A systematic review and meta-analysis of thymic mucosa-associated lymphoid tissue lymphoma
Introduction
Mucosa-associated lymphoid tissue (MALT) lymphoma is a low-grade B cell lymphoma that normally occurs in the gastrointestinal tract (50%), lung (14%), and head and neck (14%) region (1,2). The prevalence of MALT lymphoma originating from the thymus is much lower than that from the organs mentioned earlier. Thymic MALT lymphoma is so rare that most studies on it are case reports or series, whose emphasis is mainly on the pathological features or molecular findings of the tumors. Given that, treatments for thymic MALT lymphoma are primarily based on the clinician's experience. Surgery is widely considered as a reasonable option, but surgical approaches and surgical extent have not reached a consensus. In addition, the application of radiotherapy and systemic therapy also varies from patient to patient.
In this meta-analysis and systematic review, we aimed to accumulate existing evidence to learn the clinical characteristics, management, and follow-up in case of patients with thymic MALT lymphoma. We designed this study according to MOOSE guidelines (3) and present the following article in accordance with the MOOSE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-81/rc).
Methods
Study design
Considering the rarity of the disease, we included all types of studies (controlled studies, observational studies, case series and case reports) that provided information on patients histologically diagnosed with thymic MALT lymphoma. Studies in which thymic MALT lymphoma patients were analyzed with other diseases (such as other thymic lymphomas or cystic thymic lesions) as a whole were excluded, due to the unavailability of information specific to each case or aggregated data specific to thymic MALT lymphoma. We also excluded review articles, conference abstracts, and studies on animals or cell lines. There were no restrictions on the range of interventions, which included diagnostic procedures such as biopsies and treatments including drug therapy, radiotherapy and surgery, or year of publication. We did not set language restrictions while searching the literature to ensure that study inclusion was as comprehensive as possible. However, when selecting eligible studies by reviewing abstracts or full texts, we only included publications that were accessible to the authors based on their languages (Chinese and English).
Literature selection and data collection
The literature search was conducted on 15 September 2021 from seven databases (PubMed, Scopus, Embase, Cochrane Library, ClinicalTrials.gov, NCBI, and SinoMed). The search strategy was mainly as follows: (“mucosa associated lymphoid tissue lymphoma” OR “MALT” OR “marginal zone B cell lymphoma”) AND (“thymic” OR “thymus”). It should be noted that search terms were adjusted slightly from database to database, for example, in PubMed, we replaced (“thymic” OR “thymus”) with (“thym*”) to include more publications. Besides, we reviewed conference lists of obtained articles to prevent missed records.
All records were imported into EndNote X7.8 to remove duplicates. Two investigators (ZMX, CYY) independently reviewed the titles and abstracts of all records to assess their eligibility. When inconsistencies occurred, consensus was obtained by discussion. If necessary, a third investigator (HC) was consulted to make the final decision. Two other investigators (ZMX, BWL) independently screened the full-text articles that were presumed eligible. In case of inconsistencies, consensus was obtained through discussion. If necessary, a third investigator (LHS) was consulted to make final decision.
The following data were expected to be retrieved from publications: gender, age, comorbidities with autoimmune disease and hyperglobulinemia, Ann Arbor Stage, tumor size, interventions, follow-up period, and events that occurred during follow-up. Events included tumor relapse, progression, metastasis, and all-cause death. When an event occurrence was reported, the case would be recorded as “eventful”. If no event was reported at the last follow-up, the case would be recorded as “uneventful”. Two investigators (ZMX, ZJQ) extracted data and completed a designed data extraction form. Two forms were compared, and discrepancies were resolved by discussion.
Quality assessment
We used the assessment tool proposed by Murad et al. (4) to evaluate the methodological quality of the articles because all included articles were case reports/series. The tool assessed four domains of included articles, such as the selection of patients, the ascertainment of exposure (translated to specific diagnosis and treatment documents) and outcome, follow-up (longer than six months), and reporting (giving the reason of treatment or providing an insight into knowing the disease). The ascertainment of exposure and outcome was evaluated separately as two parts. Every published article was assessed based on five aspects (patient selection, exposure, outcome, follow-up, and reporting), and each part was assigned a score of 1 if it satisfied the definition.
Statistical analysis
Data extracted from case reports and case series were presented in two tables. Comparative quantitative analysis was performed between limited-stage (Ann Arbor Stage I–II) and advanced-stage (Ann Arbor Stage III–IV) and between eventful and uneventful patients in terms of age, gender, concurrent diseases, tumor size, lymph node invasion or involvement of other organs, and treatment. Student’s t-test was applied to continuous variables, and the Chi-square test and Fisher’s exact test was applied to categorical variables. Probability values that were less than 0.05 were considered to indicate statistical significance. Survival analysis was performed on survival R package (version 3.2-10) and Kaplan-Meier plots were generated using survminer package (version 0.4.9). Random-effects model was used to pool proportion of case series (4). Statistical analysis was performed on R statistic software (version 3.6.3, R foundation).
Results
Search results
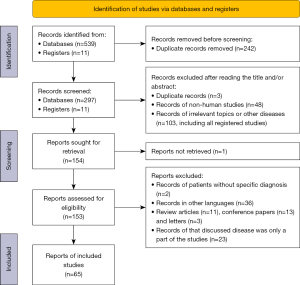
Our initial search identified 539 articles and 11 registered studies. Ultimately, 65 articles were eligible, including 52 case reports (articles that reported information about patients respectively) and 13 case series (articles that aggregated data of patients) (5). The selected procedure is depicted in Figure 1.
The quality assessment outcomes of these studies are listed in Table 1 and Table 2. Case reports were inferior to case series in terms of patient selection (P<0.001).
