
Economic evaluation of microbiological and host biomarker-based tests for the diagnosis of pleural tuberculosis in a high burden setting
Introduction
Tuberculosis (TB) remain a major global public health threat with 10 million cases and 1.4 million deaths (1). Although global rates are steadily declining, several factors threaten to reverse gains made in controlling the epidemic, including the emergence of drug resistant strains and, more recently, the COVID-19 pandemic. The latter has significantly impacted TB notification rates in 2020, with reductions of up to 75% in several high burden countries, including South Africa (1).
Although pulmonary TB is the most common form of the disease, extrapulmonary (EP) sites are often involved, especially in HIV-infected individuals, and accounts for up to 15% of active TB cases (1). Of these, pleural TB accounts for ~40% of cases and is often the most common manifestation of EP-TB (2-4). The diagnosis of pleural TB is notoriously difficult due to the paucibacillary nature of the disease and the need for invasive sampling techniques, such as image-guided pleural biopsy (5). Nonetheless, pleural fluid remains the most commonly used sample for pleural TB diagnosis.
Established microbiological tests for detection of pleural TB all suffer from suboptimal performance mainly due to the low bacterial counts usually found in pleural fluid. Smear microscopy (SM) and Mycobacterial-Growth-In-Tube liquid culture (MGIT) exhibit sensitivities of ~3% (6,7) and 40–50% (8,9), respectively. The Xpert MTB/RIF ULTRA (Xpert), an automated, rapid, real-time PCR-based assay used as the frontline TB diagnostic test in several high burden countries (and recently replacing the Xpert MTB/RIF assay), performs better than its predecessor but reported sensitivities using pleural fluid remain low (~40–50%) (6,7). Subsequently, host biomarker-based tests are being used to aid in pleural TB diagnosis. Adenosine deaminase (ADA) is often used for pleural TB, with a reported sensitivity of 85% depending on the choice of cut-point (6,10,11). While ADA is inexpensive, its lower specificity (<90%), compared to other tests, can lead to higher false positivity rates. An alternative biomarker is unstimulated interferon-gamma (IFN-γ) which performs well in extrapulmonary tuberculosis (EPTB), including pleural TB diagnosis, with a pooled sensitivity and specificity of ~90% and 97%, respectively (6,11). The IRISA-TB assay is a low-cost, same-day immunoassay developed for measuring IFN-γ in pleural TB which has been recently evaluated in a high burden setting (6).
While performance of these tests has been studied extensively for pleural TB diagnosis, there are limited data on their economic feasibility and cost-effectiveness. Only one study compared the cost of ADA to IFN-γ detection for diagnosis of pleural TB (12).
Given the relatively high EPTB burden in TB endemic countries with limited resources such as South Africa, the difficulty in diagnosing pleural TB and the lack of cost-effectiveness data on available tests in the context of pleural TB, it is important to identify the most economically viable testing strategy for pleural TB diagnosis. In order to address this gap, a cost-consequence analysis was performed to determine the cost-effectiveness of five different testing strategies for pleural TB diagnosis in South Africa. This will be important to inform health policy makers on the most appropriate testing strategy for optimal resource allocation. We present the following article in accordance with the CHEERS reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-106/rc).
Methods
A simple decision tree model (Figure S1) was used to perform a cost-consequence analysis from the South African healthcare provider perspective to evaluate five different single testing strategies for the diagnosis of pleural TB among a hypothetical cohort of 1,000 suspected TB cases presenting at primary care clinics. A hypothetical cohort was used in the analysis as empirically collected and well-characterised clinical datasets was not available. The following tests were evaluated as these are the most commonly used for pleural TB diagnosis in high burden settings: (I) Smear microscopy (SM); (II) Mycobacterial-Growth-In-tube liquid culture (MGIT); (III) Xpert MTB/RIF ULTRA (Xpert); (IV) Adenosine deaminase (ADA); (V) Interferon-gamma Release Immuno-Suspension Assay or IRISA-TB (IRISA-TB). The time horizon was approximately 6 months given that the model only assessed diagnosis and TB treatment initiation based on the available data. Calculations were performed using Microsoft Excel (Microsoft v2102, RRID:SCR_016137) and GraphPad Prism (GraphPad v6.0, RRID:SCR_002798).
