
Reliability of dynamic perfusion digital radiography as an alternative to pulmonary perfusion scintigraphy in predicting postoperative lung function and complications
Introduction
Despite advances in perioperative care, risk assessment for lung cancer surgery remains an important clinical issue due to the increasing frequency of surgery in older patients and/or in those with various comorbidities (1-4). In this regard, it is important to predict the occurrence of perioperative complications (5,6) as well as the long-term quality of life. The latter is influenced by reduced residual lung function (7), manifesting as a decreased ability to perform daily-life activities or an induction of oxygen therapy. A weakened ability to perform daily-life activities is a risk factor for reduced long-term and perioperative survival rates (8). Although the risk of lung cancer surgery has declined because of the widespread use of minimally invasive surgery and advances in perioperative management, the prediction of constantly occurring postoperative deaths and complications requires risk assessment of both preoperative pulmonary and cardiovascular functions (9,10).
Currently, postoperative complications and perioperative mortality have been predicted using each measured value by spirometry and the diffusing capacity test for carbon monoxide (DLco) (9,11-13). Furthermore, calculation of the predicted postoperative (ppo) lung function is recommended, particularly in cases with comorbidities of lung diseases, such as interstitial pneumonia and chronic obstructive pulmonary disease (COPD), or in individuals who engage in heavy smoking (14-19). However, because respiration requires a balance between pulmonary ventilation and perfusion, pulmonary perfusion status, instead of ppo lung function, which does not reflect the heterogeneity of local pulmonary perfusion, provides a meaningful addition to the spirometry measurement. Although angiography, ultrasonography, quantitative computed tomography (CT), and dynamic magnetic resonance imaging have been employed for the visualization or quantification of pulmonary blood flow (20,21), pulmonary perfusion scintigraphy (PPS) has been considered as the most reliable and conventional method to measure the pulmonary blood flow distribution. Considering the difficulties in assessing blood flow distribution, patient exposure, and the need for institutional investment, perfusion scintigraphy is now removed from the guidelines. However, a modality, which could provide easy measurement and evaluation of the pulmonary blood flow distribution and, at the same time, overcome the challenges in perfusion scintigraphy, may represent a useful substitute for this technique.
Dynamic perfusion digital radiography (DPDR) is a simple and cost-effective examination method (e.g., requiring low facility investment, no nuclide preparation, a short examination time, and low radiation exposure) to provide qualitative and quantitative information about dynamic pulmonary circulation (22,23). The analysis first obtains sequential chest radiographs with a high temporal resolution during the respiratory cycle via dynamic flat-panel detectors with a wide field of view and advanced digital image processing. This dynamic chest radiography (DCR) is performed in a setup similar to that of a standard chest radiograph. The equipment footprint is similar to that of a standard radiography suite. Therefore, this method is very advantageous as it allows DCR and simple chest radiography simultaneously. It is possible to quantify the pulmonary blood flow distribution from the cardiac-cycle related changes in pixel values in the lung field and create visually qualitative images (24). To date, correlations and similarities between angiography and perfusion scintillation in diseases, such as chronic obstructive thrombosis and pulmonary fibrosis, as well as normal lungs in animal experiments and clinical practice, have been reported (25). Preoperative screening or postoperative follow-up can be easily performed using this method. However, its utility in pulmonary perfusion measurement needs to be determined by comparison with the conventional PPS method.
The present study aimed to evaluate whether blood flow imaging using DPDR can be a viable substitute for conventional PPS. The study not only confirmed the agreement between DPDR and PPS in the blood flow ratio (BFR) and the postoperative predictive ratio (PoPR) but also compared the prediction accuracy of each BFR for postoperative lung function and perioperative complications. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-383/rc)
Methods
Patients
Patients with primary lung cancer scheduled for radical resection from May 2018 to December 2020 were recruited. Only those who were able to follow breathing instructions, which involved breath holding or forced breathing, in the sitting position were included. A total of 100 patients underwent follow-up evaluation 6 months after surgery (May 2021). Patients with a history of thoracic surgery, those younger than 20 years of age, and those at risk for adverse events due to irradiation were excluded. Ten patients were excluded due to the following reasons: presence of a disease other than lung cancer (n=3), wedge resection of the lung (n=3), and refusal to provide informed consent during the postoperative period (n=4). In this study, we selected patients who underwent preoperative PPS to confirm the reliability of DPDR as an alternative to PPS. Therefore, 44 of the 90 participants were included in the final analysis (Figure 1). Table 1 depicts the clinical variables collected from patient’s electronic medical records. Data from this patient population were previously used in a related study (22).
