidiaphragm in 20.2% of patients (20/99) at 1 month, in 78.1% of patients (50/64) at 1 year, and in 97.4% of patients (74/76) at 2 years. Efthimiou et al. (27) reported recovery of cold induced injury that was revealed at the 1 st postoperative week, in 75% of patients at 1 year, and in 94% at 2 years.
Conservative treatment and eventual recovery have been reported in bilateral cold induced diaphragmatic paralysis. Olopade and Staats (25) reported recovery of bilateral cold induced phrenic nerve paralysis after 18 months. Chandler et al. (18) reported recovery of bilateral cold induced diaphragmatic paralysis in 4 out of 5 patients after several months of disabling impairment (including mechanical ventilatory support for 4 months in one patient, and exertional dyspnoea, severe orthopnoea, insomnia, and daytime somnolence in all patients).
Abd et al. (23) reported “rocking bed ventilation” for postcardiotomy phrenic nerve dysfunction related to internal thoracic artery harvesting and/or cold induced injury. The “rocking bed” [that facilitates spontaneous breathing by tilting, head up and head down at a regular frequency, and causing cephalad and caudal gravitational movement of the abdominal viscera and thus the diaphragm (53)] was instituted at hospital and continued at home until recovery of the diaphragmatic function. Recovery, assessed by pulmonary function tests, electromyography and fluoroscopy was observed in the postcardiotomy patients within 4 to 27 months after surgery, being heralded by ability to assume the supine position without dyspnoea.
Katz et al. (49) reported that among 64 patients with COPD and phrenic nerve injury after CABG (with iced saline solution and internal thoracic artery cautery harvesting), persistent dysfunction was found in 20% (13/64) and normal function in 56% (36/64) at a mean follow-up of 33 months (9 patients died and 6 were lost to follow-up).
Deng et al. [2003] (33) reported that among 31 patients with post CABG diaphragmatic paresis associated with high free internal thoracic artery harvesting, 12 patients (38.7%) underwent plication, in 14 patients (45.2%) spontaneous recovery occurred, and 5 were lost to follow-up. After exclusion of patients in whom intraoperative transection was identified (n=4) and patients lost to follow-up (n=5) spontaneous recovery occurred in 64% of patients (14/22).
In a recent prospective randomized controlled trial, Kodric et al. (54) evaluated the impact of inspiratory muscle training in recovery of phrenic nerve paresis after CABG, valve replacement or both. The definition of diaphragmatic paralysis was based on sniff fluoroscopy and recovery was assessed by sniff fluoroscopy, pulmonary function tests and inspiratory and expiratory pressures; [n=69, 2: 1 randomization to diaphragm training using an adjustable pressure device (treatment group) vs. no training (control group), 52 completed the study assessments at 1 year, treatment group: 36 and control group: 16]. Significant improvement of diaphragm mobility was produced after 12 months in the training group (P<0.001); 77.78% of the training group patients experienced improvement (partial 41.67%, complete 36.11%) vs. partial recovery (12.5%) and no improvement (87.5%) of the control group patients.
Morbidity and mortality associated with postcardiotomy diaphragmatic paresis in adults
Generally, in unilateral diaphragmatic paralysis of all causes the prognosis is considered good in the absence of underlying lung or neurological disease (1). In many cases it is asymptomatic and in most circumstances it does not require operation (1,5). In bilateral paralysis, advanced lung disease and chronic demyelinating conditions the prognosis is considered poor (1).
Although unilateral diaphragmatic paralysis/paresis postcardiotomy in adults is usually considered benign not requiring surgical treatment, it can be poorly tolerated in some patients. Generally, postcardiotomy unilateral diaphragmatic paralysis/paresis has not been related to mortality, but it has been related to increased postoperative morbidity (mainly respiratory complications), and reduced quality of life (17, 21-23,30, 34,49).
Esposito et al. (21) reported life threatening respiratory complications (including multiple reintubations, tracheostomy, and prolonged mechanical ventilation) in 14% of patients. In a retrospective study of prospectively collected data (n=2,084, 1995-2002, (34) unilateral phrenic nerve injury (that occurred in 2,5% of patients) was identified as an independent risk factor (odds ratio 8.1) for perioperative pulmonary morbidity but not for perioperative mortality.