Table 1
| Reference (first author, year) | Selection | Exposure | Outcome | Follow-up | Reporting | Overall |
|---|---|---|---|---|---|---|
| Arai, 2017 (6) | 0 | 1 | 1 | 0 | 0 | 2 |
| Braham, 2009 (7) | 0 | 1 | 1 | 1 | 0 | 3 |
| Chang, 2012 (8) | 0 | 1 | 1 | 1 | 0 | 3 |
| Chen, 2014 (9) | 0 | 1 | 1 | 1 | 1 | 4 |
| Chen, 2020 (10) | 0 | 1 | 0 | 0 | 0 | 1 |
| Covelli, 2012 (11) | 0 | 1 | 0 | 0 | 1 | 2 |
| Di Loreto, 1996 (12) | 0 | 1 | 1 | 1 | 0 | 3 |
| Fujimoto, 2012 (13) | 0 | 1 | 1 | 1 | 1 | 4 |
| Go, 2011 (14) | 1 | 1 | 1 | 1 | 1 | 5 |
| Harigae, 2002 (15) | 0 | 1 | 1 | 1 | 0 | 3 |
| Hirokawa, 2019 (16) | 0 | 1 | 1 | 1 | 1 | 4 |
| Hu, 2017 (17) | 0 | 1 | 1 | 0 | 0 | 2 |
| Isaacson, 1990 (18) | 0 | 1 | 1 | 0† | 0† | 2 |
| Kamimura, 2002 (19) | 0 | 1 | 0 | 0 | 1 | 2 |
| Kang, 2013 (20) | 0 | 1 | 1 | 1 | 1 | 4 |
| Kim, 1998 (21) | 0 | 1 | 1 | 0 | 1 | 3 |
| Kim, 2003 (22) | 0 | 1 | 1 | 1 | 0 | 3 |
| Kinoshita, 2008 (23) | 0 | 1 | 1 | 1 | 0 | 3 |
| Kitai, 2009 (24) | 0 | 1 | 1 | 0 | 1 | 3 |
| Kurabayashi, 2010 (25) | 0 | 1 | 1 | 0 | 1 | 3 |
| Kuroki, 2004 (26) | 1 | 0 | 0 | 0 | 1 | 2 |
| Lorsbach, 2000 (27) | 1 | 1 | 0 † | 0 † | 1 | 3 |
| Maeda, 2008 (28) | 0 | 1 | 1 | 1 | 1 | 4 |
| Masunaga, 2008 (29) | 0 | 0 | 0 | 0 | 1 | 1 |
| McCluggage, 2000 (30) | 0 | 0 | 0 | 0 | 1 | 1 |
| Momoi, 2016 (31) | 0 | 1 | 0 | 0 | 1 | 2 |
| Moriyama, 2000 (2) | 0 | 1 | 1 | 1 | 1 | 4 |
| Muramatsu, 2013 (32) | 0 | 1 | 1 | 0 | 1 | 3 |
| Nagasaka, 2000 (33) | 0 | 1 | 1 | 1 | 1 | 4 |
| Naithani, 2012 (34) | 0 | 1 | 1 | 1 | 1 | 4 |
| Nakamura, 1993 (35) | 0 | 1 | 1 | 1 | 0 | 3 |
| Ortonne, 2005 (36) | 0 | 1 | 0 | 1 | 0 | 2 |
| Ota, 2013 (37) | 0 | 1 | 1 | 1 | 1 | 3 |
| Petersen, 2015 (38) | 0 | 1 | 1 | 1 | 1 | 4 |
| Rymkiewicz, 2006 (39) | 0 | 1 | 1 | 1 | 1 | 4 |
| Sakamoto, 2009 (40) | 0 | 1 | 1 | 1 | 1 | 4 |
| Song, 2011 (41) | 0 | 1 | 0 | 0 | 0 | 1 |
| Sugimoto, 2014 (42) | 0 | 1 | 1 | 1 | 1 | 4 |
| Sun, 2012 (43) | 1 | 1 | 1 | 0† | 0 | 3 |
| Sunada, 2007 (44) | 0 | 1 | 0 | 0 | 1 | 2 |
| Sunohara, 2009 (45) | 0 | 1 | 1 | 1 | 1 | 4 |
| Takagi, 1992 (46) | 0 | 1 | 1 | 1 | 1 | 4 |
| Wang, 2016 (47) | 1 | 1 | 0† | 0† | 0 | 2 |
| Yamada, 2003 (48) | 0 | 1 | 1 | 1 | 1 | 4 |
| Yamasaki, 1998 (49) | 0 | 1 | 1 | 0† | 1 | 3 |
| Yan, 2018 (50) | 0 | 1 | 1 | 0 | 0 | 2 |
| Yanagiya, 2017 (51) | 0 | 1 | 1 | 1 | 1 | 4 |
| Yang, 2021 (52) | 0 | 0 | 0 | 0 | 1 | 1 |
| Yi, 1998 (53) | 0 | 1 | 0 | 0 | 0 | 1 |
| Yokose, 1998 (54) | 0 | 1 | 1 | 1 | 1 | 4 |
| You, 2020 (55) | 0 | 1 | 0 | 0 | 1 | 2 |
| Shi, 2018 (56) | 0 | 1 | 1 | 1 | 0 | 3 |
†, follow-up period is recorded but not in all cases or is shorter than 6 months.