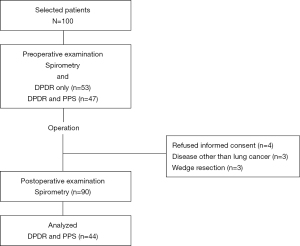
Table 1
| Variables (unit) | Subtypes | N=44 (%) |
|---|---|---|
| Age (year) | ≥75 years | 31 (70.5) |
| <75 years | 13 (29.5) | |
| Gender | Male | 38 (86.4) |
| Female | 6 (13.6) | |
| Brinkman index | ≥700 | 30 (68.2) |
| <700 | 14 (31.8) | |
| Respiratory comorbidities | Yes | 12 (27.3) |
| No | 32 (72.7) | |
| Circulatory comorbidities | Yes | 25 (56.8) |
| No | 19 (43.2) | |
| Respiratory history | Yes | 5 (11.4) |
| No | 39 (88.6) | |
| Circulatory history | Yes | 6 (13.6) |
| No | 38 (86.4) | |
| COPD stage | ≥2 | 15 (34.1) |
| <2 | 29 (65.9) | |
| KL-6 (U/mL) | ≥500 | 4 (11.1) |
| <500 | 32 (88.9) | |
| Affected side | Right | 23 (52.3) |
| Left | 21 (47.7) | |
| Resected lobe of the lung | Right upper | 11 (25.0) |
| Right middle | 3 (6.8) | |
| Right lower | 6 (13.6) | |
| Left upper | 12 (27.3) | |
| Left lower | 9 (20.5) | |
| Segmentectomy | 3 (6.8) | |
| Approach | Open thoracotomy | 11 (25.0) |
| VATS | 33 (75.0) | |
| Adjuvant therapy | Yes | 13 (29.5) |
| No | 31 (70.5) | |
| Histology | Adenocarcinoma | 32 (72.7) |
| Non-adenocarcinoma | 12 (27.3) | |
| Respiratory complications | ||
| ~1 POM | Yes | 14 (31.8) |
| No | 30 (68.2) | |
| 1–3 POM | Yes | 3 (6.8) |
| No | 41 (93.2) | |
| Circulatory complications | ||
| ~1 POM | Yes | 6 (13.6) |
| No | 38 (86.4) | |
| 1–3 POM | Yes | 0 (0.0) |
| No | 44 (100.0) | |
| LTOT | Yes | 8 (18.2) |
| No | 36 (81.8) | |
| Recurrence | Yes | 5 (11.4) |
| No | 39 (88.6) | |
| Prognosis | Dead | 1 (2.3) |
| Alive | 43 (97.7) | |
COPD, chronic obstructive pulmonary disease; VATS, video-assisted thoracic surgery; POM, postoperative month; LTOT, long-term oxygen therapy.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Shiga University of Medical Science (CRB 5180008; October 10, 2017) and informed consent was taken from all individual participants. This study was registered as a clinical trial (UMIN000029716).
