
Association between comorbid cardiomyopathy and composite endpoints of patients with congestive heart failure in the intensive care unit: a retrospective cohort study
Introduction
Heart failure (HF) is a heterogeneous disease that is estimated to occur in more than 23 million individuals worldwide (1). Although coronary artery disease is usually considered to be the primary cause of HF (2), the etiology of HF is being replaced by other diseases (3). Several studies have shown that different etiologies of HF lead to different prognoses (4,5). The prognostic factor of HF includes ischemic heart disease, hypertension, and rheumatic heart disease. However, the heterogeneous group of cardiomyopathies is usually caused by genetics, autoimmune, and infection (6). Moreover, the symptoms of HF cannot be explained by coronary artery disease, hypertension, valvular heart disease, and congenital heart disease (7). Cardiomyopathy is a disease that results in abnormal heart muscle structure and function. The prevalence of different types of cardiomyopathy in adults is 1/500 for hypertrophic cardiomyopathy (HCM), 1/250 for dilated cardiomyopathy (DCM), and 1/5,000 for arrhythmogenic right ventricular cardiomyopathy (ARVC). Restrictive cardiomyopathy (RCM) is rare (8). The incidence of all types of cardiomyopathies has increased over the past decade, and cardiomyopathy has received increasing attention as an important cause of HF (9).
Most randomized controlled trials enrolled HF patients with a reduced ejection fraction (HFrEF) but did not include those with a preserved ejection fraction (HFpEF); thus, evidence regarding the treatment of patients with HFpEF is insufficient (10). However, cardiomyopathy usually presents as phenotypes of HFpEF. A previous study reported the prognosis of patients according to whether they had ischemic or non-ischemic heart disease (11). At present, few studies have explored the effect of comorbid cardiomyopathy on the prognosis of HF.
In the intensive care unit (ICU), patients with congestive HF present with the highest risk, most complications, and most resource-intensive disease states (12). According to a previous study of children, patients with cardiomyopathy had poor outcomes, with an in-hospital mortality rate of 11% in HF-related ICU hospitalization (13). However, whether comorbid cardiomyopathy is an independent risk factor for adult HF patients in the ICU remains unclear. Therefore, in this study, we enrolled critically ill patients with congestive HF to explore the relationship between comorbid cardiomyopathy and the prognosis of patients in the ICU. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-833/rc).
Methods
Data source & ethical approval
This is a single-center retrospective cohort study. Data were obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, which is a freely-available database including data on patients admitted to the ICU of the Beth Israel Deaconess Medical Center (BIDMC) from 2008 to 2019 (14). One of the authors (Liang) of this study passed the “Protecting Human Research Participants” exam and obtained permission to access the database (Record ID: 43449634). The ethical approval of MIMIC-IV database was approved by institutional review boards of both BIDMC and Massachusetts Institute of Technology Affiliates. Moreover, all private information of subjects is hidden, and informed consent was waived by the Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patients
All adult patients included in the database were enrolled. The database only included records from the first hospitalization in the ICU. Patients were excluded according to the following criteria: (I) length of stay (LOS) of ICU <24 hours; and (II) a diagnosis of cardiomyopathy without congestive HF. The detailed procedure for patient selection is shown in Figure 1.
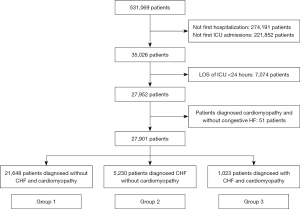
The included patients were categorized into three groups according to the diagnosis: (I) patients without cardiomyopathy and congestive HF (group 1); (II) patients diagnosed with congestive HF due to other etiologies (group 2); and (III) patients diagnosed with cardiomyopathy and congestive HF (group 3).
Data extraction
The Structured Query Language (SQL) with PostgreSQL (version 5.3; https://www.postgresql.org/; University of California Berkeley, USA) was used to extract data from the MIMIC-IV database. Patients with comorbid cardiomyopathy were diagnosed according to the Clinical Modification codes of the International Classification of Diseases, Ninth Revision and Tenth Revision (ICD-9-CM and ICD-10-CM). The diagnosis of congestive HF was extracted from the Charlson Comorbidity Index table, which records whether the symptoms of congestive HF occurred in hospitalized patients (15).
