Lost in time pulmonary metastases of renal cell carcinoma: complete surgical resection of metachronous metastases, 18 and 15 years after nephrectomy
Introduction
It was only recently observed that selective patients are candidates for resection of pulmonary metastases. These are patients with favorable histologies and resection has been accepted to prolong survival (1). There has been a case describing a 25 year survival after metastectomy (2). This anecdotal success observed has prompted others to consider and evaluate aggressive resection of metastatic pulmonary lesions, in osteogenic sarcoma and soft tissue sarcoma. An evaluation system has also been created to evaluate possible candidates (3). Pulmonary metastasectomy can be applied in selected patients, employing various approaches and resections. Complete metastasectomy with maximal pulmonary tissue preservation are the main goals.
Case series reports
Case 1
During investigation of back pain, a right lower lobe nodule was found on abdominal computed tomography (CT) of a 77 year old woman [2011]. She had history of left nephrectomy for clear cell Renal Cell Carcinoma (ccRCC) of unknown stage, 15 years ago [1996]; she also had history of left mastectomy for breast cancer 13 years ago. Thoracic and abdominal CT showed a right lower lobe nodule with a maximal diameter of 1.7 cm and a right middle lobe nodule with a maximal diameter of 0.4 cm, bilateral pleural effusion (more prominent on the right), degeneration of thoracic and lumbar vertebrae, and absence of: thoracic wall mass, loco-regional recurrence, other thoracic or abdominal mass, enlarged thoracic or abdominal lymph nodes. Co-morbidities included atrial fibrillation and diabetes mellitus. The patient was in good performance status (Karnofski index 80%). Under general anaesthesia and single contralateral lung ventilation, through a video assisted mini thoracotomy, after inspection and palpation of the lung (that revealed an additional small superficial right lower lobe nodule), she underwent wedged resection of three nodules. The pleura and the mediastinal lymph nodes appeared free of malignancy. Histology revealed ccRCC metastases in the two nodules that were revealed on CT, and adenomatous hyperplasia in one right lower lobe nodule with a maximal diameter of 0.6 cm that was not revealed on CT, but was inspected intraoperatively (Figure 1). The patient had an uneventful recovery and was discharged home on the 3rd postoperative day. She received anti-angiogenic treatment (sunitinib). She remains alive and well one year after metastasectomy (telephone follow-up), being reluctant to undergo thoracic imaging (Table 1).
Full table
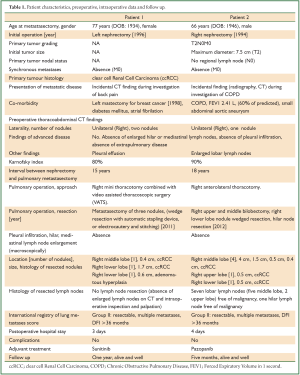
 Figure 1. Preoperative imaging, immediate postoperative specimen and patient photographs. (Case 1) A and B. Preoperative contrast CT (late arterial phase): right lower lobe nodule, maximal diameter 1.6 cm; C. Preoperative CT showing a small right middle lobe nodule, maximal diameter 0.4 cm; D. Macroscopic appearance of resected nodules (wedge resection with automatic stapling device: right lower lobe nodule, maximal diameter 1.6 cm, ccRCC), or electrocautery and stitching (right middle lobe nodule, 0.4 cm, ccRCC, and right lower lobe nodule, 0.6 cm, adenomatous hyperplasia); E. Immediate postoperative patient photograph (small lateral thoracotomy, double lumen tracheal tube still in place, left mastectomy, good nutritional status).
Figure 1. Preoperative imaging, immediate postoperative specimen and patient photographs. (Case 1) A and B. Preoperative contrast CT (late arterial phase): right lower lobe nodule, maximal diameter 1.6 cm; C. Preoperative CT showing a small right middle lobe nodule, maximal diameter 0.4 cm; D. Macroscopic appearance of resected nodules (wedge resection with automatic stapling device: right lower lobe nodule, maximal diameter 1.6 cm, ccRCC), or electrocautery and stitching (right middle lobe nodule, 0.4 cm, ccRCC, and right lower lobe nodule, 0.6 cm, adenomatous hyperplasia); E. Immediate postoperative patient photograph (small lateral thoracotomy, double lumen tracheal tube still in place, left mastectomy, good nutritional status).