Costs
Both diagnostic and treatments costs, specifically within a South African setting, were included in the analysis. Costs were expressed in USD 2019 at an exchange rate of ZAR14.45 to US$1 (https://data.worldbank.org/indicator/PA.NUS.FCRF). Where appropriate, costs were inflated to the year of analysis using the World Bank Consumer Price Index for South Africa (https://data.worldbank.org/indicator/FP.CPI.TOTL?locations=ZA). No discount rate was applied to the model as the timeframe of the model was less than 1 year. Test costs for SM, MGIT, Xpert and ADA were obtained directly from the National Health Laboratory Service (NHLS), the designated reference lab providing services for the South African public healthcare system, and thus represented the actual costs incurred by the National TB program (NTP) (13-15). As IRISA-TB is not yet readily available through the NHLS, the unit test cost was calculated from supplier quotations for equipment and laboratory consumables as well as staff time using an ingredient’s approach (Table S1). Equipment was assumed to have a 10-year life span and were annualized using a 3% discount rate. A 6-month course of anti-TB treatment was estimated from the per patient TB budget as reported in the World Health Organization (WHO) South African tuberculosis finance profile (16).
Outcomes
Model probabilities were calculated based on test sensitivities and specificities reported in the literature (Table 1). The prevalence of pleural TB was based on estimates of extra-pulmonary TB rates among TB suspects (17). These estimates were used to calculate the probabilities of a positive, negative, true positive, false positive, true negative and false negative test (further details in the Appendix 1). The probability of initiating treatment based on the test result was estimated from clinical advice (Table 1). The primary outcome measure was the number of patients correctly diagnosed and initiated on treatment. This represented the most appropriate outcome given the timeframe of the model and that no empirical clinical data (e.g., treatment outcomes) was available.
Table 1
| Estimate | Value | Range | Source |
|---|---|---|---|
| Costs (USD) | |||
| SM baseline test | $1.87 | 0.93–3.74 | National Health Laboratory Service |
| MGIT culture baseline test | $7.35 | 3.67–14.70 | |
| ADA baseline test | $3.16 | 1.58–6.33 | |
| Xpert ULTRA baseline test | $13.28 | 6.64–26.57 | |
| IRISA-TB baseline test | $23.82 | 11.91–47.64 | Antrum Biotech; calculated |
| 6-month course of DOTS-based TB treatment | $777.62 | 388.81–1,555.24 | (16) |
| Cost of those initiated on empirical TB treatment (70% patients complete 6-month course and 30% complete 3-month course) | $660.98 | 330.49–1,321.96 | Calculated |
| Probabilities | |||
| TB prevalence in TB suspects | 0.18 | – | (17) |
| Pleural TB prevalence in TB suspects | 0.0094 | 0.005–0.1 | Assume 13% of TB cases are EPTB (1) and 40% of these are pleural TB (2,3) |
| SM sensitivity | 0.03 | 0.01–0.05 | (6,7) |
| SM specificity | 0.95 | 0.75–0.99 | (6,7) |
| MGIT culture sensitivity | 0.45 | 0.25–0.65 | (7-9) |
| MGIT culture specificity | 0.95 | 0.75–0.99 | (7-9) |
| ADA sensitivity | 0.85 | 0.65–0.99 | (6,10,18) |
| ADA specificity | 0.88 | 0.68–0.99 | (6,10,18) |
| Xpert ULTRA sensitivity | 0.4 | 0.2–0.6 | (6,7) |
| Xpert ULTRA specificity | 0.98 | 0.78–0.99 | (6,7) |
| IRISA-TB sensitivity | 0.9 | 0.7–0.99 | (6,11) |
| IRISA-TB specificity | 0.97 | 0.77–0.99 | (6,11) |
| Initiation of TB treatment if test positive | 1 | – | Assumption |
| Initiation of TB treatment if test negative (empirical treatment rate) | 0.5 | 0.3–0.7 | Assumption and clinical advice |
USD, United States dollars; SM, Smear microscopy; MGIT, Mycobacterial-Growth-In-tube liquid culture; ADA, adenosine deaminase; Xpert ULTRA, Xpert MTB/RIF ULTRA; IRISA-TB, Interferon-gamma Release Immuno-Suspension Assay; TB, tuberculosis; DOTS, directly observed treatment; EPTB, extrapulmonary tuberculosis.