Unilateral persistent diaphragmatic paralysis/paresis in postcardiotomy patients with COPD, has been related to higher early and midterm morbidity and mortality ( 30,49). In a retrospective study of patients who underwent CABG [Cohen et al. (30)] matched comparisons showed that patients with COPD and phrenic nerve injury had longer intensive care unit stay, higher re-intubation rate (37.9%), longer total hospitalization, more hospital readmissions, and lower cumulative survival compared with all other groups (of patients with COPD without phrenic nerve injury, patients without COPD and with phrenic nerve injury, and patients with neither COPD nor phrenic nerve injury).
Bilateral phrenic nerve injury postcardiotomy in adults has been associated with pulmonary morbidity (prolonged mechanical ventilation and weaning, severe orthopnoea, longer postoperative hospital stay) (18,19,22) and even mortality ( 22).
Timing of plication for diaphragmatic paresis/paralysis in adults
While in diaphragmatic paralysis in infants and young children there is consensus about early plication, in adults there is less uniformity about the timing of surgical plication that is usually suggested and applied at a later time (3).
Plication after a period of 3-6 months has been suggested by some authors, while others recommended a longer waiting period in anticipation of spontaneous recovery, particularly in postcardiotomy diaphragmatic paralysis (3). Graham et al. (31) reported diaphragmatic plication (for diaphragmatic paralysis of various aetiology) after a mean time from symptom onset of 1.8 years (4 months - 4 years). Celik et al. (3) reported a mean time to plication of 32.9 months (in 13 patients, 3 postcardiotomy, 9 after trauma, 1 idiopathic). The long period was attributed to late diagnosis and referral, rather than long waiting period for surgery. According to Celik et al. (3), the waiting period should be at least 1 year depending on the aetiology of paralysis. Mouroux et al. (35) advocated an observation period of 18-24 months in symptomatic patients with recent diaphragmatic elevation before suggesting surgery. Groth et al. (55) advocated 1 year observation period for adults with phrenic nerve injury postcardiotomy in anticipation of improvement within this timeframe (laparoscopic plication). Groth and Andrade (2) in their review reported that in postcardiotomy diaphragmatic paresis an observation period of 1-2 years is often recommended.
Deng Byth et al. (33) reported their treatment algorithm for right diaphragmatic paresis/paralysis associated with high free internal thoracic artery harvesting, diagnosed postoperatively. They waited for spontaneous recovery, evaluating the patients with fluoroscopy and pulmonary function tests at upright and supine position, trying to determine whether it was paresis or complete paralysis. If there was not paradoxical motion on fluoroscopy and the supine to upright FEV1 and FVC ratios were higher than 0.8, phrenic neuropraxia and diaphragmatic paresis was diagnosed, and waiting was suggested, unless there was disabling dyspnoea. If diaphragmatic paralysis was diagnosed (paradoxical movement, supine to upright ratios <0.8) a waiting period of 3 months for signs of improvement was suggested. If by that time there were no signs of recovery plication was recommended.
Early plication in postcardiotomy diaphragmatic paresis/paralysis in adults
There are rare reports of early postcardiotomy diaphragmatic plication in adults.
Deng Byth et al. (33) reported right hemidiaphragmatic plication during the same operation (CABG) in 4 adults, when transection of the phrenic nerve was identified intraoperatively (during harvesting of the right internal thoracic artery).
Glassman et al. (56) reported early diaphragmatic plication for postcardiotomy right diaphragmatic paresis that allowed successful weaning from mechanical ventilation and discharge in an adult patient. The patient had undergone resternotomy, aortic valve replacement and CABG; plication was performed on the 34 th postoperative day, through a 10 cm thoracotomy, after significant postoperative respiratory morbidity (including re-intubation, tracheostomy and fever) that resolved soon after plication. The authors suggested that early plication should be considered in adults with postcardiotomy respiratory failure and diaphragmatic dysfunction.
Takara et al. (57) reported early plication (during initial hospitalization) of an adult with unilateral diaphragmatic paresis after ascending aorta replacement, who was re-intubated and ventilated for 2 months (after re-intubation). Plication allowed successful weaning from mechanical ventilation in 4 days.
Kuniyoshi et al. (58) reported early postcardiotomy (within 30 to 61 days from cardiac surgery) diaphragmatic plication, through a muscle sparing approach in 4 adults with postocardiotomy diaphragmatic paralysis resulting in difficulty to wean from mechanical ventilation. After plication, they noted dramatic improvement of mean forced tidal volume in all patients and were able to wean all patients from mechanical ventilation. One patient with obstructive pulmonary dysfunction died due to aspiration pneumonia, the other 3 patients were discharged without symptoms.