Table 2
| Reference (first author, year) | Selection | Exposure | Outcome | Follow-up | Reporting | Score |
|---|---|---|---|---|---|---|
| Inagaki, 2002 (57) | 1 | 1 | 1 | 1 | 1 | 5 |
| Kominato, 2012 (58) | 1 | 0 | 0 | 0 | 1 | 2 |
| Yang, 2020 (59) | 1 | 1 | 1 | 1 | 1 | 5 |
| Shimizu, 2005 (60) | 0 | 1 | 1 | 1 | 1 | 4 |
| Shimizu, 2010 (61) | 1 | 1 | 0 | 0 | 1 | 3 |
| Takino, 2013 (62) | 1 | 1 | 1 | 1 | 1 | 5 |
| Wang, 2019 (63) | 1 | 1 | 1 | 0† | 0 | 3 |
| Weissferdt, 2011 (64) | 1 | 1 | 1 | 1 | 1 | 5 |
| Xu, 2020 (65) | 1 | 1 | 1 | 1 | 0 | 4 |
| Wu, 2020 (66) | 1 | 0 | 0 | 0 | 1 | 2 |
| Ye, 2015 (67) | 1 | 1 | 1 | 1 | 0 | 5 |
| Yoshida, 2006 (68) | 1 | 1 | 1 | 1 | 1 | 5 |
| Wang, 1993 (69) | 1 | 0 | 0 | 0 | 1 | 2 |
†, follow-up period is recorded but not in all cases or is shorter than 6 months.
Overall, we studied 69 cases from case reports, and the information extracted from them is listed in the Table S1. Three cases (26,46,53) (with marked references in Table S1) may have been reported more than once because the patients had similar information and the authors were from the same institution. We did not include articles with inadequate information. Individuals from case series were also shared in case reports or other series. As a result, rather than displaying the data of each patient in a case series, we tabulated the aggregated data and reported pooled proportion with I-squared values (Table 3). We also sorted data from case reports in Table 3.
Table 3
| Reference | Case | Female | Age range (years) | Asymptomatic† | Autoimmune disease† | Hyperglobulinemia† | Tumor size range (cm) | Surgery only† | Limited-stage† | Follow-up (months) | Uneventful† |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case reports | 69 | 53 | 11–75 | 42/58 | 44/63 | 30/33 | 1.5–17.5 | 36/66 | 39/47 | 2–132 | 48/53 |
| Yang, 2020 (59) | 9 | 7 | 35–72 | 5/7 | 2/2 | NA | 7.2–13.5 | 9/9 | NA | 10–96 | 5/5 |
| Shimizu, 2005 (60) | 4 | 3 | 30–75 | 3/4 | 1/4 | 3/4 | 7.5–12.5 | 4/4 | 4/4 | 8–96 | 4/4 |
| Inagaki, 2002 (57) | 15 | 12 | 36–75 | 10/15 | 9/13 | 9/13 | 3–17 | NA | 13/15 | 8–252 | 11/13 |
| Xu, 2020 (65) | 7 | 6 | 33–56 | 4/7 | 5/7 | 3/7 | 1.5–8.9 | 7/7 | 4/7 | 6–20 | 7/7 |
| Weissferdt, 2011 (64) | 6 | 6 | 48–59 | 3/6 | 3/6 | NA | 4–6 | 6/6 | NA | 1–3 years | 6/6 |
| Takino, 2013 (62) | 18 | 16 | 23–68 | 14/18 | 16/18 | NA | 3–19 | 11/18 | 17/18 | 6–252 | 17/17 |
| Wang, 2019 (63) | 4 | 4 | 29–53 | 2/4 | NA | 2/2 | 3–14 | 4/4 | NA | 2–19 | 4/4 |
| Yoshida, 2006 (68) | 9 | 8 | 45–75 | 9/9 | 7/7 | NA | 3–17 | 6/9 | 9/9 | 9–252 | 9/9 |
| Shimizu, 2010 (61) | 9 | 6 | 30–75 | 8/9 | 5/6 | 8/8 | NA | 8/9 | NA | NA | NA |
| Kominato, 2012 (58) | 14 | 14 | 38–68 | NA | 12/13 | NA | 1.7–14 | NA | 8/8 | NA | NA |
| Wang, 1993 (69) | 8 | 3 | 24–61 | 4/7 | NA | NA | 5.5–10 | NA | NA | NA | NA |
| Ye, 2015 (67) | 5 | 5 | 37–63 | NA | 5/5 | NA | NA | 4/5 | NA | 2–124 | 5/5 |
| Pooled proportion (95% CI) | N/A | 83.5% (72.2–90.8%) |
N/A | 72.2% (64.4–78.9%) |
78.6% (65.5–87.7%) |
82.3% (60.9–93.3%) |
N/A | 87.7% (64.9–96.5%) |
84.2% (66.4–93.5%) |
N/A | 95.0% (76.2–99.1%) |
| I-squared (95% CI) | N/A | 0% (0–56.6%) |
N/A | 0% (0–60.2%) |
0% (0–60.2%) |
33.1% (0–73.1%) |
N/A | 0% (0–62.4%) |
41.4% (0–75.2%) |
N/A | 0% (0–62.4%) |
†, the data is presented in the form of the number of patients with the character in the table header/the number of all patients with relative information. NA, information not available; CI, confidence interval; N/A, not applicable.
Clinical characteristics
Female patients account for 66.7% to 100% in the case series and 76.8% in case reports. Twenty-four patients had nationality/race records in case reports, among whom 19 (79.2%) were Asians (8 from China, 9 from Japan, 1 from Korea and 1 from Laos), while in the case series, only 1 author (64) reported 5 Caucasian patients. Other authors recorded all the cases coming from Japan or China. The median age at diagnosis of cases from case reports was 50 years (range, 11–75 years).
More than half of the patients in the case series and 60.9% of cases in case reports were asymptomatic. Patients usually accidentally found an anterior mass during check-ups. Lorsbach et al. (27) reported a case whose thymic tumor was identified and resected during heart surgery. Symptomatic patients generally complained of non-specific presentations. The most common symptoms were chest discomfort (6.9%) and cough (6.9%). Symptoms of lymphoma, including fever, weight loss, and night sweat, were not frequent in thymic MALT lymphoma. Only 2 (3.4%) patients had a fever, and 1 (1.7%) had weight loss.