Pulmonary perfusion analysis and BFR measurement
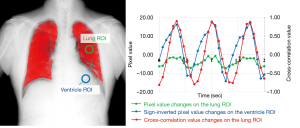
DCR for the pulmonary perfusion analysis was performed as previously described (22). The dynamic imaging device in this study was a prototype system (Konica Minolta, Inc., Tokyo, Japan) with an indirect-conversion flat-panel detector, an X-ray tube, and a pulsed X-ray generator. As participants held their breath in the sitting position, they were scanned by the pulsed X-ray at 15 frame per second for approximately 10 seconds to capture perfusion-induced changes in the pixel values. To avoid excessive exposure, the total radiation dose of the participants was adjusted to <1.5 mGy. Furthermore, pulmonary perfusion was analyzed by cross-correlation using these dynamic images (Figure 2). This process calculated the degree of waveform correlation between changes in the pixel values in pulmonary and ventricle regions. Cross-correlation values (CCv, from −1.0 to 1.0) detect periodic changes in pixel values corresponding to the cardiac cycle in the lung field. A positive CCv indicates the presence of blood flow. Higher CCv indicates increased similarity in the blood flow changes in the cardiac cycle. After the maximum CCv (MaxCCv) for each pixel had been computed for all frames, BFR was calculated as the ratio of the sum of the MaxCCv on the affected side to the sum of MaxCCv in both the left and right lung fields. BFR was used as the left-right ratio because the left-right difference is less affected by the imaging position despite of the gravitational influence on pulmonary blood flow.
PPS
PPS was performed using a dual-head gamma camera (Discovery630; GE Healthcare Life Sciences, Amersham Place, Little Chalfont, Buckinghamshire HP7 9NA, England). Patients were administered with half of the 200-MBq 99mTc microalbumin/99mTc-macroaggregate solution intravenously in the prone position, and with the other half in the supine position. Subsequently, the images obtained by plane scan were subjected to quantitative perfusion analysis. Geometric mean values were calculated from anterior-to-posterior and posterior-to-anterior projections of the bilateral lungs, which were divided into six regions of interest.
The BFR, calculated by planar perfusion scintigraphy, did not consider the spatial overlap of blood flow in each lung lobe due to the low spatial resolution of imaging under respiratory movement.
Pulmonary function tests
All patients underwent pulmonary function tests within 2 months prior to surgery and at 1, 3, and 6 months after surgery, using a computerized spirometer (FUDAC-77; Fukuda Denshi Co., Ltd., Tokyo, Japan). Preoperative spirometry values included vital capacity (VC) and %VC, forced vital capacity (FVC) and %FVC, forced expiratory volume in one second (FEV1) and %FEV1, DLco and %DLco.
Calculation of postoperative predicted lung function values
PoPR was calculated using the number of remaining lung segments, in consideration of the left–right BFR from DPDR and PPS, with the following formula (22):
The number of lung segments was defined as follows: right upper lobe, 3; right middle lobe, 2; right lower lobe, 5; left upper segment, 3; left lingular segment, 2; and left lower lobe, 4. Furthermore, ppo lung function values were calculated using the PoPR and various preoperative spirometry values with the following formula, as shown in FEV1 as an example Eq. [2].
Statistical analysis
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) (26). Data are reported as means ± standard deviations. Continuous data were examined for normality using the Shapiro-Wilk test. Correlations between DPDR and PPS in preoperative BFRs or PoPRs were evaluated using Pearson’s correlation and regression analysis. Bland-Altman analysis was used to assess the consistency of the PoPR between DPDR and PPS (27). Outcome comparisons between two groups were performed using Fisher’s exact test. Receiver operating characteristic (ROC) analysis was performed to assess the best spirometry threshold for the occurrence of perioperative respiratory and circulatory complications. Sensitivity and specificity of the analysis were calculated. All statistical analyses were two-tailed, with a significance threshold of 0.05.
Results
Clinical characteristics
Table 1 shows the preoperative clinicopathological factors of 44 patients who underwent DPDR and PPS. Brickman Index ≥700, COPD comorbidity with stage 2 or higher, and respiratory comorbidity were observed in 68.18%, 34.09%, and 27.27% of patients, respectively. In most patients, the respiratory comorbidity was COPD, and only one patient had idiopathic pulmonary fibrosis. In each patient, respiratory and/or circulatory-related events, occurring up to 6 months after surgery, were extracted. Complications of Clavien-Dindo classification II or higher were targeted. In this study, there were no deaths due to complications, and no complications occurred after 3 postoperative months. In the 1st postoperative month, complications occurred in 18 cases, of which 14 were respiratory-system related (hypoxemia, 7; pleuritis, 5; bacterial pneumonia, 3; exacerbation of interstitial pneumonia, 1; pneumothorax, 1; difficulty in expectoration, 1; prolonged air leakage, 1) and six were cardiovascular-system related (paroxysmal atrial fibrillation, 5; pulmonary thrombosis, 1). All three complications, occurring between the 1st and 3rd postoperative months, were respiratory-system related (pleuritis, 2; exacerbation of interstitial pneumonia, 1).