Shiraishi et al. (59) treated bilateral postcardiotomy phrenic nerve paralysis resulting in severe respiratoty distress in an adult by early plication (on the 43rd postoperative day), which was followed by early weaning from the ventilator.
Sismansky et al. (16) applied diaphragmatic plication early after cardiothoracic operations (42-52nd postoperative day) due to failure to wean from mechanical ventilation in 4 adults, only 1 of whom was successfully weaned.
Ohta et al. (60) reported bilateral diaphragmatic plication in a 44-year-old man with bilateral diaphragmatic paralysis early after complete resection of a large thymoma infiltrating both phrenic nerves. The plication allowed weaning from the ventilator on the 4 th postoperative day, rehabilitation and return to full time work 5 weeks postoperatively. No restrictive or obstructive pattern of lung function was noted. The clinical status and the pulmonary function tests indicated that the thoracic cage ventilatory movement can compensate loss of bilateral diaphragmatic function for at least 18 months.
Surgical treatment - diaphragmatic plication
The established surgical treatment of diaphragmatic paresis/paralysis in children and adults is diaphragmatic plication, performed through various approaches and various plication techniques ( 1-16,31,32,35,37,44,45,55,60-68).
In adults, open, endoscopic and minimally invasive, transthoracic or transabdominal approaches have been used. The same techniques have been applied in diaphragmatic paralysis and true eventration ( 2). Open transthoracic approaches include thoracotomies of various lengths, at various intercostal spaces (3,16,31,32,36,42,44,58,66), including conventional posterolateral (31) limited lateral (66) and muscle sparing ( 58), but also sternotomy and hemi clam shell incision ( 37). The time honoured approach is thoracotomy (2, 3,16,31,32,36,42,44,58,66), but more recently applied thoracoscopic techniques ( 35,36,61-64,67,68) are gaining acceptance (45), while good results have been reported with laparoscopic techniques (2, 55). Thoracoscopic and minimally invasive techniques (video-assisted thoracoscopic surgery (VATS) facilitated by a 4 cm mini-thoracotomy without rib spreading ( 35,62), mini-thoracotomy with thoracoscopic assistance (65), but also open transthoracic approaches (particularly) in technically demanding cases are currently applied ( 3,16,36, 37,64).
Various plication suture lines, transverse (35), oblique (posterolateral to anteromedial (63), from the costophrenic to the cardiophrenic angle that has been considered the classic direction (37 ), but also anterolateral to posteromedial (67), anterior- transverse (37), posteroanterior plus transverse (creating a T shaped line) (2,55) have been described. Continuous (35,62 ,67) or interrupted ( 37,55,63), usually non absorbable (2, 55,67) but also absorbable ( 37), buttressed and non-buttressed sutures have been used. Stapling devices have also been applied (68). Coverage of the plication area with various patches has also been reported (69).
According to Leo et al. (70) “the best suture line runs from the midline of the posterolateral diaphragmatic dome to the level of the phrenic nerve”. A matter of great significance is the degree of plication. Some authors described that the plication is continued until the diaphragm is taut, as tight as possible, or tense on palpation (31,66,67). Leo et al. (70) highlighted the significance of achieving a flattened dome but allowing the diaphragm to assume it normal curvature at the costodiaphragmatic portion.
Results of diaphragmatic plication
Most authors reported that diaphragmatic plication in symptomatic unilateral diaphragmatic paresis/paralysis in the adult, usually offered after an observation period, is a safe and effective procedure, with good early (3, 4,31,32, 35-37,44,55,63), and long term results ( 3,31,32, 35,64,66) Most long term results come from studies that reported open plication (3,31, 32,66). Nevertheless in the prospective study of Mouroux et al. (35) (n=12), thoracoscopic plication yielded excellent short and long term results (at a mean follow-up of 64.4+/-46 months, while 6 patients were followed-up for >5 years). In the retrospective study by Freeman et al. (n=41) (64) in which the majority of patients underwent thoracoscopic plication (n=30) excellent short and long term results have been reported at a mean follow-up of 57+/-10 months (range, 49-80 months).