Case 2
During investigation of chronic obstructive pulmonary disease, a right middle lobe tumor was found on chest x-ray and CT of a 66 year old man, 16 years after right nephrectomy for ccRCC (performed in 1994). Renal tumor staging had been T2N0M0 (maximal initial renal tumor diameter: 7.5 cm). When initially revealed [2010], the lung tumor had a maximal diameter of 3.5 cm, but CT guided needle biopsy was negative for malignancy. At 1-year follow-up [2011] it appeared mildly increased, and at 2-year follow up [2012] it further increased to a maximal diameter of 4.5 cm. Other findings of thoracic and abdominal CT were enlarged right middle lobar lymph nodes, centrilobular emphysema, a small abdominal aortic aneurysm, and absence of: thoracic wall infiltration, loco-regional recurrence or other abdominal mass, enlarged mediastinal or abdominal lymph nodes. The patient was in good clinical and performance status [Forced Expiratory Volume in 1 second (FEV1) 2.41 L (60% of predicted), Karnofski index 90%]. On general anesthesia and single contralateral lung ventilation, through a right anterolateral thoracotomy, after inspection and palpation of the lung (that revealed enlargement of lobar lymph nodes of the right middle and upper lobes, and a right lower lobe nodule), he underwent right upper and middle bilobectomy plus right lower lobe nodule wedge resection. One hilar lymph node was also resected for staging. The pleura and the mediastinal lymph nodes appeared free of malignancy. Histology revealed ccRCC pulmonary metastases in 6 nodules identified (four at the right middle lobe with maximal diameters of 0.4-4 cm, one at the right upper lobe with a maximal diameter of 0.5 cm, one from the right lower lobe with a maximal diameter of 0.5 cm), and absence of malignancy in seven lobar lymph nodes and one hilar lymph node. The resection was deemed complete. The patient had an uneventful recovery and was discharged home on the 4th postoperative day. He received systemic anti-angiogenic treatment (pazopanib). The chest CT one month postoperatively was clear. The patient remains alive and well 5 months after metastasectomy (Figure 2).
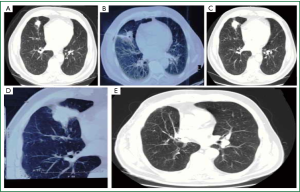 Figure 2. Serial CT imaging of the tumor, within a period of two years. Despite negative for malignancy CT guided needle biopsy, the tumor proved to be a late renal cell carcinoma pulmonary metastasis. (Case 2) A. Chest computed tomography (CT) in 2010, 16 years after initial nephrectomy for T2N0M0 clear cell Renal Cell Carcinoma, maximal tumor diameter 3.5 cm; B. CT guided needle aspiration [2010]; C. Follow up chest CT in 2011, tumor size somewhat increased (fuller contour, without significant change in maximal diameter); D. Chest CT in 2012, 18 years after initial nephrectomy, tumor size increased to a maximal diameter of 4.5 cm; E. Chest CT one month after right upper and middle bilobectomy and right lower lobe nodule wedged resection. Metastasectomy was deemed complete.
Figure 2. Serial CT imaging of the tumor, within a period of two years. Despite negative for malignancy CT guided needle biopsy, the tumor proved to be a late renal cell carcinoma pulmonary metastasis. (Case 2) A. Chest computed tomography (CT) in 2010, 16 years after initial nephrectomy for T2N0M0 clear cell Renal Cell Carcinoma, maximal tumor diameter 3.5 cm; B. CT guided needle aspiration [2010]; C. Follow up chest CT in 2011, tumor size somewhat increased (fuller contour, without significant change in maximal diameter); D. Chest CT in 2012, 18 years after initial nephrectomy, tumor size increased to a maximal diameter of 4.5 cm; E. Chest CT one month after right upper and middle bilobectomy and right lower lobe nodule wedged resection. Metastasectomy was deemed complete.
