Pulmonary arterioplasty using video-assisted thoracic surgery mechanical suture technique
Introduction
Lung cancer invading pulmonary trunk is not uncommon. Traditionally, the removal of the superior vena cava requires occlusion of a part of the sidewall by a clamp or complete occlusion of the pulmonary trunk from the proximal to distal ends. When a video-assisted thoracic surgery (VATS) manual suture technique is applied, more operation ports are required to facilitate the blockage of the pulmonary trunk; or, many surgical devices need to pass through the same port; in addition, the suturing needs to be performed using needle holders. Thus, this technique is highly challenging for most thoracic surgeons. Few articles have reported the application of complete VATS techniques in patients with lung cancer invading the pulmonary trunk. Automatic mechanical suturing enables the direct resection of the lateral wall of pulmonary trunk and avoids the blockage of pulmonary trunk and manual suturing, thus lowering the difficulty of minimally invasive surgery.
Case 1 presentation
Patient
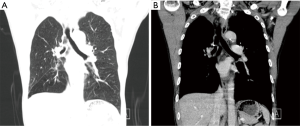
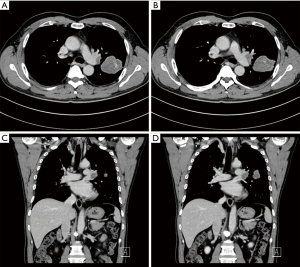
A 60-year-old smoking male patient was admitted due to recurrent cough for over 2 months. Chest CT indicated the presence of right upper lobe carcinoma and obstructive pneumonia (Figure 1). Bronchofiberscopy indicated that the lesion was a squamous cell carcinoma. No distant metastasis was detected. In order to achieve satisfactory effectiveness of radical treatment, we further discussed the disease condition and treatment protocol with the patient and his family and with other colleagues in our department and then decided to carry out Preoperative neoadjuvant chemotherapy. After three cycles of treatment, chest CT showed that the tumor remarkably shrank (Figure 2). Preoperative assessment showed good heart and lung function. Pulmonary arterioplasty by VATS was then scheduled.
Anesthesia
After the induction of general anesthesia, the patient was under double-lumen endotracheal intubation.
Body position
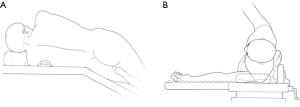
The patients were often placed in a lateral decubitus position on the unaffected side (Figure 3). The waist bridge is elevated to maximize the intercostal spaces and thus facilitate the operation.
Design of incisions
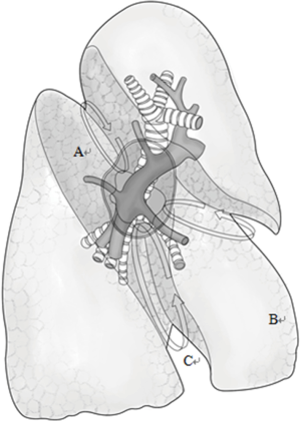
Typically three incisions will be more feasible (Figure 4). Observation port (incision): in the 6th or 7th intercostal space at anterior axillary line, about 1 cm in length. Main operation incision: in the 3rd or 4th intercostal space at anterior axillary line, about 4 cm in length. Auxiliary operation incision: within the same intercostal space with the observation port; in the 6th or 7th intercostal space at posterior axillary line, about 0.5 or 1 cm in length.
Surgical process
The detail of the surgical process was as follow:
- Dissect the posterior mediastinal pleura using an electrocautery hook, and then open up the tunnels in and outside the upper oblique fissure of lung; finally, divide the upper oblique fissure using the endoscopic cutter/stapler (Figure 5). Notice:
- During the dissection of the posterior mediastinal pleura, the electrocautery hook should be closer to lung till the bronchus;
- The key of the first approach is to free a channel for the creation of an artificial fissure within the lung parenchyma (Figure 6). The beginning and end of the channel used for creating artificial lung fissure in right lung were demonstrated in Table 1;
- If the lung fissure is poorly developed and the inter-fissure tissues are basically fused, parts of the tissue can be divided using cutter/stapler at the site of lung fissure before creating the channel.
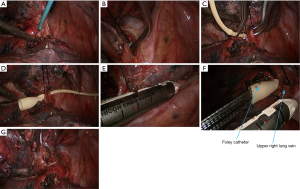
- The gap between right upper lung vein and pulmonary trunk was dissected using an electrocautery hook. The right upper lung vein was dissociated with a right-angle clamp and then suspended with a suture. Under the guidance of a urinary catheter, the right upper lung vein was transected with the endoscopic cutter/stapler (Figure 7);
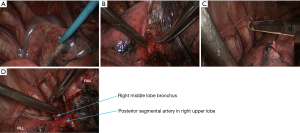
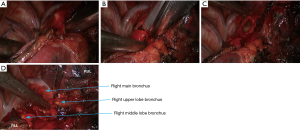
- Dissociate the right intermediate bronchus and right main bronchus using the vascular clamp, followed by the transection of right intermediate bronchus and right main bronchus (Figure 8);
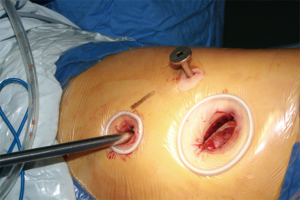
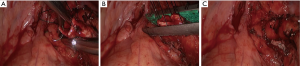
- Thoroughly dissociate the adjacent tissues. Lift the pulmonary artery, cross the endoscopic cutter/stapler, and then resect the arterial branch and side-wall of part of pulmonary trunk in right upper lung (Figure 9). The detail of the surgery was demonstrated in the video (Figure 10).