Diaphragmatic plication offers improvement in pulmonary function tests (2,3,31,32,35,36,45,62,64,66) symptomatic improvement (mainly relief of dyspnoea and orthopnoea) (3,32,44,45,63-66) and improved quality of life (3,32). Patients that may not have significant symptomatic improvement (despite improved pulmonary function tests) are morbidly obese patients and patients with long standing bilateral paralysis (64).
The early mortality after diaphragmatic plication performed for paresis/paralysis of various aetiologies varied among studies. Zero mortality was reported by Wright et al. (0/7) (44), Graham et al. (0/17) (31), Higgs et al. (0/19) (32), Leo et al. (0/10) (37), Mouroux et al. (0/12) (35) Groth et al. 0/25 (55), Freeman et al. (0/25) (36), Freeman et al. (0/41 including the patients of their previous study) (64), Lai et al. (0/5) (65) and Kim et al. (0/4) (63). Celik et al. (3), reported 1 death in a patient with COPD and obesity among 13 patients (7.6%). Simansky et al. (16) reported zero mortality among 7 adult patients who were not mechanically ventilated before plication, but death of 2 among 4 (50%) adult patients who were on mechanical ventilation before plication (18% overall). Versteegh et al. (66) reported death of 3 patients (due to pulmonary embolism, acute myocardial infarction, renal and cardiac failure) among 22 patients (13.63%). Kuniyoshi et al. (58) reported death from aspiration pneumonia in one patient with COPD among 4 patients that were operated early after cardiac operations (25%).
According to the 2010 review by Groth and Andrade (2) minimally invasive diaphragmatic plication has emerged as an equally effective and less morbid alternative to open plication, although “long-term results have yet to be published”.
According to the 2012 review by Gazala et al. (45) diaphragmatic plication can improve dyspnoea, functional status and pulmonary function tests of patients with unilateral diaphragm paralysis. Thoracoscopic approaches appear to have more advantages in subjective and objective measures (including dyspnoea score, pulmonary function tests, length of hospital stay and postoperative complications). Further research was suggested with high-quality study designs, focussing mainly on the long-term results and the health-related quality of life.
Aims of plication and respiratory mechanics after plication
Diaphragmatic plication has been considered as a “symptomatic” rather than a curative treatment. Although long-term, overtime return of diaphragmatic function after early postcardiotomy plication has been reported in children (8,15) this is probably attributed to phrenic nerve recovery rather than a direct curative effect of plication. In the absence of post-plication functional recovery of the phrenic nerve, there is no expectation for postoperative diaphragmatic function (attributed to the surgical procedure), and the effects of plication on respiratory mechanics are probably indirect. Plication aims to restore the position of the paralyzed diaphragm, relieve the compression of the lung parenchyma, allow its re-expansion, stabilize the thoracic base and the mediastinum, strengthen and render effective the respiratory function of the intercostal, perithoracic, and abdominal muscles (3, 42,47). Even in bilateral diaphragmatic plication (in a young man) the thoracic cage inspiratory function could compensate for the non-functioning plicated hemidiaphragms (60).
Phrenic nerve repair
Restoration of diaphragmatic function by phrenic nerve repair represents a curative treatment.
Merav et al. (71) described the feasibility of restoration of diaphragmatic motor function by early repair of a transected phrenic nerve. Brouillette et al. (72) reported return of right diaphragmatic function after end-to-end anastomosis of a transected phrenic nerve in a 16-month-old infant with respiratory failure due to bilateral diaphragmatic paralysis after resection of a thoracic teratoma.
Kaufman et al. (73) reported re-inervation of the paralysed diaphragm by phrenic nerve reconstruction (neurolysis, sural nerve interposition, end-to-side neurotization) in carefully selected patients with symptomatic unilateral phrenic nerve paralysis after surgery (including CABG in one patient), chiropractic manipulation, trauma, or anesthetic blocks.
Comments on our case and personal perspective
The incidence of phrenic nerve injury after in valve operation without local cooling is extremely low (34,50). Nevertheless, diaphragmatic paresis after valve surgery still occurs (46, 54) and reoperation has been related with increased risk of phrenic nerve injury (10, 12,13,56).
In out patient phrenic nerve injury was not identified intraoperatively, and it was probably related to thermal and/or traction injury. Since transection is highly unlikely, recovery was expected, but according to all literature reports it would require a minimum of several months to occur.