Correlation between blood flow distributions using DPDR and PPS
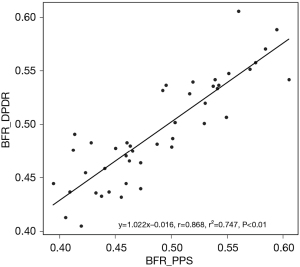
Correlation between the preoperative BFRs of the affected side, obtained from DPDR and PPS, was examined in 44 patients. Figure 3 shows the linear regression analysis with excellent correlation between the distributions of affected-side-to-total ratios, obtained by DPDR (y) and PPS (x) (r=0.868, P<0.01).
Correlation between the measured and predicted spirometry values using DPDR and PPS
Ppo lung function was obtained by multiplying the PoPR, calculated using the left–right BFR from DPDR and PPS, with the preoperative spirometry measurements. While there was a high correlation between PoPRs calculated from DPDR and PPS (r=0.975, P<0.01), four out of 44 (9.1%) cases deviated from the limits of agreement in the Bland-Altman analysis (Figure S1). The relationship between the measured values, such as FEV1 and DLco, which have been frequently used to predict postoperative lung function and perioperative risk, and predictive values, using BFRs from DPDR and PPS, was examined among 44 patients who underwent simultaneous DPDR and PPS at 1, 3, and 6 months postoperatively. Table 2 presents a summary of the correlation coefficients obtained using DPDR and PPS. The correlation coefficients between the predicted values, obtained from DPDR or PPS, and the measured values at postoperative months 1, 3, and 6 were high (r>0.8). Furthermore, these results also showed a high correlation between DPDR and PPS.
Table 2
| Modality | FEV1 | DLco | |||||
|---|---|---|---|---|---|---|---|
| 1POM | 3POM | 6POM | 1POM | 3POM | 6POM | ||
| DPDR | 0.809 | 0.845 | 0.855 | 0.847 | 0.848 | 0.854 | |
| PPS | 0.808 | 0.839 | 0.848 | 0.847 | 0.849 | 0.853 | |
DPDR, dynamic perfusion digital radiography; PPS, pulmonary perfusion scintigraphy; FEV1, forced expiratory volume in one second; DLco, diffusing capacity for carbon monoxide; POM, postoperative month.
Predicting postoperative complications using the BFR
Ppo lung function values were calculated using the number of segments before and after surgery, and the left-right BFR from DPDR and PPS. An ROC curve was generated, and the area under the curve (AUC) was calculated to determine the best discriminating level of its various indices for predicting the occurrence of postoperative respiratory and circulatory complications. Indicators of lung function with an AUC greater than 0.6 were extracted. The cut-off values, sensitivity, and specificity are summarized in Table 3. In addition, the table includes the results of Fisher’s exact test on the association with complications during each postoperative period, when divided into two groups using each spirometry cut-off value. The excellent prediction values with an AUC exceeding 0.7 were ppo%DLco_PPS and ppo%DLco_DPDR for respiratory complications within 1 month. However, the cut-off values were significantly different from 65.3 and 49.19, respectively. Therefore, the number of cases below each cut-off value was different. However, the same cases with respiratory complications were detected. The prediction of respiratory complications within 1 and 3 months after surgery using ppo% DLco as an index was good for both DPDR and PPS (DPDR: P=0.008 and 0.025, respectively, for 1 and 3 months postoperatively; PPS: P=0.034 and 0.006, respectively, for 1 and 3 months postoperatively).