Concurrent autoimmune disease was identified in 69.8% of patients in case reports. Sjogren’s syndrome (SS) was most common and observed in 50.8% of patients. Most case series reported that more than half of patients had autoimmune diseases except one reported by Shimizu et al. (60) in 2005. The team found 1 patient had SS while the other 3 were healthy. However, in 2010, the same team reported that 83.3% of patients had autoimmune diseases with an expanded sample size. Hyperglobulinemia was presented in 90.9% of cases, 21 of whom were polyclonal. Five case series had reported hyperglobulinemia incidence. The percentage of patients who had this complication was 33–100%.
Tumor characteristics
The median size of tumors in the case reports was 7.45 cm (range, 1.5–17.5 cm). In the case series, the minimum size was 1.5 cm (65) while the maximum size was 19 cm (62). Forty-seven cases recorded stage information according to the Ann Arbor staging system, with 63.8% presented as stage I, 19.1% as stage II, 4.3% as stage III, and 12.8% as stage IV. Six case series had records of Ann Arbor stage, and most patients (57.1–100%) presented as limited-stage. There was no difference between patients at limited- and advanced-stage in terms of age, gender, tumor size, or presence of an autoimmune disease or hyperglobulinemia.
Diagnosis
According to clinical and imaging impression, 23 cases had reports of suspected diagnosis, among whom 60.9% patients were initially suspected of having thymoma. Only 2 case series (52,63) had reported suspected diagnosis, and all patients were suspected to have thymoma. Ota et al. (37) diagnosed a patient with MALT lymphoma based on image findings before any treatment.
Preoperative biopsy was documented being performed in 7 cases in case reports. Three patients underwent needle biopsy, two of whom did not acquire a confirmed diagnosis (41,45), and one of whom was diagnosed with lymphoma without recognizing specific subtype (43). Two patients received a biopsy by video-assisted thoracotomy surgery (VATS). One case was misdiagnosed with plasmacytic neoplasm. Without any treatment, the tumor enlarged and compressed surrounding tissue, leading to surgical treatment, after which a final definite diagnosis was made (15). In another case with an accurate diagnosis, the extent of the biopsy was not described, and we could not rule out the possibility of tumor resection (19). Two cases have not documented the approach of biopsy, but both patients were diagnosed with thymic MALT lymphoma. Three cases were described in two case series as not receiving surgical treatment, although the basis of diagnosis was not clear (62,68).
Treatments
In case reports, 54.5% of patients accepted surgery alone, while in case series, this percentage was higher, ranging from 61.1% to 100%. Among all 67 surgical cases, 56.1% received tumor resection (tumors were removed, but the extent of the surgeries was unknown), 21.2% received total thymectomy (both thymus and tumor were removed, without surrounding lymph nodes), and 16.7% received extended thymectomy (tumor, thymus, and surrounding lymph nodes were removed). In terms of surgery approaches, 21 (60%) in 35 cases who had relative information had median sternotomy while 11 (32.4%) had VATS. The stage of the tumor did not influence the choice of either surgical extent (P=0.616) or surgical approach (P=1.000). Moreover, surgical extent (P=0.530) and surgical approach (P=0.538) did not differ among eventful and uneventful patients.
Postoperative treatments, including radiotherapy, systemic therapy, and a combination of them, were applied to 20 (35.7%) patients. Among these patients, 14 received systemic therapy alone, 4 received radiotherapy alone and 2 received combination therapy. We found no difference when these therapies were used in limited- and advanced-stage cancers (P=0.187). However, when we compared patients with Ann Arbor I to patients with other stages, the latter received postoperative treatments more frequently (P=0.011). Furthermore, the administration of these therapies did not differ between eventful and uneventful patients (P=0.637).
Two patients (both classified as Ann Arbor II) were reported as not having undergone surgery and receiving concomitant radiation and systemic therapy. One patient was lost during follow-up, and another achieved complete remission in 13 months after surgery.
Prognosis
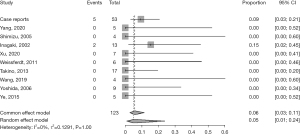
The median overall survival (OS) time was not determined, and the median progression-free survival (PFS) time was 132 months. Of 53 patients, the condition of 48 (90.6%) who had prognosis documents was uneventful. Two patients (one with no stage information and another classified as Ann Arbor I) were found to have lymph node metastasis at 132 and 2 months after surgery, respectively (19,36). Two patients (both classified as Ann Arbor IV) had metastasis of other organs: one in the skin 12 months after surgery and another in the liver 6 years after surgery (12,39). One patient (classified as Ann Arbor III) (33) relapsed and died of cardiac shock eight months after his surgery, perhaps induced by tumor compression. The pooled event rate of case series was 5.01% (95% CI: 0.88–23.81%), and the forest plot was depicted in Figure 2.
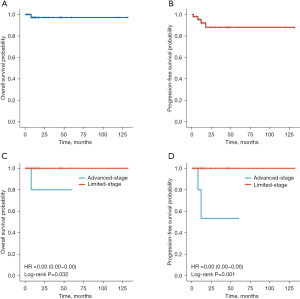
The five-year OS rate and five-year PFS rate were 97.2% and 88.4%, respectively (Figure 3A,3B). Advanced-stage patients suffered more events than limited-stage patients (P=0.009). And advanced-stage disease was associated with worse OS (P=0.032) and PFS (P=0.001) (Figure 3C,3D). Patients with concurrent MALT lymphoma in other non-lymphoid organs (classified as Ann Arbor IV), such as lung, stomach, and salivary gland, were liable to the events (P=0.047). However, lymph node metastasis (classified as Ann Arbor II or III) diagnosis did not correlate with the occurrence of events (P=0.508).
Discussion
Thymic MALT lymphoma is a rare disease, accounting for only about 3% of anterior mediastinum mass (70). To the best of our knowledge, there are only about 100 cases reported. Because some cases were reported multiple times, specific data could not be obtained.