In addition to the clinical outcome variables, the following variables were also extracted: age, sex, ethnicity, insurance, marriage, admission types, ICU types, other comorbidities [coronary heart disease, valve heart disease, congenital heart disease, hypertension, atrial fibrillation or atrial flutter, diabetes, peripheral vascular disease, chronic pulmonary disease, stroke, dementia, paraplegia, rheumatic disease, mild liver disease, severe liver disease, renal disease, malignant cancer, metastatic solid tumor, acquired immune deficiency syndrome (AIDS), and sepsis], vital signs on first day after ICU admission [weight, mean heart rate (HR), mean systolic blood pressure (SBP), diastolic blood pressure (DBP), mean respiratory rate (RR)], laboratory tests on first day after ICU admission [white blood cell (WBC), hemoglobin, platelet, creatinine, glucose, sodium (Na), potassium (K), bicarbonate (HCO3−)], scoring systems on first day after ICU admission [the Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation III scores (APACHE III)], and treatments [renal replacement treatment (RRT), mechanical ventilation, vasoactive drug, antibiotics, β-receptor blockers (β-RB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), diuretics, and digoxin].
Definitions and outcomes
Cardiomyopathy diagnoses were extracted from ICD-9-CM and ICD-10-CM codes generated for billing purposes following the hospital stay, which included both primary and specific cardiomyopathies (ICD-9 code: 425 and ICD-10: I42) (16-18). Primary cardiomyopathies include HCM, DCM, RCM, and unclassified cardiomyopathies, (including endocardial fibroelastosis and endomyocardial fibrosis). Specific cardiomyopathies included metabolic cardiomyopathy, alcoholic cardiomyopathy, and cardiomyopathy due to drugs and external agents.
The following outcome variables were extracted: in-hospital mortality, cardiac arrest, readmission to ICU, re-hospitalization, tracheal intubation, RRT on the first day, and LOS in the ICU. Cardiac arrest was diagnosed according to the ICD-9-CM and ICD-10-CM (ICD-9 code: 4275 and ICD-10: I46). The composite endpoints included in-hospital mortality, cardiac arrest, and readmission to the ICU.
Statistical analysis
Continuous variables were expressed as means ± standard deviations (SDs) or medians [interquartile ranges (IQRs)] and compared using Student’s t-tests (normal distribution) or Kruskal-Wallis rank tests (non-normal distribution). Categorical variables were presented as numbers (percentages) and compared using chi-squared tests (or Fisher’s exact tests). The associations between comorbid cardiomyopathy and outcomes were assessed using logistic regression models. Three different models were used to adjust for potential confounders: model 1, including age, sex, ethnicity, insurance, marriage, admission types, and ICU types; model 2, including age, sex, ethnicity, insurance, marriage, admission types, ICU types, other comorbidities, SOFA, APACHE III, and treatments; model 3, propensity-score matching (PSM).
PSM was used to reduce the imbalance within each group, with a 1:1:1 nearest neighbor matching and a caliper width of 0.05 (19). PSM was used to prevent potential bias due to significant differences in the baseline characteristics. A propensity score for each patient was calculated to estimate the probability of matching (using multivariable logistic regression models) given the following factors: age, sex, ethnicity, insurance, marriage, admission types, ICU types, other comorbidities, vital signs, laboratory tests, as well as SOFA and APACHE III scores. Subgroup analyses were used to assess whether different ICU types could change the association between cardiomyopathy and composite endpoints. Variables with >10% missing data, such as brain natriuretic peptide (BNP) and left ventricular ejection fraction (LVEF), were excluded. Meanwhile, variables with <10% missing data, such as HCO3−, hemoglobin, platelet, Na, and K levels, were assessed using the regression model. All data were integrated and analyzed using STATA 15.0 (STATA, College Station, TX, USA). All tests were two-sided, and P<0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
A total of 27,901 critically ill patients were enrolled in this study (Figure 1), among which 1,023 (3.67%) patients were diagnosed with congestive HF and cardiomyopathy. The average age of this cohort was 64.37±17.36 years, 58.13% were men, and the ethnicity was predominantly white (64.67%). The ICUs in this study included the cardiac vascular ICU (CVICU) (23.18%), coronary care unit (CCU) (10.51%), medical ICU (MICU) (16.30%), MICU/surgical ICU (SICU) (12.83%), SICU (15.71%), trauma ICU (TSICU) (14.00%), and neurological ICU (NICU) (7.47%).
The patients were divided into three groups based on the diagnosis (Table 1). Patients with congestive HF and cardiomyopathy were younger than those with congestive HF alone (73.82±12.96 vs. 65.49±15.05, P<0.001). The incidence of coronary heart disease, valvular heart disease, stroke, atrial fibrillation, peripheral artery disease, hypertension, diabetes, chronic pulmonary disease, renal disease, dementia, malignant cancer, and sepsis was higher in patients with congestive HF alone. Patients with cardiomyopathy and congestive HF tended to have higher weight, faster HR and RR, higher DBP, and lower SBP than those in the other two groups (P<0.05).