Table 3
| Type of complications | Period | Ppo lung function values | AUC | Cut-off value | Specificity | Sensitivity | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|---|---|---|---|
| Respiratory complications | −1 POM | %VC_DPDR | 0.610 | 87.150 | 0.833 | 0.429 | 0.276 | 0.050–1.408 | 0.132 |
| %FEV1_DPDR | 0.633 | 60.110 | 0.800 | 0.571 | 3.858 | 0.816–19.649 | 0.074 | ||
| %DLco_DPDR | 0.731 | 49.190 | 0.967 | 0.429 | 14.292 | 1.419–783.269 | 0.008 | ||
| %VC_PPS | 0.654 | 77.400 | 0.467 | 0.857 | 0.281 | 0.042–1.363 | 0.104 | ||
| %FVC_PPS | 0.630 | 80.600 | 0.533 | 0.786 | 0.314 | 0.058–1.407 | 0.111 | ||
| %FEV1_PPS | 0.630 | 63.500 | 0.767 | 0.571 | 4.216 | 0.928–21.003 | 0.042 | ||
| %DLco_PPS | 0.714 | 65.300 | 0.800 | 0.571 | 5.098 | 1.091–26.627 | 0.034 | ||
| −3 POM | %FEV1_DPDR | 0.603 | 60.110 | 0.778 | 0.471 | 2.398 | 0.533–11.324 | 0.309 | |
| %DLco_DPDR | 0.671 | 49.190 | 0.963 | 0.353 | 10.239 | 0.994–529.784 | 0.025 | ||
| %VC_PPS | 0.624 | 77.400 | 0.481 | 0.824 | 0.294 | 0.055–1.282 | 0.114 | ||
| %FEV1_PPS | 0.611 | 65.000 | 0.630 | 0.588 | 2.378 | 0.595–10.117 | 0.218 | ||
| %DLco_PPS | 0.660 | 46.600 | 1.000 | 0.294 | Inf | 1.704–Inf | 0.006 | ||
| Circulatory complications | −1 POM | %VC_PPS | 0.621 | 69.500 | 0.949 | 0.400 | 11.011 | 0.607–207.942 | 0.057 |
| %FVC_PPS | 0.615 | 76.800 | 0.795 | 0.600 | 5.517 | 0.538–76.563 | 0.091 | ||
| −3 POM | %VC_PPS | 0.621 | 69.500 | 0.949 | 0.400 | 11.011 | 0.607–207.942 | 0.057 | |
| %FVC_PPS | 0.615 | 76.800 | 0.795 | 0.600 | 5.517 | 0.538–76.563 | 0.091 |
Ppo, predicted postoperative; AUC, area under the curve; CI, confidence interval; POM, postoperative month; VC, vital capacity; DPDR, dynamic perfusion digital radiography; FEV1, forced expiratory volume in one second; DLco, diffusing capacity for carbon monoxide; FVC, forced vital capacity; PPS, pulmonary perfusion scintigraphy; Inf, infinity.
Predicting postoperative long term oxygen therapy induction using the BFR
Additionally, an ROC curve was generated using ppo lung function values. The AUC was calculated to determine the best discriminating level of its various indices for predicting the induction of postoperative long term oxygen therapy. Table 4 shows the cut-off values, 95% confidence interval, sensitivity, and specificity of each lung function indicator with an AUC >0.6. The ppo%DLco using BFR from both DPDR and PPS was a good indicator (AUC >0.8). There was no difference in sensitivity or specificity between DPDR and PPS. The ppo values of %VC and %FVC were cut-off values with the AUC >0.7. However, both values were not considered useful indicators as they were within the normal range for both DPDR and PPS.