In 2010, Shimizu et al. (61) depicted a diagnostic flowchart for thymic MALT lymphoma, proposing that Asian patients with cystic thymic mass, autoimmune disease, and hyperglobulinemia should be considered to have thymic MALT lymphoma. However, preoperative diagnosis based on clinical and imaging impression was frequently prone to errors.
Thymic MALT lymphoma should be distinguished from other types of lymphoma and other thymic cystic neoplasms. Generally, lymphomas originating from the thymus mainly include Hodgkin’s lymphoma, lymphoblastic lymphoma, and large B cell lymphoma (27,70). Patients diagnosed with the former two diseases are younger than patients with MALT lymphoma, and the occurrence of autoimmune diseases in such patients is also rare. Large B cell lymphoma was diagnosed at a similar age as thymic MALT lymphoma. However, large B cell lymphoma often presents with solid lesions and invasive behavior (66), which were not common in thymic MALT lymphoma. There were only two thymic MALT lymphoma cases (4.5%) reporting the invasion of surrounding tissues by the tumor. Other thymic neoplasms such as thymic hyperplasia, thymic cysts, and thymic epithelial tumor could be manifested as cystic lesions in image findings, which were hard to differentiate with thymic MALT lymphoma (14). Therefore, it is well-recognized that a definite diagnosis mainly depends on the pathological factors and at times on immunohistochemistry and gene arrangement tests (2,64,69).
Even with the use of a microscope, the diagnosis was challenging; therefore, the quantity of tissue available was crucial. Biopsy analysis was conducted on seven patients in our study, and five of them did not have a definitive diagnosis. Thus, to obtain a sufficient quantity of tissue to allow a precise diagnosis, resection of the tumor by surgery is recommended rather than biopsy (15,53,71). Surgery is also considered highly effective as a therapeutic method (24,37,57). For patients with limited-stage disease, features of indolent course, intracapsular tumor, and less aggressive behavior have made surgery an optimal therapy. For patients with advanced-stage disease, surgery was also helpful to avoid tumor enlargement and compression of the surrounding tissue.
The surgical approach and extent have not reached a consensus. In our study, neither of them correlate with the occurrence of events, which might be because of the infrequency of events and inexplicit records. Most cases recorded tumor resection instead of the specific surgical extent; as a result, the difference among partial thymectomy, thymectomy, and extended thymectomy could not be evaluated. Thymectomy is generally agreeable for thymic MALT lymphoma patients in all ages as long as the tumor is not invading surrounding vessels or organs. The meaning of regional lymph node dissection is unclear. In the current study, five patients were found to have lymph node metastasis during surgery, and more patients who did not receive extended thymectomy may have invisible lymph node metastasis (37). Therefore, we assumed that regional lymph node sampling could help assess the disease stage. Additionally, the diagnosis might be obscured by intraoperative frozen pathology. For patients with a suspected diagnosis of other thymic malignancy, such as thymic carcinoma, lymph node dissection might be recommended (72). In terms of surgical approach, median sternotomy was used most of time, which was reasonable given the lack of a definitive diagnosis. Surgeons could use VATS to explore thorax and tumors now that VATS has been developed. Thoracotomy should be used if the tumor is invasive or if VATS is unable to remove the whole tumor (48).
Apart from surgery, radiotherapy is also an important local treatment for limited-stage MALT lymphoma (73). Some authors claimed that thymic MALT lymphoma patients could benefit from radiotherapy, though the relevant evidence is insufficient (50). In the current study, no patient received radiotherapy alone. Six patients in case reports received postoperative radiation, and five of these cases were uneventful during follow-up. The follow-up status of the remaining one was not reported. We were unable to determine if postoperative radiation could improve prognosis or whether surgery alone is sufficient due to lack of evidence.
Systemic treatment, with or without surgery, was recommended on limited-stage patients according to evidence from MALT lymphoma at other sites (73). In the current study, systemic therapy was not applied alone. Together with radiotherapy, it was proved effective in one patient, which could not determine its value in thymic MALT lymphoma. Sixteen patients received postoperative systemic treatment in case reports, two of them had tumor relapse (33,39) and one patient died (33). Similarly, we could not evaluate the efficacy of postoperative systemic therapy in this rare disease. However, by referring to the treatment modality of MALT lymphoma at other sites, we considered it as a preferred therapy for patients with advanced-stage disease or those whose tumors could not be resected completely. We need more studies to validate the efficacy of systemic therapy in thymic MALT lymphoma.
The regimen of systemic treatment varied. For indolent and low-grade SS-associated lymphoma, rituximab (RTX), an anti-CD20 drug, may achieve a good effect (75). Three patients in the study received RTX only after surgery, and they all had good prognoses (7,11,42). Other systemic therapies included CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (rituximab, cyclophosphamide, vincristine, and prednisolone) and their combination with RTX, and differences among them were not pronounced. Yan et al. (50) reported an interesting case in which the tumor shrank after using glucocorticoids for erythema. Because many individuals require glucocorticoids to treat autoimmune diseases, it was hypothesized that glucocorticoids would limit tumor growth. However, more research is needed to confirm this conclusion.
The prognosis for thymic MALT lymphoma is considered to be good. Inagaki et al. (57) reported that the 3- and 5-year OS rates as 89% and 85% respectively. In the present study, only one patient died. The 5-year OS and PFS rates were 97.2% and 88.4%, respectively. These outcomes may further confirm that thymic MALT lymphoma is an indolent disease. However, MALT lymphoma in multiple non-lymphoid organs may lead to a worse prognosis, which was indicated in our study and another study by Aria et al. (76).
The limitation of our study is obvious. Cases reports and case series increase the risk of bias, and the certainty of evidence provided by a systematic review of case reports and case series can be very low (77). However, because of no available higher evidence, an analysis of published cases may offer more evidence for decision-making than cases from a single institution (4). We excluded some studies due to the language restriction of the authors, including 33 Japanese studies, two French studies and one German study, which may have resulted in some cases being lost.