In our opinion, when diaphragmatic paresis/paralysis is causing disabling dyspnoea in adults postcardiotomy, not allowing weaning from non invasive ventilation and oxygen supplementation, hindering mobilization, rehabilitation and hospital discharge, early plication should be considered. Although the injury may be reversible the pulmonary morbidity can be life threatening not only in patients with COPD, but also in older patients with limited cardiac and physical reserve, who may not survive until phrenic nerve recovery is achieved, but succumb to respiratory complications. Lung atelectasis, prolonged hospitalization, inappropriate food intake (due to dyspnoea), inappropriate sleep (due to orthopnoea), inappropriate mobilization (due to exertional dyspnoea) predispose to infections and cause muscle wasting and further dyspnoea.
In our opinion, when plication is performed the main goal is to restore the normal anatomical position of the plicated hemidiaphragm at the position of full inspiration as described by Celik et al. (3), without rendering it excessively taut (overcorrection) or leaving it elevated (undercorrection) as highlighted by Leo et al. (70).
In our opinion, an anteromedial to posterolateral oblique plication suture line from the cardiophrenic angle, posteriorly to the phrenic nerve, to the costophrenic recess, being parallel to the long axis of the right leaflet of the central tendon appears “anatomically correct”. Interrupted, non absorbable, Teflon felt pledgeted U stiches offer safe suturing in thin, friable and/or oedematous tissues.
Conclusions
In most patients with postcardiotomy unilateral diaphragmatic paresis/paralysis surgical treatment is not required. In a significant percentage of patients spontaneous recovery of diaphragmatic function occurs (17,18,22-25,27,28,33,49,54). Spontaneous recovery of cold induced injury occurred in more than 94% of patients at 2 years (18, 22,25,27) while recovery of mammary artery harvesting related injury occurred in more than 50% of patients after the first year (33,49)
In persistent symptomatic unilateral diaphragmatic paresis/paralysis in the adult, diaphragmatic plication, applied after various observation periods commonly exceeding 6 months, was usually associated with low morbidity and mortality (31,32, 35-37,44,63-65), and offered subjective ( 3,32,44,45,63-66) and objective (2, 3,31, 32,35,36,45,62,64,66) improvement, preserved overtime (31,32, 35,45,62,64,66).
Persistent unilateral postcardiotomy paresis may remain asymptomatic or cause mild symptoms in the absence of underlying diseases. In patients with COPD though, phrenic nerve injury after CABG had a major negative impact on the immediate and the midterm results, regarding both morbidity and mortality ( 30). We speculate that postcardiotomy phrenic nerve injury in high risk patients, such as elderly, patients with compromised/borderline cardiac and/or hepatic and/or renal function, but also in patients with limited cardiac and organ reserve may also be associated with poor prognosis.
Early postcardiotomy plication is the established treatment in infants and young children and is mainly applied due to failure to wean from mechanical ventilation. Early postcardiotomy plication in adults has been very rarely reported, mainly applied in patients with severe respiratory failure. The results were encouraging, although mortality was reported in high risk patients, including an obese patient with COPD and 2 patients who underwent plication due to failure to wean from mechanical ventilation (16,56-59). In our opinion consideration of early postcardiotomy plication is justified in high risk patients, preferentially through minimally invasive approaches and before exhaustion of remaining reserve.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Qureshi A., Diaphragm paralysis. Semin Respir Crit Care Med. 2009;315-20. [PubMed ]
- Groth SS, Andrade RS, Diaphragm plication for eventration or paralysis: a review of the literature. Ann Thorac Surg. 2010;S2146-50. [PubMed ]
- Celik S, Celik M, Aydemir B, Long-term results of diaphragmatic plication in adults with unilateral diaphragm paralysis. J Cardiothorac Surg. 2010;5:111. [PubMed ]