Table 4
| Modality | Ppo lung function values | AUC | Cut-off value | 95% CI | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| DPDR | %VC | 0.733 | 91.670 | 0.485–0.981 | 0.889 | 0.625 |
| %FVC | 0.726 | 91.240 | 0.482–0.969 | 0.889 | 0.625 | |
| %FEV1 | 0.625 | 60.110 | 0.366–0.884 | 0.750 | 0.625 | |
| %DLco | 0.840 | 47.260 | 0.671–1.000 | 0.972 | 0.625 | |
| PPS | %VC | 0.722 | 90.300 | 0.469–0.975 | 0.861 | 0.625 |
| %FVC | 0.708 | 93.500 | 0.459–0.958 | 0.944 | 0.500 | |
| %FEV1 | 0.625 | 60.300 | 0.374–0.876 | 0.778 | 0.625 | |
| %DLco | 0.851 | 46.600 | 0.688–1.000 | 1.000 | 0.625 |
Ppo, predicted postoperative; AUC, area under the curve; CI, confidence interval; DPDR, dynamic perfusion digital radiography; PPS, pulmonary perfusion scintigraphy; VC, vital capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; DLco, diffusing capacity for carbon monoxide.
Discussion
This study compared the following factors, obtained from DPDR and PPS, to assess whether DPDR could be used as a substitute for conventional PPS: (I) BFR; (II) actual spirometry and ppo values using PoPR calculated from BFR; and (III) prediction accuracy of postoperative respiratory and circulatory complications. BFRs and PoPRs, obtained from DPDR and PPS, showed an excellent correlation (r=0.868, P<0.01 and r=0.975, P<0.01, respectively). Correlation coefficients between the predicted lung function values obtained from these two methods and measured values were similar throughout the postoperative course (Table 2). Although a high correlation in PoPR was observed, a small number of cases outside the range of the limits of agreement were found in the Bland-Altman analysis, possibly because of the differences in evaluation targets and measurement methods.
In PPS, the distribution of 99mTc-macroaggregated albumin, which transiently accumulated in peripheral capillaries as microemboli after intravenous injection, is proportional to the pulmonary artery blood flow (28,29). In contrast, in the lung perfusion analysis by DPDR, local perfusion was evaluated as a CCv based on the correlation between changes in the pixel values of the pulmonary and ventricular regions during the cardiac cycle. BFR was calculated as the ratio of the sum of the MaxCCv on the left and right lung fields (22). Cross-correlation is an assessment of local pulmonary pulsation, which indicates the presence of local pulmonary artery blood flow. Therefore, some differences between DPDR and PPS findings are not surprising, as both have completely different imaging targets and mechanisms. In addition, DPDR imaging can be strongly affected by pulsations at the margins of cardiac shadows or large blood vessels in the hilum. It is important to develop a simple and minimally invasive alternative modality by increasing the measurement accuracy with improved analytical techniques.
The prediction of perioperative complications, including the postoperative induction of oxygen therapy based on ppo%FEV1 and ppo%DLco, were markedly similar between DPDR and PPS. The non-functional lung region, indicated by the low attenuation area on chest CT, is a region of no or low blood flow. Therefore, DLco, strongly influenced by pulmonary blood flow, is likely correlated with the predicted lung function using the BFR. Although minimally invasive surgery for lung cancer has recently been associated with fewer critical complications (19,30), the preoperative prediction of postoperative complications calls for attention to postoperative management. However, many thoracic surgeons do not believe that a single method can yield precise predictions (9,10). In this regard, a highly accurate prediction for postoperative complications is expected to be achieved by complication prediction model of risk factors, including the ppo lung function. There have been many reports of predictions of postoperative lung function and perioperative complications using various modalities, each of which has advantages and disadvantages, such as exposure dose or inspection time (20,21,28,29). Therefore, it is more reasonable to select a simpler and less invasive method than to obtain data to calculate a predicted value from a method with certain disadvantages. Modality should be used as an alternative to examination methods with previously accumulated data. In addition, the captured images can be used to obtain additional information, such as thoracic and diaphragmatic movements by DCR, ventilation status by ventilation imaging, or thromboembolism by blood flow imaging (31-34). As such, a lot of information could be easily obtained not only from a normal moving image but also from ventilation/perfusion dynamic image-by-image processing.