Conclusions
Thymic MALT lymphoma is so rare that a firm conclusion of treatment and prognosis is difficult to make. However, from current evidence, it seems that thymectomy is a reasonable method to diagnose and treat this disease, while lymph node sampling/dissection could be performed on selected patients. Postoperative therapy might not provide a better prognosis, but for patients with advanced-stage disease, it should be considered radically. Given the rarity of thymic MALT lymphoma, randomized trials could be difficult to perform, but a nationwide or even worldwide database may help physicians learn more about this disease.
Acknowledgments
The authors would like to express their gratitude to the clinicians and authors of previous studies who provided precious information and experience and made this study possible. The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-81/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-81/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-81/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplastic diseases of the haematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Histopathology 2000;36:69-86. [Crossref] [PubMed]
- Moriyama E, Yokose T, Kodama T, et al. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue in the thymus of a patient with pulmonary amyloid nodules. Jpn J Clin Oncol 2000;30:349-53. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60-3. [Crossref] [PubMed]
- Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet 2002;359:145-9. [Crossref] [PubMed]
- Arai H, Tajiri M, Kaneko S, et al. Two surgical cases of thymic MALT lymphoma associated with multiple lung cysts: possible association with Sjögren's syndrome. Gen Thorac Cardiovasc Surg 2017;65:229-34. [Crossref] [PubMed]
- Braham E, Capron J, Sene D, et al. Thymic marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue-type in a patient with Sjögren's syndrome and cryoglobulinaemia. Pathology 2009;41:701-3. [Crossref] [PubMed]
- Chang XY, Chen J, Jiang Y, et al. Mucosa-associated Lymphoid Tissue Lymphoma of the Thymus: A Stuty of 2 Cases. Medical Journal of Peking Union Medical College Hospital 2012;3:41-6.
- Chen PH, Chiang BL, Lu MY, et al. An 11-year and 10-month-old girl with purpura and chest pain. J Microbiol Immunol Infect 2014;47:438-40. [Crossref] [PubMed]
- Chen Q, Du Y, Prince S, et al. Primary thymic mucosa-associated lymphoid tissue lymphoma complicated with renal amyloidosis: A first case report. Medicine (Baltimore) 2020;99:e19462. [Crossref] [PubMed]
- Covelli M, Lanciano E, Tartaglia P, et al. Rituximab treatment for Sjogren syndrome-associated non-Hodgkin's lymphoma: case series. Rheumatol Int 2012;32:3281-4. [Crossref] [PubMed]
- Di Loreto C, Mariuzzi L, De Grassi A, et al. B cell lymphoma of the thymus and salivary gland. J Clin Pathol 1996;49:595-7. [Crossref] [PubMed]
- Fujimoto M, Yuba Y, Huang CL, et al. Coexistence of Epstein-Barr virus-associated lymphoproliferative disorder and marginal zone B-cell lymphoma of the thymus in a patient with rheumatoid arthritis treated with methotrexate. Histopathology 2012;61:1230-2. [Crossref] [PubMed]
- Go H, Cho HJ, Paik JH, et al. Thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: a clinicopathological and genetic analysis of six cases. Leuk Lymphoma 2011;52:2276-83. [Crossref] [PubMed]
- Harigae H, Ichinohasama R, Miura I, et al. Primary marginal zone lymphoma of the thymus accompanied by chromosomal anomaly 46,X,dup(X)(p11p22). Cancer Genet Cytogenet 2002;133:142-7. [Crossref] [PubMed]
- Hirokawa Y, Fujikawa R, Arai Y, et al. Primary thymic MALT lymphoma in a patient with Sjögren's syndrome and multiple lung cysts: a case report. Surg Case Rep 2019;5:138. [Crossref] [PubMed]
- Hu S, Li ZY, Lang ZJ. Two cases: mucosa-associated lymphoid tissue lymphoma of the thymus. J Pract Radiol 2017;33:676-7.
- Isaacson PG, Chan JK, Tang C, et al. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the thymus. A thymic lymphoma mimicking myoepithelial sialadenitis. Am J Surg Pathol 1990;14:342-51. [Crossref] [PubMed]
- Kamimura K, Nakamura N, Ishibashi T, et al. Somatic hypermutation of immunoglobulin heavy chain variable region genes in thymic marginal zone B-cell lymphoma of MALT type of a patient with Sjögren's syndrome. Histopathology 2002;40:294-6. [Crossref] [PubMed]
- Kang LY, Ho SP, Chou YP. Primary thymic mucosa-associated lymphoid tissue lymphoma with multiple thin walled lung cysts: case report and literature review. Chin J Cancer Res 2013;25:354-7. [PubMed]
- Kim DH, Nam ES, Yi JG, et al. Mucosa-associated lymphoid tissue (MALT) lymphoma of the thymus: Case report and literature review. Int J Surg Pathol 1998;6:229-34. [Crossref]
- Kim JM. Primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue-type in the thymus of a patient with Sjögren's syndrome and rheumatoid arthritis. J Korean Med Sci 2003;18:897-900. [Crossref] [PubMed]
- Kinoshita N, Ashizawa K, Abe K, et al. Mucosa-associated lymphoid tissue lymphoma of the thymus associated with Sjögren's syndrome: report of a case. Surg Today 2008;38:436-9. [Crossref] [PubMed]