Table 1
| Variables | All patients (n=27,901) | Group 1 (n=21,648) | Group 2 (n=5,230) | Group 3 (n=1,023) | P |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 64.37±17.36 | 62.04±17.36 | 73.82±12.96 | 65.49±15.05 | <0.001 |
| Males, n (%) | 16,220 (58.13) | 12,625 (58.32) | 2,899 (55.43) | 696 (68.04) | <0.001 |
| Ethnicity, n (%) | <0.001 | ||||
| Asian | 725 (2.60) | 606 (2.80) | 102 (1.95) | 17 (1.66) | |
| Black | 1,762 (6.32) | 1,394 (6.44) | 284 (5.43) | 84 (8.21) | |
| Hispanic | 861 (3.09) | 727 (3.36) | 105 (2.01) | 29 (2.83) | |
| White | 18,044 (64.67) | 13,908 (64.25) | 3,499 (66.90) | 637 (62.27) | |
| Other | 6,509 (23.33) | 5,013 (23.16) | 1,240 (23.71) | 256 (25.02) | |
| Insurance, n (%) | <0.001 | ||||
| Medicaid | 1,980 (7.10) | 1,690 (7.81) | 216 (4.13) | 74 (7.23) | |
| Medicare | 11,296 (40.49) | 7,804 (36.05) | 3,080 (58.89) | 412 (40.27) | |
| Other | 14,625 (52.42) | 12,154 (56.14) | 1,934 (36.98) | 537 (52.49) | |
| Marriage, n (%) | <0.001 | ||||
| Divorced | 1,876 (6.72) | 1,427 (6.59) | 377 (7.21) | 72 (7.04) | |
| Married | 12,614 (45.21) | 9,866 (45.57) | 2,276 (43.52) | 472 (46.14) | |
| Single | 6,922 (24.81) | 5,726 (26.45) | 937 (17.92) | 259 (25.32) | |
| Widowed | 2,987 (10.71) | 1,907 (8.81) | 991 (18.95) | 89 (8.70) | |
| Unknown | 3,502 (12.55) | 2,722 (12.57) | 649 (12.41) | 131 (12.81) | |
| Admission type, n (%) | <0.001 | ||||
| Elective | 1,136 (4.07) | 829 (3.83) | 245 (4.68) | 62 (6.06) | |
| Emergency | 17,057 (61.13) | 13,562 (62.65) | 2,950 (56.41) | 545 (53.27) | |
| Surgical | 3,357 (12.03) | 2,962 (13.68) | 309 (5.91) | 86 (8.41) | |
| Urgent | 6,351 (22.76) | 4,295 (19.84) | 1,726 (33.00) | 330 (32.26) | |
| ICU type, n (%) | <0.001 | ||||
| CVICU | 6,468 (23.18) | 4,875 (22.52) | 1,339 (25.60) | 254 (24.83) | |
| CCU | 2,932 (10.51) | 1,325 (6.12) | 1,279 (24.46) | 328 (32.06) | |
| MICU | 4,549 (16.30) | 3,427 (15.83) | 968 (18.51) | 154 (15.05) | |
| MICU/SICU | 3,580 (12.83) | 2,781 (12.85) | 691 (13.21) | 108 (10.56) | |
| SICU | 4,382 (15.71) | 3,857 (17.82) | 441 (8.43) | 84 (8.21) | |
| TSICU | 3,905 (14.00) | 3,497 (16.15) | 342 (6.54) | 66 (6.45) | |
| NICU | 2,085 (7.47) | 1,886 (8.71) | 170 (3.25) | 29 (2.83) | |
| Comorbidity, n (%) | |||||
| Coronary heart disease | 9,743 (34.92) | 6,081 (28.09) | 3,161 (60.44) | 501 (48.97) | <0.001 |
| Valve heart disease | 4,301 (15.42) | 2,285 (10.56) | 1,715 (32.79) | 301 (29.42) | <0.001 |
| Congenital heart disease | 864 (3.10) | 668 (3.09) | 153 (2.93) | 43 (4.20) | 0.096 |
| Hypertension | 17,153 (61.48) | 12,544 (57.95) | 3,968 (75.87) | 641 (62.66) | <0.001 |
| Atrial fibrillation | 7,427 (26.62) | 4,364 (20.16) | 2,589 (49.50) | 474 (46.33) | <0.001 |
| Diabetes | 7,202 (25.81) | 4,890 (22.59) | 2,001 (38.26) | 311 (30.40) | <0.001 |
| Peripheral artery disease | 2,934 (10.52) | 2,014 (9.30) | 786 (15.03) | 134 (13.10) | <0.001 |
| Chronic pulmonary disease | 6,239 (22.36) | 3,964 (18.31) | 2,004 (38.32) | 271 (26.49) | <0.001 |
| Stroke | 5,150 (18.46) | 4,214 (19.47) | 791 (15.12) | 145 (14.17) | <0.001 |
| Dementia | 881 (3.16) | 629 (2.91) | 224 (4.28) | 28 (2.74) | <0.001 |
| Paraplegia | 1,809 (6.48) | 1,525 (7.04) | 232 (4.44) | 52 (5.08) | <0.001 |
| Rheumatic disease | 782 (2.80) | 549 (2.54) | 202 (3.86) | 31 (3.03) | <0.001 |
| Peptic ulcer | 715 (2.56) | 562 (2.60) | 134 (2.56) | 19 (1.86) | 0.344 |