Full table
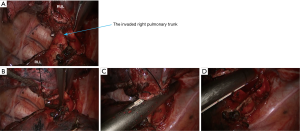

Postoperative diagnosis and follow-up
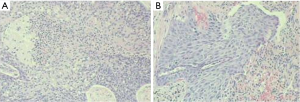
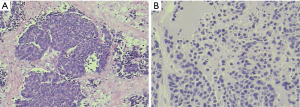
Pathology: moderately-differentiated squamous cell carcinoma in the right upper lung. The tumor invaded the bronchus, while no cancer was seen in the bronchial stump (Figure 11).
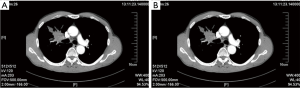
Post-operative CT showed that the ventilation was good in both lungs; the right middle bronchus did not become narrow or twisted; the pulmonary artery was patent, and no luminal stricture was observed (Figure 12).
Case 2 presentation
Patient
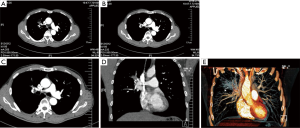
A 57-year-old smoking male patient was admitted due to left chest pain for over 3 months. Chest CT indicated the presence of carcinoma at the apical and posterior segments of the left upper lobe and obstructive pneumonia (Figure 13). No distant metastasis was seen in further preoperative examinations. The heart and lung functions were good. Pulmonary arterioplasty by VATS was then scheduled.
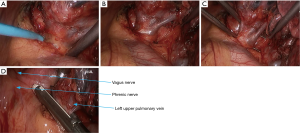
- The mediastinal pleura were dissected using electric surgical knife. Tissues around the left upper pulmonary vein were thoroughly dissociated to achieve nearly skeletonization. The left upper pulmonary vein was dissociated using a right-angled clamp (Figure 14) and then transected using the endoscopic cutter/stapler;
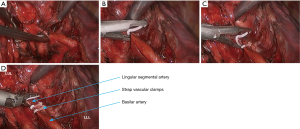
- After the left upper lobe was lifted upwards, the sheath of pulmonary artery was dissected to expose the pulmonary arterial branch at the lingular segment of left upper lobe, which was blocked with vascular clamps and then transected using ultrasonic scalpel (Figure 15). The pulmonary arterial branch at the posterior segment of left upper lobe was handled using the same method, followed by the division of the oblique fissure;
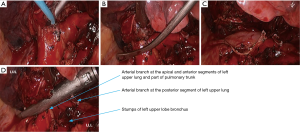
- After the left upper lung was lifted upwards, the right upper lobe bronchus was dissociated and then transected (Figures 16,17);
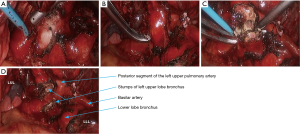
- After the posterior mediastinal pleura at the pulmonary hilum was cut open and its surrounding tissues were dissociated, the endoscopic cutter/stapler was placed to remove the first branch of left pulmonary artery and the side-wall of part of pulmonary trunk (Figure 18). Pulmonary arterioplasty using mechanical suturing technique in left upper lobectomy was performed on June 6th 2013 (Figure 19). The pathology result demonstrated that small cell lung cancer (Figure 20).

Discussion
The method used in this study has two key technical concerns: first, how to ensure safe resection without bleeding margins of the sidewall, and second, how to ensure thoracoscopic resection with negative margins while maintaining sufficient blood reflux in the residual vessel.
Regarding the first technical issue, in thoracoscopic lung surgery, cutting blood vessels of different diameters and thicknesses with a stapler is a common operation (3-5). From a technical point of view, resection of the sidewall of the pulmonary trunk with a stapler is essentially nothing different from transection of handling the pulmonary artery (6). Therefore, we do not worry whether the cutting margin can be properly stapled and if bleed leakage occurs.
Regarding the second technical issue, from the technical view for resection, we are most concerned about the length and the circumference of invasions in the pulmonary trunk, especially the latter. In theory, after one third of the circumference of pulmonary trunk is resected, the area of the channel formed by the remaining sidewall of the pulmonary artery is about half of the sectional area of the original pulmonary trunk, which is sufficient to deliver blood to the lower pulmonary lobe. Therefore, after the application of endoscopic cutter/stapler, if the lower margin of the clamped part is no more than one third of the original lumen of pulmonary trunk, the blood supply of the pulmonary artery will not be affected.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Xu X, Huang J, He J, et al. Pulmonary arterioplasty using mechanical suturing technique in right lung. Asvide 2016;3:160. Available online: http://www.asvide.com/articles/918
- Xu X, Huang J, He J, et al. Pulmonary arterioplasty using mechanical suturing technique in left upper lobectomy. Asvide 2016;3:161. Available online: http://www.asvide.com/articles/920
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Nakanishi R, Yamashita T, Oka S. Initial experience of video-assisted thoracic surgery lobectomy with partial removal of the pulmonary artery. Interact Cardiovasc Thorac Surg 2008;7:996-1000. [Crossref] [PubMed]
- Kamiyoshihara M, Nagashima T, Ibe T, et al. A tip for controlling the main pulmonary artery during video-assisted thoracic major pulmonary resection: the outside-field vascular clamping technique. Interact Cardiovasc Thorac Surg 2010;11:693-5. [Crossref] [PubMed]
- Jones DR. Technique of superior vena cava resection for lung carcinomas. Operative Techniques in Thoracic and Cardiovascular Surgery 2008;13:274-82. [Crossref]