- Tripp HF, Bolton JW, Phrenic nerve injury following cardiac surgery: a review. J Card Surg. 1998;218-23. [PubMed ]
- Fell SC, Surgical anatomy of the diaphragm and the phrenic nerve. Chest Surg Clin N Am. 1998;281-94. [PubMed ]
- de Vries TS, Koens BL, Vos A, Surgical treatment of diaphragmatic eventration caused by phrenic nerve injury in the newborn. J Pediatr Surg. 1998;602-5. [PubMed ]
- Shoemaker R, Palmer G, Brown JW, Aggressive treatment of acquired phrenic nerve paralysis in infants and small children. Ann Thorac Surg. 1981;250-9. [PubMed ]
- Stone KS, Brown JW, Canal DF, Long-term fate of the diaphragm surgically plicated during infancy and early childhood. Ann Thorac Surg. 1987;62-5. [PubMed ]
- Hamilton JR, Tocewicz K, Elliott MJ, Paralysed diaphragm after cardiac surgery in children: value of plication. Eur J Cardiothorac Surg. 1990;487-90 ;:-;. [PubMed ]
- Mok Q, Ross-Russell R, Mulvey D, Phrenic nerve injury in infants and children undergoing cardiac surgery. Br Heart J. 1991;287-92. [PubMed ]
- Yellin A, Lieberman Y, Barzilay Z., Postoperative unilateral diaphragmatic paralysis in children, a plea for early plication. Thorac Cardiovasc Surg. 1991;221-3. [PubMed ]
- Tönz M, von Segesser LK, Mihaljevic T, Clinical implications of phrenic nerve injury after pediatric cardiac surgery. J Pediatr Surg. 1996;1265-7. [PubMed ]
- de Leeuw M, Williams JM, Freedom RM, Impact of diaphragmatic paralysis after cardiothoracic surgery in children. J Thorac Cardiovasc Surg. 1999;510-7. [PubMed ]
- Joho-Arreola AL, Bauersfeld U, Stauffer UG, Incidence and treatment of diaphragmatic paralysis after cardiac surgery in children. Eur J Cardiothorac Surg. 2005;53-7. [PubMed ]
- Baker CJ, Boulom V, Reemtsen BL, Hemidiaphragm plication after repair of congenital heart defects in children: quantitative return of diaphragm function over time. J Thorac Cardiovasc Surg. 2008;56-61. [PubMed ]
- Simansky DA, Paley M, Refaely Y, Diaphragm plication following phrenic nerve injury: a comparison of paediatric and adult patients. Thorax. 2002;613-6. [PubMed ]
- Dajee A, Pellegrini J, Cooper G, Phrenic nerve palsy after topical cardiac hypothermia. Int Surg. 1983;345-8. [PubMed ]
- Chandler KW, Rozas CJ, Kory RC, Bilateral diaphragmatic paralysis complicating local cardiac hypothermia during open heart surgery. Am J Med. 1984;243-9. [PubMed ]
- Kohorst WR, Schonfeld SA, Altman M, Bilateral diaphragmatic paralysis following topical cardiac hypothermia. Chest. 1984;65-8. [PubMed ]
- Rousou JA, Parker T, Engelman RM, Phrenic nerve paresis associated with the use of iced slush and the cooling jacket for topical hypothermia. J Thorac Cardiovasc Surg. 1985;921-5. [PubMed ]
- Esposito RA, Spencer FC, The effect of pericardial insulation on hypothermic phrenic nerve injury during open-heart surgery. Ann Thorac Surg. 1987;303-8. [PubMed ]
- Curtis JJ, Nawarawong W, Walls JT, Elevated hemidiaphragm after cardiac operations: incidence, prognosis, and relationship to the use of topical ice slush. Ann Thorac Surg. 1989;764-8. [PubMed ]
- Abd AG, Braun NM, Baskin MI, Diaphragmatic dysfunction after open heart surgery: treatment with a rocking bed. Ann Intern Med. 1989;881-6. [PubMed ]
- Wilcox PG, Paré PD, Pardy RL, Recovery after unilateral phrenic injury associated with coronary artery revascularization. Chest. 1990;661-6. [PubMed ]
- Olopade CO, Staats BA, Time course of recovery from frostbitten phrenics after coronary artery bypass graft surgery. Chest. 1991;1112-5. [PubMed ]
- Allen BS, Buckberg GD, Rosenkranz ER, Topical cardiac hypothermia in patients with coronary disease. An unnecessary adjunct to cardioplegic protection and cause of pulmonary morbidity. J Thorac Cardiovasc Surg. 1992;626-31. [PubMed ]
- Efthimiou J, Butler J, Woodham C, Phrenic nerve and diaphragm function following open heart surgery: a prospective study with and without topical hypothermia. Q J Med. 1992;845-53. [PubMed ]
- Nikas DJ, Ramadan FM, Elefteriades JA, Topical hypothermia: ineffective and deleterious as adjunct to cardioplegia for myocardial protection. Ann Thorac Surg. 1998;28-31. [PubMed ]
- Mazzoni M, Solinas C, Sisillo E, Intraoperative phrenic nerve monitoring in cardiac surgery. Chest. 1996;1455-60. [PubMed ]