| Mild liver disease | 2,668 (9.56) | 2,120 (9.79) | 426 (8.15) | 122 (11.93) | <0.001 |
| Severe liver disease | 1,069 (3.83) | 908 (4.19) | 126 (2.41) | 35 (3.42) | <0.001 |
| Renal disease | 3,974 (14.24) | 2,046 (9.45) | 1,683 (32.18) | 245 (23.95) | <0.001 |
| Malignant cancer | 2,820 (10.11) | 2,312 (10.68) | 435 (8.32) | 73 (7.14) | <0.001 |
| Metastatic tumor | 1,310 (4.70) | 1,128 (5.21) | 153 (2.93) | 29 (2.83) | <0.001 |
| AIDS | 111 (0.40) | 100 (0.46) | 6 (0.11) | 5 (0.49) | 0.001 |
| Sepsis | 13,873 (49.72) | 10,271 (47.45) | 3,056 (58.43) | 5,456 (53.37) | <0.001 |
| Vital signs, mean ± SD | |||||
| Weight (kg) | 82.19±22.69 | 81.66±21.81 | 83.65±25.65 | 85.94±24.16 | <0.001 |
| HR (bpm) | 84.54±15.42 | 84.40±15.29 | 84.40±15.28 | 88.32±18.21 | <0.001 |
| SBP (mmHg) | 118.75±15.60 | 119.66±15.46 | 116.02±15.75 | 113.25±15.13 | <0.001 |
| DBP (mmHg) | 63.57±11.02 | 64.28±10.94 | 60.37±10.71 | 64.78±11.33 | <0.001 |
| RR (bpm) | 19.02±3.70 | 18.76±3.64 | 19.87±3.73 | 20.02±3.87 | <0.001 |
| Laboratory tests, mean ± SD | |||||
| WBC (×109/L) | 11.47±6.90 | 11.47±6.79 | 11.51±7.38 | 11.26±6.61 | 0.563 |
| Hemoglobin (g/dL) | 11.31±2.23 | 11.39±2.22 | 10.92±2.20 | 11.66±2.28 | <0.001 |
| Platelet (×109/L) | 214.02±101.00 | 214.27±101.82 | 214.42±99.48 | 206.55±90.69 | 0.055 |
| Creatinine (mg/dL) | 1.22±1.24 | 1.13±1.18 | 1.53±1.37 | 1.50±1.43 | <0.001 |
| Glucose (mg/dL) | 138.65±66.44 | 136.99±64.82 | 145.39±72.67 | 139.19±64.91 | <0.001 |
| Na (mmol/L) | 138.79±4.82 | 138.84±4.78 | 138.66±5.00 | 138.41±4.65 | 0.001 |
| K (mmol/L) | 4.14±0.65 | 4.11±0.63 | 4.25±0.70 | 4.25±0.74 | <0.001 |
| HCO3− (mmol/L) | 23.80±4.45 | 23.55±4.20 | 24.79±5.19 | 24.09±4.86 | <0.001 |
| Scoring system, median [IQR] | |||||
| SOFA | 4 [2, 7] | 4 [2, 6] | 5 [3, 8] | 5 [3, 9] | <0.001 |
| APACHE III | 40 [29, 56] | 38 [28, 54] | 47 [36, 64] | 46 [34, 66] | <0.001 |
| Treatments, n (%) | |||||
| RRT | 1,278 (4.58) | 781 (3.61) | 413 (7.90) | 84 (8.21) | <0.001 |
| Mechanical ventilation | 12,113 (43.41) | 9,247 (42.72) | 2,409 (46.06) | 457 (44.67) | – |
| Vasoactive drugs | 10,810 (38.74) | 7,733 (35.72) | 2,545 (48.66) | 532 (52.00) | <0.001 |
| Antibiotics | 19,109 (68.49) | 14,474 (66.86) | 3,930 (75.14) | 705 (68.91) | <0.001 |
| β-RB | 6,636 (23.78) | 4,466 (20.63) | 1,807 (34.55) | 363 (35.48) | <0.001 |
| ACEI/ARB | 3,600 (12.90) | 2,396 (11.07) | 945 (18.07) | 259 (25.32) | <0.001 |
| Diuretics | 7,204 (25.82) | 4,646 (21.46) | 2,155 (41.20) | 403 (39.39) | <0.001 |
| Digoxin | 453 (1.62) | 151 (0.70) | 217 (4.15) | 85 (8.31) | <0.001 |
Group 1, patients without cardiomyopathy and congestive HF; group 2, patients only with congestive HF; group 3, patients with cardiomyopathy and congestive HF. SD, standard deviation; ICU, intensive care unit; CVICU, cardiac vascular ICU; CCU, coronary care unit; MICU, medical ICU; SICU, surgical ICU; TSICU, trauma ICU; NICU, neurological ICU; AIDS, acquired immune deficiency syndrome; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; RR, respiratory rate; WBC, white blood cell; Na, sodium; K, potassium; HCO3−, bicarbonate; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; APACHE III, Acute Physiology and Chronic Health Evaluation III scores; RRT, renal replace treatment; β-RB, β-receptor blockers; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; HF, heart failure.