Cost-effectiveness
A simple decision tree model incorporating cost and outcome data was used to determine the cost-effectiveness of each testing strategy. Cost-effectiveness was expressed as the cost per pleural TB case diagnosed and initiated on treatment (per 1,000 TB suspects screened) for each strategy.
Sensitivity analysis
A univariate sensitivity analysis was performed where a single parameter was changed to determine its effect on the cost per pleural TB patient diagnosed and initiated on treatment. Input values for probability estimates were varied based on clinical advice and on estimates from the literature. Cost estimates were either halved or doubled for the low and high input values, respectively.
Assumptions
The following assumptions were made for the analysis: (I) any patient with a positive test was assumed to be immediately initiated on treatment, which is in line with current clinical practice; (II) all patients initiating treatment based on a positive test result completed a full 6-month course of first line anti-TB therapy; (III) 50% of patients with a negative test result were initiated on TB treatment empirically (this percentage was extensively varied in the sensitivity analysis). Furthermore, based on clinical advice, 70% of empirically treated patients completed a full 6-month course of anti-TB treatment whereas 30% will complete a 3-month course (costs were adjusted accordingly); (IV) costs associated with drug resistant TB were not included in the analysis; (V) additional costs, including pleural fluid collection, chest radiography or HIV-associated clinical management, were not included as they were assumed to be equivalent in each of the strategies; (VI) treatment outcomes (cure, died, etc.) were not included in the outcomes due to a lack of empiric clinical data.
Results
Costs, outcomes and cost-effectiveness
The total cost of testing strategies (per 1,000 patients screened) ranged from $354,632 (SM) to $390,363 (ADA). IRISA-TB had the second highest strategy costs ($371,365). Test costs were the highest for IRISA-TB because it was the most expensive test. However, treatment costs made up the majority (>95%) of the total strategy costs. ADA had the highest overall treatment costs ($387,200) as it exhibited the lowest test specificity resulting in more false positives initiating treatment. Conversely, IRISA-TB and Xpert ULTRA has the lowest costs associated with false positives ($23,110 and $15,407, respectively) as these were the most specific tests (Table 2).
Table 2
| Description | Smear Microscopy | MGIT | Xpert ULTRA | ADA† | IRISA-TB |
|---|---|---|---|---|---|
| Costs (per 1,000 patients with suspected TB) | |||||
| A. Total cost of each strategy | $354,632 | $361,869 | $354,307 | $390,363 | $371,365 |
| Diagnostic costs | $1,870 | $7,349 | $13,284 | $3,163 | $23,821 |
| Treatment costs | $352,762 | $354,520 | $341,022 | $387,200 | $347,544 |
| B. Costs associated with unnecessary treatment | $349,543 | $349,543 | $336,255 | $380,550 | $340,684 |
| Costs incurred by false positives initiating treatment | $38,517 | $38,517 | $15,407 | $92,441 | $23,110 |
| Costs incurred by true negatives initiating treatment | $311,026 | $311,026 | $320,848 | $288,108 | $317,574 |
| Outcomes (per 1,000 patients with suspected TB) | |||||
| C. Number of patients correctly diagnosed and initiated on treatment | 0.3 | 4.2 | 3.7 | 8.0 | 8.4 |
| D. Number of missed TB cases | 4.5 | 2.6 | 2.8 | 0.7 | 0.5 |
| E. Number of patients empirically treated‡ | 475.1 | 473.1 | 488.2 | 436.6 | 480.9 |
| F. Number of patients without TB who were unnecessarily treated | 520.1 | 520.1 | 505.2 | 554.8 | 510.2 |
| Cost-effectiveness | |||||
| Cost per pleural TB patient diagnosed and initiated on treatment (A/C) | $1,262,935 | $85,914 | $94,633 | $49,065 | $44,084 |
Costs are expressed in 2019 USD. †, based on the 30 IU/L cut-point used in many TB endemic countries including South Africa; ‡, test negative patients started by clinicians on treatment at a ‘best guess’ based on existing data (thus, this is not necessarily reflective of, or linked to specificity). USD, United States dollars; MGIT, Mycobacterial-Growth-In-tube liquid culture; ADA, adenosine deaminase; Xpert ULTRA, Xpert MTB/RIF ULTRA; IRISA-TB, Interferon-gamma Release Immuno-Suspension Assay; TB, tuberculosis.