- Cohen AJ, Katz MG, Katz R, Phrenic nerve injury after coronary artery grafting: is it always benign? . Ann Thorac Surg. 1997;148-53. [PubMed ]
- Graham DR, Kaplan D, Evans CC, Diaphragmatic plication for unilateral diaphragmatic paralysis: a 10-year experience. Ann Thorac Surg. 1990;248-51. [PubMed ]
- Higgs SM, Hussain A, Jackson M, Long term results of diaphragmatic plication for unilateral diaphragm paralysis. Eur J Cardiothorac Surg. 2002;294-7. [PubMed ]
- Deng Y, Byth K, Paterson HS, Phrenic nerve injury associated with high free right internal mammary artery harvesting. Ann Thorac Surg. 2003;459-63. [PubMed ]
- Deng Y, Sun Z, Ma J, Semi-skeletonized internal mammary grafts and phrenic nerve injury: cause-and-effect analysis. J Huazhong Univ Sci Technolog Med Sci. 2006;455-9. [PubMed ]
- Mouroux J, Venissac N, Leo F, Surgical treatment of diaphragmatic eventration using video-assisted thoracic surgery: a prospective study. Ann Thorac Surg. 2005;308-12. [PubMed ]
- Freeman RK, Wozniak TC, Fitzgerald EB. Functional and physiologic results of video-assisted thoracoscopic diaphragm plication in adult patients with unilateral diaphragm paralysis. Ann Thorac Surg 2006;81:1853-7; discussion 1857.
- Leo F, Girotti P, Tavecchio L, Anterior diaphragmatic plication in mediastinal surgery: the “reefing the mainsail” technique. Ann Thorac Surg. 2010;2065-7. [PubMed ]
- Cheng DC, Martin J, Lal A, Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila). 2011;84-103. [PubMed ]
- Kaufman MR, Elkwood AI, Rose MI, Reinnervation of the paralyzed diaphragm: application of nerve surgery techniques following unilateral phrenic nerve injury. Chest. 2011;191-7. [PubMed ]
- Rochester DF, Esau SA, Assessment of ventilatory function in patients with neuromuscular disease. Clin Chest Med. 1994;751-63. [PubMed ]
- Lyall RA, Donaldson N, Polkey MI, Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain. 2001;2000-13. [PubMed ]
- Ribet M, Linder JL, Plication of the diaphragm for unilateral eventration or paralysis. Eur J Cardiothorac Surg. 1992;357-60. [PubMed ]
- Ko MA, Darling GE, Acquired paralysis of the diaphragm. Thorac Surg Clin. 2009;501-10. [PubMed ]
- Wright CD, Williams JG, Ogilvie CM, Results of diaphragmatic plication for unilateral diaphragmatic paralysis. J Thorac Cardiovasc Surg. 1985;195-8. [PubMed ]
- Gazala S, Hunt I, Bédard EL, Diaphragmatic plication offers functional improvement in dyspnoea and better pulmonary function with low morbidity. Interact Cardiovasc Thorac Surg. 2012;505-8. [PubMed ]
- Summerhill EM, El-Sameed YA, Glidden TJ, Monitoring recovery from diaphragm paralysis with ultrasound. Chest. 2008;737-43. [PubMed ]
- Le Pimpec-Barthes F, Brian E, Vlas C, Surgical treatment of diaphragmatic eventrations and paralyses. Rev Mal Respir. 2010;565-78. [PubMed ]
- Yemisci OU, Cosar SN, Karatas M, A prospective study of temporal course of phrenic nerve palsy in children after cardiac surgery. J Clin Neurophysiol. 2011;222-6. [PubMed ]
- Katz MG, Katz R, Schachner A, Phrenic nerve injury after coronary artery bypass grafting: will it go away? . Ann Thorac Surg. 1998;32-5. [PubMed ]
- Cheng DC, Martin J, Lal A, Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila). 2011;84-103. [PubMed ]
- Gayan-Ramirez G, Gosselin N, Troosters T, Functional recovery of diaphragm paralysis: a long-term follow-up study. Respir Med. 2008;690-8. [PubMed ]
- Marco JD, Hahn JW, Barner HB, Topical cardiac hypothermia and phrenic nerve injury. Ann Thorac Surg. 1977;235-7. [PubMed ]
- Chalmers RM, Howard RS, Wiles CM, Use of the rocking bed in the treatment of neurogenic respiratory insufficiency. QJM. 1994;423-9. [PubMed ]
- Kodric M, Trevisan R, Torregiani C, Inspiratory muscle training for diaphragm dysfunction after cardiac surgery. J Thorac Cardiovasc Surg. 2012; . [PubMed ]
- Groth SS, Rueth NM, Kast T, Laparoscopic diaphragmatic plication for diaphragmatic paralysis and eventration: an objective evaluation of short-term and midterm results. J Thorac Cardiovasc Surg. 2010;1452-6. [PubMed ]
- Glassman LR, Spencer FC, Baumann FG, et al. Successful plication for postoperative diaphragmatic paralysis in an adult. Ann Thorac Surg 1994;58:1754-5; discussion 1757-8.