The laboratory tests showed that the patients with cardiomyopathy and congestive HF had higher levels of hemoglobin and K and lower levels of creatinine, glucose, and Na compared to patient with congestive HF alone. In addition, patients with cardiomyopathy comorbidity had similar severity scores to those with congestive HF alone. Moreover, the use of RRT, vasoactive drugs, β-RB, ACEI/ARB, and digoxin was more prevalent in patients with cardiomyopathy and congestive HF.
Clinical outcomes of different groups
A total of 8,572 composite endpoints were recorded in this cohort. There were 2,932 (10.51%) in-hospital deaths, 805 (2.89%) events of cardiac arrest, 5,835 (20.91%) readmissions to the ICU, 10,913 (39.11%) events of re-hospitalization, 5,252 (18.82%) events of tracheal intubation, and 505 (1.81%) events of RRT on the first day after ICU admission. Compared to patients with congestive HF alone, those with cardiomyopathy and congestive HF had higher composite endpoints (40.52% vs. 43.30%, P<0.001).
In addition, the incidences of cardiac arrest, readmission to the ICU, re-hospitalization, and RRT on the first day were higher in patients with congestive HF and cardiomyopathy (P<0.05). However, patients with congestive HF alone had higher rates of in-hospital death (14.84% vs. 13.29%, P<0.001) and tracheal intubation (20.17% vs. 19.35%, P=0.017). Moreover, the LOS of ICU in patients with cardiomyopathy and congestive HF was longer than that in the other two groups (P<0.001; Table 2).
Table 2
| Outcomes | All patients (n=27,901) | Group 1 (n=21,648) | Group 2 (n=5,230) | Group 3 (n=1,023) | P |
|---|---|---|---|---|---|
| Composite endpoints, n (%) | 8,572 (30.72) | 6,031 (27.86) | 2,105 (40.25) | 436 (42.62) | <0.001 |
| In-hospital mortality, n (%) | 2,932 (10.51) | 2,020 (9.33) | 776 (14.84) | 136 (13.29) | <0.001 |
| Cardiac arrest, n (%) | 805 (2.89) | 530 (2.45) | 207 (3.96) | 68 (6.65) | <0.001 |
| Re-admission to the ICU, n (%) | 5,835 (20.91) | 4,108 (18.98) | 1,424 (27.23) | 303 (29.62) | <0.001 |
| Re-hospitalization, n (%) | 10,913 (39.11) | 8,165 (37.72) | 2,240 (42.83) | 508 (49.66) | <0.001 |
| Tracheal intubation, n (%) | 5,252 (18.82) | 3,999 (18.47) | 1,055 (20.17) | 198 (19.35) | 0.017 |
| RRT on first day, n (%) | 505 (1.81) | 326 (1.51) | 142 (2.72) | 37 (3.62) | <0.001 |
| LOS in the ICU (days), median [IQR] | 2.33 [1.50, 4.38] | 2.21 [1.44, 4.09] | 2.93 [1.80, 5.23] | 3.07 [1.80, 5.93] | <0.001 |
Group 1, patients without cardiomyopathies and congestive HF; group 2, patients only with congestive HF; group 3, patients with cardiomyopathy and congestive HF. ICU, intensive care unit; RRT, renal replacement treatment; LOS, length of stay; IQR, interquartile range; HF, heart failure.
Relationship between cardiomyopathies and clinical outcomes
The logistic regression model results indicated that comorbid cardiomyopathy was a risk factor for composite endpoints, with a crude odds ratio (OR) of 1.92 [95% confidence interval (CI): 1.69–2.18; P<0.001], whereas the OR of patients with congestive HF due to other etiologies was 1.74 (95% CI: 1.64–1.86; P<0.001). After adjustment using different models, similar findings were observed. The risk of composite endpoints in patients with cardiomyopathy and congestive HF was higher than that in those with congestive HF alone (OR =1.87; 95% CI: 1.62–2.15; P<0.001; adjustment by model 2).