In terms of outcomes, the most sensitive tests correctly diagnosed the most patients which were subsequently initiated on TB treatment. As such, both IRISA-TB and ADA diagnosed a similar number of patients per 1,000 TB suspects screened (8.4 and 8.0 patients, respectively). Similarly, IRISA-TB and ADA also missed the fewest TB cases (0.5 and 0.7 patients, respectively). SM, MGIT and Xpert ULTRA had the lowest sensitivities (<50%), diagnosed the fewest patients and missed the most cases (Table 2).
The most cost-effective strategy was IRISA-TB at $44,084 per pleural TB case diagnosed and initiated on treatment. This was ~$5,000 less than ADA, the second most cost-effective strategy ($49,065). MGIT and Xpert ULTRA, primarily due to their poor sensitivity, were less cost-effective than any of the biomarker-based assays ($85,914 and $94,633 respectively). SM was the least cost effective of all strategies by far ($1,262,935) and was thus excluded from the sensitivity analyses.
The cost savings of using IRISA-TB, over ADA, to diagnose and initiate treatment in a single pleural TB patient (savings of $4,981) was extrapolated to the total case burden of pleural TB in South Africa. A total of 208,032 notified TB cases were reported in South Africa in 2020 (WHO). Using pleural TB prevalence values reported in Table 1 (which equates to 9,153 pleural TB cases per year), it is estimated that implementing an IRISA-TB testing strategy could save the South African NTP up to ~$45 million per year.
Sensitivity analysis
A univariate sensitivity analysis performed for each strategy indicated that pleural TB prevalence, empirical treatment rate and TB treatment costs were the most influential parameters on cost-effectiveness (Figure 1). Both MGIT and Xpert ULTRA cost-effectiveness were also influenced by changes in their respective test sensitivities (Figure 1A,1B). Tests costs had the least impact on overall cost-effectiveness.
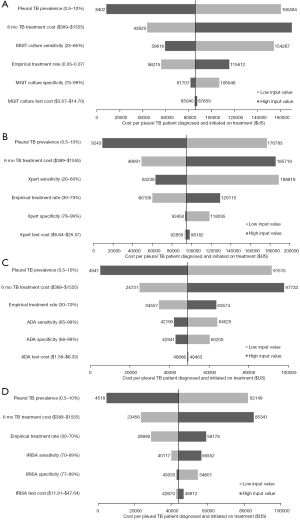
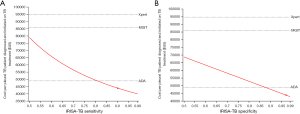
In order to make our finding more generalizable, the cost per pleural TB patient diagnosed and initiated on treatment of each strategy was plotted against a wide range of pleural TB prevalence (0.2–20%) and empirical TB treatment rate (0–80%) estimates as these parameters tend to vary considerably across different settings and in different populations. In both cases, IRISA-TB remained the most cost-effective strategy, followed by ADA, up to a pleural TB prevalence of 20% (Figure 2A) and empirical treatment rate up to 80% (Figure 2B) that was assessed in the model. The effect of varying IRISA-TB sensitivity and specificity on cost-effectiveness was also determined [given published data on IRISA-TB test performance is limited to 2 studies (6,11)]. IRISA-TB became less cost-effective than ADA if IRISA-TB sensitivity drops below 81% (Figure 3A). Similarly, IRISA-TB becomes less cost-effective than ADA if IRISA-TB specificity falls below 88% (Figure 3B). IRISA-TB remained more cost effective than both MGIT and Xpert ULTRA for the entire range of values assessed in the sensitivity analysis (Figure 3).

Discussion
We evaluated the cost-effectiveness of five different testing strategies for the diagnosis of pleural TB. The key findings of the study were that: (I) IRISA-TB was the most cost-effective strategy per pleural TB case diagnosed and initiated on treatment, and was associated with a ~US$ 45 million in annual cost savings if IRISA-TB was implemented into the South African national TB programme, (II) IRISA-TB was most sensitive to changes in pleural TB prevalence, treatment costs and empiric treatment rates, and (III) the unit cost of each individual assay made only a minor contribution to the cost-effectiveness of the specified test strategy.