- Takara I, Ooshiro M, Iha H, A successful weaning from mechanical ventilation by diaphragmatic plication for unilateral diaphragmatic paralysis. Masui. 2004;184-7. [PubMed ]
- Kuniyoshi Y, Yamashiro S, Miyagi K, Diaphragmatic plication in adult patients with diaphragm paralysis after cardiac surgery. Ann Thorac Cardiovasc Surg. 2004;160-6. [PubMed ]
- Shiraishi Y, Miyamoto T, Shimada I, Bilateral diaphragmatic plication for an adult patient. Nihon Kyobu Geka Gakkai Zasshi. 1991;1927-31. [PubMed ]
- Ohta M, Ikeda N, Tanaka H, Satisfactory results of diaphragmatic plication for bilateral phrenic nerve paralysis. Ann Thorac Surg. 2007;1029-31. [PubMed ]
- Gharagozloo F, McReynolds SD, Snyder L, Thoracoscopic plication of the diaphragm. Surg Endosc. 1995;1204-6. [PubMed ]
- Mouroux J, Padovani B, Poirier NC, Technique for the repair of diaphragmatic eventration. Ann Thorac Surg. 1996;905-7. [PubMed ]
- Joo Hwang J, Kim KD. Thoracoscopic diaphragmatic plication using three 5 mm ports. Interact Cardiovasc Thorac Surg. 2007;280-1. [PubMed ]
- Freeman RK, Van Woerkom J, Vyverberg A, Long-term follow-up of the functional and physiologic results of diaphragm plication in adults with unilateral diaphragm paralysis. Ann Thorac Surg. 2009;1112-7. [PubMed ]
- Lai DT, Paterson HS, Mini-thoracotomy for diaphragmatic plication with thoracoscopic assistance. Ann Thorac Surg. 1999;2364-5. [PubMed ]
- Versteegh MI, Braun J, Voigt PG, Diaphragm plication in adult patients with diaphragm paralysis leads to long-term improvement of pulmonary function and level of dyspnea. Eur J Cardiothorac Surg. 2007;449-56. [PubMed ]
- Hwang Z, Shin JS, Cho YH, A simple technique for the thoracoscopic plication of the diaphragm. Chest. 2003;376-8. [PubMed ]
- Moon SW, Wang YP, Kim YW, Thoracoscopic plication of diaphragmatic eventration using endostaplers. Ann Thorac Surg. 2000;299-300. [PubMed ]
- Balci AE, Ozyurtkan MO, Clinical and surgical specifications of adult unilateral diaphragmatic eventration according to their aetiology in 28 patients. Importance of using diaphragmatic patch and minimal thoracotomy incision. Eur J Cardiothorac Surg. 2010;606-12. [PubMed ]
- Leo F, Venissac N, Morales F, Plication for diaphragmatic eventration: a simple technique, not a simple problem. Chest. 2004;1170-author reply 1170-1 ;:;. [PubMed ]
- Merav AD, Attai LA, Condit DD, Successful repair of a transected phrenic nerve with restoration of diaphragmatic function. Chest. 1983;642-4. [PubMed ]
- Brouillette RT, Hahn YS, Noah ZL, Successful reinnervation of the diaphragm after phrenic nerve transection. J Pediatr Surg. 1986;63-5. [PubMed ]
- Kaufman MR, Elkwood AI, Rose MI, Reinnervation of the paralyzed diaphragm: application of nerve surgery techniques following unilateral phrenic nerve injury. Chest. 2011;191-7. [PubMed ]