After reducing the imbalance among the three groups by PSM, similar severities of disease were observed based on the scoring systems (P>0.05; Table S1). The risk of composite endpoints was still higher in patients with cardiomyopathy (OR =1.64; 95% CI: 1.33–2.02; P<0.001; adjustment by model 3).
The risk of outcomes was also respectively explored. Compared to patients with congestive HF alone (OR =1.43; 95% CI: 1.26–1.62; P<0.001; adjustment by model 2), those with cardiomyopathy had a similar risk of in-hospital death (OR =1.35; 95% CI: 1.06–1.71; P=0.014; adjustment by model 2). However, the risk of in-hospital death was not significant after PSM (P>0.05). The risks of cardiac arrest (OR =1.53; 95% CI: 1.01–2.34; P=0.029; adjustment by model 3) and re-admission to the ICU (OR =1.74; 95% CI: 1.39–2.17; P<0.001; adjustment by model 3) were both higher in patients with cardiomyopathy and congestive HF compared to those in the other groups (Table 3).
Table 3
| Outcomes | Group | OR | 95% CI | P |
|---|---|---|---|---|
| Composite endpoints | ||||
| Crude | Refa | 1 | ||
| Only HFb | 1.74 | 1.64–1.86 | <0.001 | |
| Bothc | 1.92 | 1.69–2.18 | <0.001 | |
| Model 1 | Ref | 1 | ||
| Only HF | 1.72 | 1.61–1.84 | <0.001 | |
| Both | 2.07 | 1.82–2.36 | <0.001 | |
| Model 2 | Ref | 1 | ||
| Only HF | 1.55 | 1.44–1.68 | <0.001 | |
| Both | 1.87 | 1.62–2.15 | <0.001 | |
| Model 3 | Ref | 1 | ||
| Only HF | 1.30 | 1.04–1.62 | 0.019 | |
| Both | 1.64 | 1.33–2.02 | <0.001 | |
| In-hospital mortality | ||||
| Crude | Ref | 1 | ||
| Only HF | 1.69 | 1.55–1.85 | <0.001 | |
| Both | 1.49 | 1.24–1.79 | <0.001 | |
| Model 1 | Ref | 1 | ||
| Only HF | 1.44 | 1.31–1.58 | <0.001 | |
| Both | 1.61 | 1.33–1.96 | <0.001 | |
| Model 2 | Ref | 1 | ||
| Only HF | 1.43 | 1.26–1.62 | <0.001 | |
| Both | 1.35 | 1.06–1.71 | 0.014 | |
| Model 3 | Ref | 1 | ||
| Only HF | 0.98 | 0.69–1.39 | 0.917 | |
| Both | 1.04 | 0.74–1.45 | 0.822 | |
| Cardiac arrest | ||||
| Crude | Ref | 1 | ||
| Only HF | 1.64 | 1.39–1.93 | <0.001 | |
| Both | 2.84 | 2.19–3.68 | <0.001 | |
| Model 1 | Ref | 1 | ||
| Only HF | 1.60 | 1.35–1.91 | <0.001 | |
| Both | 2.58 | 1.98–3.36 | <0.001 | |
| Model 2 | Ref | 1 | ||
| Only HF | 1.34 | 1.11–1.62 | 0.003 | |
| Both | 2.06 | 1.54–2.75 | <0.001 | |
| Model 3 | Ref | 1 | ||
| Only HF | 0.88 | 0.53–1.46 | 0.617 | |
| Both | 1.53 | 1.01–2.34 | 0.029 | |
| Re-admission to the ICU (%) | ||||
| Crude | Ref | 1 | ||
| Only HF | 1.60 | 1.49–1.71 | <0.001 | |
| Both | 1.80 | 1.56–2.06 | <0.001 | |
| Model 1 | Ref | 1 | ||
| Only HF | 1.66 | 1.54–1.78 | <0.001 | |
| Both | 1.89 | 1.64–2.17 | <0.001 | |
| Model 2 | Ref | 1 | ||
| Only HF | 1.52 | 1.40–1.65 | <0.001 | |
| Both | 1.77 | 1.53–2.05 | <0.001 | |
| Model 3 | Ref | 1 | ||
| Only HF | 1.45 | 1.14–1.83 | 0.002 | |
| Both | 1.74 | 1.39–2.17 | <0.001 | |
The composite endpoints included all-cause death, cardiac arrest, and re-admission of ICU. a, the patient was diagnosed without cardiomyopathies and congestive HF; b, the patient was diagnosed with congestive HF; c, the patient was diagnosed with cardiomyopathies and congestive HF. Model 1 was adjusted by age, sex, ethnicity, insurance, marital status, type of admission, and type of ICU; model 2 was adjusted by age, sex, ethnicity, insurance, marriage, admission types, ICU types, other comorbidities, SOFA, APACHE III, and treatments; model 3 was adjusted by age, sex, ethnicity, insurance, marriage, admission types, ICU types, other comorbidities, SOFA, APACHE III, and treatments after PSM. ICU, intensive care unit; HF, heart failure; OR, odds ratio; CI, confidence interval; SOFA, Sequential Organ Failure Assessment; APACHE III, Acute Physiology and Chronic Health Evaluation III scores; PSM, propensity-score matching.