- Kitai R, Sasaki M, Sato K, et al. Thymic mucosa-associated lymphoid tissue lymphoma associated with bilateral orbital pseudotumor. Gen Thorac Cardiovasc Surg 2009;57:566-9. [Crossref] [PubMed]
- Kurabayashi A, Iguchi M, Matsumoto M, et al. Thymic mucosa-associated lymphoid tissue lymphoma with immunoglobulin-storing histiocytosis in Sjogren's syndrome. Pathol Int 2010;60:125-30. [Crossref] [PubMed]
- Kuroki S, Nasu K, Murakami K, et al. Thymic MALT lymphoma: MR imaging findings and their correlation with histopathological findings on four cases. Clin Imaging 2004;28:274-7. [Crossref] [PubMed]
- Lorsbach RB, Pinkus GS, Shahsafaei A, et al. Primary marginal zone lymphoma of the thymus. Am J Clin Pathol 2000;113:784-91. [Crossref] [PubMed]
- Maeda A, Hayama M, Nakata M, et al. Mucosa-associated lymphoid tissue lymphoma in the thymus of a patient with systemic lupus erythematosus. Gen Thorac Cardiovasc Surg 2008;56:288-91. [Crossref] [PubMed]
- Masunaga A, Iwamoto S, Nakamura H, et al. Thymic epithelial cells expressed unusual follicular dendritic cell markers: thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Pathol Int 2008;58:402-5. [Crossref] [PubMed]
- McCluggage WG, McManus K, Qureshi R, et al. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) of thymus. Hum Pathol 2000;31:255-9. [Crossref] [PubMed]
- Momoi A, Nagai K, Isahai N, et al. Thymic Extranodal Marginal Zone Lymphoma of Mucosa-associated Lymphoid Tissue with 8q24 Abnormality. Intern Med 2016;55:799-803. [Crossref] [PubMed]
- Muramatsu T, Tanaka Y, Higure R, et al. Thymic and pulmonary mucosa-associated lymphoid tissue lymphomas. Ann Thorac Surg 2013;95:e69-70. [Crossref] [PubMed]
- Nagasaka T, Lai R, Harada T, et al. Coexisting thymic and gastric lymphomas of mucosa-associated lymphoid tissues in a patient with Sjögren syndrome. Arch Pathol Lab Med 2000;124:770-3. [Crossref] [PubMed]
- Naithani R, Ngan BY, Roifman C, et al. Thymic mucosa-associated lymphoid tissue lymphoma in an adolescent girl. J Pediatr Hematol Oncol 2012;34:552-5. [Crossref] [PubMed]
- Nakamura S, Koshikawa T, Kaba S, et al. Imprint cytology of low-grade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the thymus: a case report. Diagn Cytopathol 1993;9:665-7. [Crossref] [PubMed]
- Ortonne N, Copie-Bergman C, Remy P, et al. Mucosa-associated lymphoid tissue lymphoma of the thymus: a case report with no evidence of MALT1 rearrangement. Virchows Arch 2005;446:189-93. [Crossref] [PubMed]
- Ota H, Kawai H, Tsubasa M. Thymic mucosa-associated lymphoid tissue lymphoma involving lymph nodes. Int J Surg Case Rep 2013;4:250-2. [Crossref] [PubMed]
- Petersen JK, Larsen TS, Møller MB, et al. Primary thymic extranodal marginal zone B cell lymphoma as an incidental finding in a Caucasian woman. BMJ Case Rep 2015;2015:bcr2015211469. [Crossref] [PubMed]
- Rymkiewicz G, Ptaszyński K, Walewski J, et al. Unusual cyclin D1 positive marginal zone lymphoma of mediastinum. Med Oncol 2006;23:423-8. [Crossref] [PubMed]
- Sakamoto T, Yamashita K, Mizumoto C, et al. MALT lymphoma of the thymus with Sjögren's syndrome: biphasic changes in serological abnormalities over a 4-year period following thymectomy. Int J Hematol 2009;89:709-13. [Crossref] [PubMed]
- Song WA, Wang W, Zhou NK. Clinicopathological analysis of thymic mucosa-associated lymphoid tissue lymphoma. Chinese Clinical Oncology 2011;16:829-32.
- Sugimoto KJ, Asahina M, Shimada A, et al. IgG3 subclass-positive primary thymic MALT lymphoma without trisomy 3 and trisomy 18: report of a case and review of literature. Int J Clin Exp Pathol 2014;7:8980-7. [PubMed]
- Sun L, Shi HY, Wei LX. Clinicopathologic features of primary thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type. Zhonghua Bing Li Xue Za Zhi 2012;41:234-8. [PubMed]
- Sunada K, Hasegawa Y, Kodama T, et al. Thymic and pulmonary mucosa-associated lymphoid tissue lymphomas in a patient with Sjögren's syndrome and literature review. Respirology 2007;12:144-7. [Crossref] [PubMed]
- Sunohara M, Hara K, Osamura K, et al. Mucosa associated lymphoid tissue (MALT) lymphoma of the thymus with trisomy 18. Intern Med 2009;48:2025-32. [Crossref] [PubMed]
- Takagi N, Nakamura S, Yamamoto K, et al. Malignant lymphoma of mucosa-associated lymphoid tissue arising in the thymus of a patient with Sjögren's syndrome. A morphologic, phenotypic, and genotypic study. Cancer 1992;69:1347-55. [Crossref] [PubMed]
- Wang Z, Li H, Zeng Z, et al. Clinicopathologic study of primary thymic extranodal marginal zone lymphoma of mucosa associated lymphoid tissue and lymphoepithelial sialadenitis-like thymic hyperplasia. J Clin Exp Pathol 2016;32:1338-42.
- Yamada S, Tadasu K, Mingyon M, et al. Mucosa-associated lymphoid tissue lymphoma of the thymus resected using combined thoracoscopic and transcervical approaches. Ann Thorac Surg 2003;76:293-5. [Crossref] [PubMed]
- Yamasaki S, Matsushita H, Tanimura S, et al. B-cell lymphoma of mucosa-associated lymphoid tissue of the thymus: a report of two cases with a background of Sjögren's syndrome and monoclonal gammopathy. Hum Pathol 1998;29:1021-4. [Crossref] [PubMed]
- Yan TM, Zuo YG, Liu J, et al. A case of thymus mucosa-associated lymphoid tissue lymphoma accompanied with eosinophilia. J Clin Dermatol 2018;47:96-9.