Discussion
In 21 studies published between 1961 and 2011, the five-year survival after RCC pulmonary metastasectomy ranged from 21% to 60% (1-3). Chen et al. [2008] reported a five-year survival of 83% among eight selected patients without extrapulmonary metastases, who underwent complete resection of solitary or multiple, unilateral or bilateral RCC pulmonary metastases (seven out of eight patients had one or two unilateral metastases) (4).
In most studies, complete resection of metastases was a good prognostic factor (1-3). Other good prognostic factors included solitary metastasis (vs. multiple), smaller number of metastases (<3 or <7), lower size of metastases (<3 cm), metachronous (vs. synchronous) metastases, longer disease free interval (>12-36 months), absence of positive hilar and/or mediastinal lymph nodes, absence of pleural infiltration, and absence of positive lymph nodes at initial nephrectomy (1-3).
Resectability itself may be associated with better prognosis, but reduction of tumor burden may also play a role (1). Higher number of metastases and lymph node involvement indicates advanced disease (3) and probably compromises complete resectability.
In metachronous metastases the disease free interval (DFI), defined as the time interval between nephrectomy and pulmonary metastasectomy or between nephrectomy and diagnosis of pulmonary metastases varies, but in the majority of cases it does not exceed five years (2,3). Among others, Kanzaki et al. reported a maximum DFI longer than 15 years (maximum 19.7 years, mean 4.1 years) (2). Shiono et al. reported RCC pulmonary metastasectomy, pleural metastasectomy, and repeat pulmonary metastasectomy, at 16, 24, and 25 years respectively after the primary nephrectomy in a patient who remained alive and free of disease 8 months after the third metastasectomy (5). Reviewing the literature (in 2003) they cited 5 more cases with time interval between nephrectomy and pulmonary metastasectomy between 20 and 28 years. Death from RCC was reported at 1 and 2 years after metastasectomy in 2 of those cases, while disease free survival of 1.5 and 4 years was reported in 2 cases (5).
Longer DFI may reflect “a less aggressive cancer” (3), but “it does not always imply slow growth or absence of other metastases” (5). Very late metastasectomy has been rarely reported, and more over the long term results are practically lacking.
Conclusions
RCC pulmonary metastases can occur very late after “curative” nephrectomy. The metastases may increase in size to more than 4 cm and still remain asymptomatic. The conventional CT may not show small metastases, and needle biopsy can be false negative. In selected patients with good clinical and performance status, and completely resectable disease, metastasectomy appears beneficial, associated with low postoperative mortality and acceptable short term results.
Acknowledgements
Disclosure: The authors declare no conflict of interest .
References
- Pogrebniak HW, Haas G, Linehan WM, Renal cell carcinoma: resection of solitary and multiple metastases. Ann Thorac Surg. 1992;33-8. [PubMed ]
- Kanzaki R, Higashiyama M, Fujiwara A, Long-term results of surgical resection for pulmonary metastasis from renal cell carcinoma: a 25-year single-institution experience. Eur J Cardiothorac Surg. 2011;167-72. [PubMed ]
- Meimarakis G, Angele M, Staehler M, Evaluation of a new prognostic score (Munich score) to predict long-term survival after resection of pulmonary renal cell carcinoma metastases. Am J Surg. 2011;158-67. [PubMed ]
- Chen F, Fujinaga T, Shoji T, Pulmonary resection for metastasis from renal cell carcinoma. Interact Cardiovasc Thorac Surg. 2008;825-8. [PubMed ]
- Shiono S, Yoshida J, Nishimura M, Late pulmonary metastasis of renal cell carcinoma resected 25 years after nephrectomy. Jpn J Clin Oncol. 2004;46-9. [PubMed ]