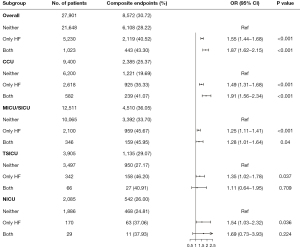
The subgroup analysis of the different types of ICU showed that the association between comorbid cardiomyopathy and composite endpoints among patients admitted to the CCU and MICU/SICU was consistent (as shown in Figure 2). However, the difference between the TSICU and NICU was not statistically significant (P>0.05).
Discussion
The main findings of this study were as follows. Firstly, comorbid cardiomyopathy was an independent risk factor for critically ill patients with congestive HF. Critically ill patients with congestive HF and cardiomyopathy had an increased risk of composite endpoints, which included in-hospital mortality, cardiac arrest, and re-admission to the ICU. The risk of cardiac arrest and re-admission to the ICU was also respectively increased. However, compared to patients with congestive HF due to other etiologies, patients with cardiomyopathy had no significant increase in the risk of in-hospital mortality. In addition, comorbid cardiomyopathy increased the risk of composite outcomes in the CCU and MICU/SICU subgroups.
Although several types of cardiomyopathies, such as HCM [which is clinically asymptomatic and has an average life expectancy in daily life (20,21)], occur in adolescents and young adults, critically ill patients often have serious clinical conditions such that cardiomyopathy may be a risk of worsening prognosis in the ICU. In our study, patients with cardiomyopathies were younger than those in the HF cohort. However, patients with comorbid cardiomyopathy likely have a longer history of HF than other group and experience refractory symptoms of congestive HF.
Cardiomyopathies are a heterogeneous group of heart muscle diseases with different clinical phenotypes. Patients diagnosed with cardiomyopathies, such as HCM and RCM (22,23), tend to present with HFpEF, whereas those diagnosed with DCM tend to present with HFrEF (24). However, whether the prognoses of HFpEF and HFrEF are different remains controversial. According to a study from the Mayo Clinic, the outcomes of patients with reduced LVEF improve over time but not for those with preserved LVEF (25). In contrast, the findings of other studies demonstrate an improved prognosis for patients with HFpEF compared to those with HFrEF (26,27). Our study found that in-hospital mortality was similar in patients with and without cardiomyopathies (13.29% vs. 14.84%), but an increased incidence of cardiac arrest (6.65% vs. 3.96%) worsened the outcome. The incidence of re-admission to the ICU implied a deterioration of disease (28), with a higher rate in patients with cardiomyopathy (29.62% vs. 27.23%).
Currently, HFpEF remains an unexplored area. ACEI/ARB and β-RB do not improve the outcomes of HFpEF patients (29). In our study, critically ill patients with cardiomyopathy and congestive HF had higher usage rates of vasoactive drugs, beta-blockers, ACEI/ARB, and digoxin, without an improvement in outcomes. This is because the etiology of cardiomyopathy is caused by genetic, immunologic, and systemic reasons (30,31). We consider that critically ill patients with cardiomyopathy may have a lesser response to classical treatments, which is a potential underlying mechanism.
To the best of our knowledge, this study is the first to clarify that comorbid cardiomyopathy is related to the poor prognosis of critically ill patients with congestive HF, providing a real-world study of patients with cardiomyopathy in the ICU. However, this study had some limitations that should be noted. Firstly, this study was a retrospective, single-center study that included only 1,023 patients with congestive HF and cardiomyopathies. Moreover, most of the included patients were white. Secondly, the primary diagnoses of patients admitted to the ICU could not be obtained from the MIMIC-IV database. Thirdly, the LVEF data were not obtained from the MIMIC-IV database, and the type of HF in this cohort could not be distinguished. Fourthly, the determinants of the severity of congestive HF, such as BNP, could not be evaluated using the data available from the MIMIC-IV database.
Conclusions
Comorbid cardiomyopathy increased the risk of composite endpoints in patients with congestive HF admitted to the ICU. The risk of comorbid cardiomyopathy was consistently observed in patients admitted to the CCU and MICU/SICU. Cardiomyopathy is related to the poor outcomes of critically ill patients with congestive HF.
Acknowledgments
We acknowledge Charlesworth for linguistic editing and proofreading in the preparation of this manuscript.