- Yanagiya M, Matsumoto J, Hashimoto H, et al. Surgery of Thymic Tumor with Persistent Left Superior Vena Cava. Ann Thorac Cardiovasc Surg 2017;23:316-9. [Crossref] [PubMed]
- Yang F, He Z, Cao L, et al. Thymic MALT lymphoma mimicking parathyroid adenoma uptake on 99mTc-MIBI parathyroid scintigraphy. Hell J Nucl Med 2021;24:157-8. [PubMed]
- Yi JG, Kim DH, Choi CS. Malignant lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) arising in the thymus: radiologic findings. AJR Am J Roentgenol 1998;171:899-900. [Crossref] [PubMed]
- Yokose T, Kodama T, Matsuno Y, et al. Low-grade B cell lymphoma of mucosa-associated lymphoid tissue in the thymus of a patient with rheumatoid arthritis. Pathol Int 1998;48:74-81. [Crossref] [PubMed]
- You S, Sun JS, Park KJ, et al. Amyloid deposition in thymic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in a patient with myasthenia gravis: A case report. Thorac Cancer 2020;11:781-4. [Crossref] [PubMed]
- Shi XJ, Sun WW, Zhang N, et al. Two cases of primary SjÖgren's syndrome complicated with thymus mucosa-associated lymphoid tissue. Chin J Gen Pract 2018;17:936-7.
- Inagaki H, Chan JK, Ng JW, et al. Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol 2002;160:1435-43. [Crossref] [PubMed]
- Kominato S, Nakayama T, Sato F, et al. Characterization of chromosomal aberrations in thymic MALT lymphoma. Pathol Int 2012;62:93-8. [Crossref] [PubMed]
- Yang SH, Zhi CC, Ye SB, et al. Thymic extranodal marginal zone lymphoma of mucosa associated lymphoid tissue: a clinicopathologic analysis of 9 cases. J Clin Exp Pathol 2020;36:657-61.
- Shimizu K, Ishii G, Nagai K, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in the thymus: report of four cases. Jpn J Clin Oncol 2005;35:412-6. [Crossref] [PubMed]
- Shimizu K, Yoshida J, Kakegawa S, et al. Primary thymic mucosa-associated lymphoid tissue lymphoma: diagnostic tips. J Thorac Oncol 2010;5:117-21. [Crossref] [PubMed]
- Takino H, Li C, Yamada S, et al. Thymic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue: a gene methylation study. Leuk Lymphoma 2013;54:1742-6. [Crossref] [PubMed]
- Wang X, Zhang GJ, Wang HL, et al. Clinicopathological features of primary thymic extranodal marginal zone B cell lymphoma of mucosa associated lymphoid tissue. Zhonghua Bing Li Xue Za Zhi 2019;48:315-7. [PubMed]
- Weissferdt A, Moran CA. Primary MALT-type lymphoma of the thymus: a clinicopathological and immunohistochemical study of six cases. Lung 2011;189:461-6. [Crossref] [PubMed]
- Xu DM, Wang L, Zhu HY, et al. Primary thymic mucosa-associated lymphoid tissue lymphoma: 7 clinical cases report and a review of the literature. Zhonghua Xue Ye Xue Za Zhi 2020;41:54-8. [PubMed]
- Wu Y, Zhang W, Shen J, et al. CT diagnosis of primary mucosa-associated lymphoid tissue lymphoma of thymus. J Med Imaging 2020;30:213-6.
- Ye YZ, Sun K, Wang ZM, et al. Analysis of Clinicopathologic Features of Primary Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue(MALT lymphoma) in the Thymus. Journal of Chinese Oncology 2015;21:533-6.
- Yoshida M, Okabe M, Eimoto T, et al. Immunoglobulin VH genes in thymic MALT lymphoma are biased toward a restricted repertoire and are frequently unmutated. J Pathol 2006;208:415-22. [Crossref] [PubMed]
- Wang Z, Wang RN, Xu TR, et al. Lymphoma of mucosa-associated lymphoid tissue (MALT) in the thymus (case report and review of literature). Journal of Practical Oncology 1993;21-4.
- Maeshima AM, Taniguchi H, Suzuki T, et al. Distribution of malignant lymphomas in the anterior mediastinum: a single-institution study of 76 cases in Japan, 1997-2016. Int J Hematol 2017;106:675-80. [Crossref] [PubMed]
- Song WA, Zhou NK, Tian XD. The role of surgery in the management of primary thymic mucosa-associated lymphoid tissue (MALT) lymphoma. J Thorac Oncol 2010;5:1109-author reply 1109-10. [Crossref] [PubMed]
- Hwang Y, Kang CH, Park S, et al. Impact of Lymph Node Dissection on Thymic Malignancies: Multi-Institutional Propensity Score Matched Analysis. J Thorac Oncol 2018;13:1949-57. [Crossref] [PubMed]
- Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin 2016;66:153-71. [Crossref] [PubMed]
- Quartuccio L, Fabris M, Salvin S, et al. Controversies on rituximab therapy in sjögren syndrome-associated lymphoproliferation. Int J Rheumatol 2009;2009:424935. [Crossref] [PubMed]
- Zufferey P, Meyer OC, Grossin M, et al. Primary Sjögren's syndrome (SS) and malignant lymphoma. A retrospective cohort study of 55 patients with SS. Scand J Rheumatol 1995;24:342-5. [Crossref] [PubMed]
- Arai H, Tajiri M, Kikunishi N, et al. A spectrum of Thymic mucosa-associated lymphoid tissue lymphoma and Thymic amyloidosis in the patient with Auto immune disease: a case series. Mediastinum 2021;5:12. [Crossref] [PubMed]
- Murad MH. Clinical Practice Guidelines: A Primer on Development and Dissemination. Mayo Clin Proc 2017;92:423-33. [Crossref] [PubMed]