DCR studies can considerably stimulate the exploration of respiratory and cardiovascular physiology. Therefore, this technical has many potential applications, from disease diagnosis to functional evaluation (31). Notably, DCR analysis can provide information in all supine, standing, and sitting positions, which are more reflective of the normal respiratory and circulatory states. A recent study on pulmonary blood flow distribution using other modalities reported that conventional gravity and vessel anatomy are major determinants of regional pulmonary blood flow (35). However, since these were measured in the lateral and supine positions, the actual influence of body position on pulmonary circulation is incompletely understood (36). In contrast, DPDR can provide definitive data on the magnitude of the impact of standing and sitting positions on the pulmonary blood flow distribution in spontaneously breathing and awake humans. After radical surgery for lung cancer, the pulmonary blood flow distribution is expected to change as a result of right heart dysfunction, due to the reduction of the pulmonary vascular bed, or increased pulmonary vascular resistance, due to flexion of the bronchi and pulmonary blood vessels associated with residual lung repositioning. DPDR also provides new insights into these situations.
The current study has some limitations. First, this study was a single-center analysis with a small sample size. There was a possibility of selection bias for the following reasons: (I) all cases were indicated for surgery; and (II) PPS was given to patients with smoking history and pulmonary dysfunction, such as comorbidity to avoid unnecessary radiation exposure. Therefore, although the results from this study may differ from those involving low-risk and standard lung function, cases requiring perioperative prediction in clinical practice appear to resemble the target cases in this study. However, indications for lung cancer patients with representative lung cancer demographics should also be evaluated. Since this study emphasized on the measurement and prediction of postoperative lung function by a simple method, lung function was predicted by the segmental method considering the BFR. However, a method for calculating residual lung volume using CT volumetry may be useful to further improve the accuracy (21). Therefore, additional studies are required in the future to examine these issues.
Conclusions
Our study showed that simple and minimally invasive DPDR can be a good alternative to PPS for predicting postoperative pulmonary function values and the risk of postoperative respiratory complications. While this study only demonstrated a partial utilization of DPDR, this new imaging system has the potential to provide new insights and many functional analyses.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing. Katsunori Miyata, a radiological technician at our institution, contributed substantially by assisting with DCR.
Funding: This work was supported by Konica Minolta, Inc.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-383/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-383/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-383/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-383/coif). JH received a research grant from Konica Minolta Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Shiga University of Medical Science (CRB 5180008; October 10, 2017) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370-7. [Crossref] [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Onaitis MW, Furnary AP, Kosinski AS, et al. Prediction of Long-Term Survival After Lung Cancer Surgery for Elderly Patients in The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2018;105:309-16. [Crossref] [PubMed]
- Ferguson MK, Watson S, Johnson E, et al. Predicted postoperative lung function is associated with all-cause long-term mortality after major lung resection for cancer. Eur J Cardiothorac Surg 2014;45:660-4. [Crossref] [PubMed]
- Sawabata N, Nagayasu T, Kadota Y, et al. Risk assessment of lung resection for lung cancer according to pulmonary function: republication of systematic review and proposals by guideline committee of the Japanese association for chest surgery 2014. Gen Thorac Cardiovasc Surg 2015;63:14-21. [Crossref] [PubMed]
- Bolliger CT, Gückel C, Engel H, et al. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration 2002;69:482-9. [Crossref] [PubMed]
- Kawaguchi Y, Hanaoka J, Oshio Y, et al. Decrease in performance status after lobectomy mean poor prognosis in elderly lung cancer patients. J Thorac Dis 2017;9:1525-30. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;64:e77-137. [Crossref] [PubMed]
- Ferguson MK, Siddique J, Karrison T. Modeling major lung resection outcomes using classification trees and multiple imputation techniques. Eur J Cardiothorac Surg 2008;34:1085-9. [Crossref] [PubMed]
- Ferguson MK, Gaissert HA, Grab JD, et al. Pulmonary complications after lung resection in the absence of chronic obstructive pulmonary disease: the predictive role of diffusing capacity. J Thorac Cardiovasc Surg 2009;138:1297-302. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg 2011;142:1143-51. [Crossref] [PubMed]
- Ali MK, Mountain CF, Ewer MS, et al. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980;77:337-42. [Crossref] [PubMed]
- Wernly JA, DeMeester TR, Kirchner PT, et al. Clinical value of quantitative ventilation-perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg 1980;80:535-43. [Crossref] [PubMed]
- Beckles MA, Spiro SG, Colice GL, et al. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest 2003;123:105S-14S. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Different diffusing capacity of the lung for carbon monoxide as predictors of respiratory morbidity. Ann Thorac Surg 2009;88:405-10; discussion 410-1. [Crossref] [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91; discussion 1091. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28, dicussion 28-29.e1.