Funding: This work was supported by the Science and Technology Planning Project of Tianjin (grant numbers 0010404, 303070100404, 15YFYZSY00020).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-833/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-833/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethical approval of MIMIC-IV database was approved by institutional review boards of both BIDMC and Massachusetts Institute of Technology Affiliates. Moreover, all private information of subjects is hidden, and informed consent was waived by the Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30-41. [Crossref] [PubMed]
- Jenča D, Melenovský V, Stehlik J, et al. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail 2021;8:222-37. [Crossref] [PubMed]
- Jentzer JC, Bennett C, Wiley BM, et al. Predictive Value of the Sequential Organ Failure Assessment Score for Mortality in a Contemporary Cardiac Intensive Care Unit Population. J Am Heart Assoc 2018;7:008169. [Crossref] [PubMed]
- Pecini R, Møller DV, Torp-Pedersen C, et al. Heart failure etiology impacts survival of patients with heart failure. Int J Cardiol 2011;149:211-5. [Crossref] [PubMed]
- Kajimoto K, Minami Y, Sato N, et al. Etiology of Heart Failure and Outcomes in Patients Hospitalized for Acute Decompensated Heart Failure With Preserved or Reduced Ejection Fraction. Am J Cardiol 2016;118:1881-7. [Crossref] [PubMed]
- Arrigo M, Jessup M, Mullens W, et al. Acute heart failure. Nat Rev Dis Primers 2020;6:16. [Crossref] [PubMed]
- Brieler J, Breeden MA, Tucker J. Cardiomyopathy: An Overview. Am Fam Physician 2017;96:640-6. [PubMed]
- McKenna WJ, Judge DP. Epidemiology of the inherited cardiomyopathies. Nat Rev Cardiol 2021;18:22-36. [Crossref] [PubMed]
- Brownrigg JR, Leo V, Rose J, et al. Epidemiology of cardiomyopathies and incident heart failure in a population-based cohort study. Heart 2021; Epub ahead of print. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Silverdal J, Sjöland H, Bollano E, et al. Prognostic impact over time of ischaemic heart disease vs. non-ischaemic heart disease in heart failure. ESC Heart Fail 2020;7:264-73. [Crossref] [PubMed]
- Metkus TS, Lindsley J, Fair L, et al. Quality of Heart Failure Care in the Intensive Care Unit. J Card Fail 2021;27:1111-25. [Crossref] [PubMed]
- Shamszad P, Hall M, Rossano JW, et al. Characteristics and outcomes of heart failure-related intensive care unit admissions in children with cardiomyopathy. J Card Fail 2013;19:672-7. [Crossref] [PubMed]
- Johnson A, Bulgarelli L, Pollard T, et al. Mimic-iv (version 0.4). 2020. Available online: https://physionet.org/content/mimiciv/0.4/
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996;93:841-2. [Crossref] [PubMed]
- Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807-16. [Crossref] [PubMed]
- Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270-6. [Crossref] [PubMed]
- Scotina AD, Gutman R. Matching algorithms for causal inference with multiple treatments. Stat Med 2019;38:3139-67. [Crossref] [PubMed]
- Maron BJ, Rowin EJ, Maron MS. Global Burden of Hypertrophic Cardiomyopathy. JACC Heart Fail 2018;6:376-8. [Crossref] [PubMed]
- Ommen SR, Semsarian C. Hypertrophic cardiomyopathy: a practical approach to guideline directed management. Lancet 2021;398:2102-8. [Crossref] [PubMed]
- González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585-94. [Crossref] [PubMed]
- Maron BJ, Rowin EJ, Udelson JE, et al. Clinical Spectrum and Management of Heart Failure in Hypertrophic Cardiomyopathy. JACC Heart Fail 2018;6:353-63. [Crossref] [PubMed]
- Tayal U, Ware JS, Lakdawala NK, et al. Understanding the genetics of adult-onset dilated cardiomyopathy: what a clinician needs to know. Eur Heart J 2021;42:2384-96. [Crossref] [PubMed]
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9. [Crossref] [PubMed]
- Smith GL, Masoudi FA, Vaccarino V, et al. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol 2003;41:1510-8. [Crossref] [PubMed]
- Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260-9. [Crossref] [PubMed]
- Giraud T, Dhainaut JF, Vaxelaire JF, et al. Iatrogenic complications in adult intensive care units: a prospective two-center study. Crit Care Med 1993;21:40-51. [Crossref] [PubMed]
- Palazzuoli A, Caravita S, Paolillo S, et al. Current gaps in HFpEF trials: Time to reconsider patients' selection and to target phenotypes. Prog Cardiovasc Dis 2021;67:89-97. [Crossref] [PubMed]
- Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 2021;18:169-93. [Crossref] [PubMed]
- Yamada T, Nomura S. Recent Findings Related to Cardiomyopathy and Genetics. Int J Mol Sci 2021;22:12522. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