- Ohno Y, Koyama H, Nogami M, et al. Postoperative lung function in lung cancer patients: comparative analysis of predictive capability of MRI, CT, and SPECT. AJR Am J Roentgenol 2007;189:400-8. [Crossref] [PubMed]
- Oswald NK, Halle-Smith J, Mehdi R, et al. Predicting Postoperative Lung Function Following Lung Cancer Resection: A Systematic Review and Meta-analysis. EClinicalMedicine 2019;15:7-13. [Crossref] [PubMed]
- Hanaoka J, Yoden M, Hayashi K, et al. Dynamic perfusion digital radiography for predicting pulmonary function after lung cancer resection. World J Surg Oncol 2021;19:43. [Crossref] [PubMed]
- Tanaka R. Dynamic chest radiography: flat-panel detector (FPD) based functional X-ray imaging. Radiol Phys Technol 2016;9:139-53. [Crossref] [PubMed]
- Tanaka R, Sanada S, Okazaki N, et al. Evaluation of pulmonary function using breathing chest radiography with a dynamic flat panel detector: primary results in pulmonary diseases. Invest Radiol 2006;41:735-45. [Crossref] [PubMed]
- Yamasaki Y, Kamitani T, Abe K, et al. Diagnosis of Pulmonary Hypertension Using Dynamic Chest Radiography. Am J Respir Crit Care Med 2021;204:1336-7. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [Crossref] [PubMed]
- Kovacević-Kuśmierek K, Kozak J, Pryt Ł, et al. Perfusion lung scintigraphy for the prediction of postoperative residual pulmonary function in patients with lung cancer. Nucl Med Rev Cent East Eur 2015;18:70-7. [Crossref] [PubMed]
- Toney LK, Wanner M, Miyaoka RS, et al. Improved prediction of lobar perfusion contribution using technetium-99m-labeled macroaggregate of albumin single photon emission computed tomography/computed tomography with attenuation correction. J Thorac Cardiovasc Surg 2014;148:2345-52. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Yamada Y, Ueyama M, Abe T, et al. Time-Resolved Quantitative Analysis of the Diaphragms During Tidal Breathing in a Standing Position Using Dynamic Chest Radiography with a Flat Panel Detector System ("Dynamic X-Ray Phrenicography"): Initial Experience in 172 Volunteers. Acad Radiol 2017;24:393-400. [Crossref] [PubMed]
- Watase S, Sonoda A, Matsutani N, et al. Evaluation of intrathoracic tracheal narrowing in patients with obstructive ventilatory impairment using dynamic chest radiography: A preliminary study. Eur J Radiol 2020;129:109141. [Crossref] [PubMed]
- Ohkura N, Kasahara K, Watanabe S, et al. Dynamic-Ventilatory Digital Radiography in Air Flow Limitation: A Change in Lung Area Reflects Air Trapping. Respiration 2020;99:382-8. [Crossref] [PubMed]
- Yamasaki Y, Abe K, Hosokawa K, et al. A novel pulmonary circulation imaging using dynamic digital radiography for chronic thromboembolic pulmonary hypertension. Eur Heart J 2020;41:2506. [Crossref] [PubMed]
- Glenny RW, Robertson HT. Determinants of pulmonary blood flow distribution. Compr Physiol 2011;1:39-59. [PubMed]
- Galvin I, Drummond GB, Nirmalan M. Distribution of blood flow and ventilation in the lung: gravity is not the only factor. Br J Anaesth 2007;98:420-8. [Crossref] [PubMed]