Biomarker-based tests (ADA and IRISA-TB) remained substantially more cost-effective than microbiological tests like MGIT (bacterial culture) and Xpert (DNA-based detection) for the diagnosis of pleural TB. The main drivers of the higher cost per case diagnosed and initiated on treatment for these microbiological tests were (I) fewer patients were diagnosed and initiated on treatment due to their lower test sensitivity; (II) a higher proportion of individuals underwent unnecessary TB treatment either because they were initiated on empirical TB treatment (true negatives initiating treatment) or there were more false positives initiating TB treatment (Table 2). Thus, tests with a low sensitivity were naturally associated with high empiric treatment rates. There are two key reasons underpinning this phenomenon. Firstly, pleural TB is a paucibacillary disease with a very low bacterial load that is below the threshold detection limit of Xpert Ultra and smear microscopy. Indeed, smear microscopy using pleural fluid has a sensitivity of <5% in pleural TB (7,19). Performance data of Xpert Ultra on pleural fluid (when using pleural biopsy as a reference standard) was limited to 2 studies from TB endemic settings with a sensitivity of ~40% to 50% (6,7). Pooled sensitivities of Xpert MTB/RIF (the majority of data on test performance using pleural fluid is available for this version of the assay) in a recent meta-analysis were similarly low (20). Culture has a sensitivity of ~45% when using pleural biopsy as a reference standard (7,8). Secondly, pleural TB is an immunologically mediated disease (like TB meningitis), where a hypersensitivity reaction to very a low burden of antigen results in the accumulation of fluid between the serosal membranes encapsulating the lung, heart and abdominal organs (e.g., parietal and visceral pleura in the case of pleural TB), and in the subarachnoid space (between the pia and arachnoid mater membranes) in the case of TB meningitis. A recent review summarises the concept of the immunopathological components of TB (e.g., TB meningitis and TB serositis) where immunodiagnostic biomarkers fared much better than microbiological tests given the very low TB antigen burden in the relevant compartment (21).
IRISA-TB was found to be more cost-effective than ADA despite both tests having high sensitivity. Compared to ADA, several studies have shown that unstimulated IFN-γ-based assays (including IRISA-TB) has a higher specificity and negative predictive value (6,11,18,22). Studies in the same setting showed that ADA suffered from a sub-optimal specificity at a cut point of 30 IU/litre. In these settings, ADA specificity ranged from ~75% to 90% (6,10,18,23,24). This leads to false positives (in those without TB) and unnecessary initiation of treatment, which contributed to IRISA-TB being more cost effective compared to ADA. Only one economic evaluation study compared the detection on unstimulated IFN-γ with ADA (12). While this study found ADA to be more economical for the detection of pleural TB, it was severely limited by restricting itself to only the cost of the test and did not include any outcome measures or incorporation of test performance (as was undertaken in our study). Furthermore, it used the existing pricing of kits available in a research setting and not an assay commercially optimised and validated for the detection of pleural TB in a clinical setting (lower cost allowing for single use in one patient thus minimising wastage). Thus, while the unit cost of IRISA-TB is higher than that of ADA (as far as the test itself is concerned), the treatment costs were lower with IRISA-TB mainly due to fewer false positive patients undergoing unnecessary treatment. Furthermore, the ADA performance in various studies is dependent on the cut point used. Our estimates involve the use of a cut point of 30 IU/litre which is used in the South African setting (6). However, higher cutpoints, i.e., 40 IU/litre are used in other settings, which increases specificity but reduces sensitivity (23). However, at these higher cut points, IRISA-TB remains more cost-effective than ADA based on the modelling that we have conducted.
There are several factors that impacted the cost-effectiveness of each diagnostic strategy. Firstly, for example, the overall cost-effectiveness estimates of each strategy decreased when pleural TB prevalence increased and empiric treatment rates decreased. However, our sensitivity analysis showed that with differing TB prevalence, the cost rankings remained unchanged, i.e., IRISA-TB was the most cost-effective strategy compared to ADA, MGIT and Xpert. Second, although pleural TB prevalence was based on national estimates [using WHO Global TB Report and other South African studies (1-3)] it is well documented that rates of pleural and extrapulmonary TB are higher in certain sub-populations such as HIV-infected persons (1,25). It is therefore expected that tests like IRISA-TB [which have been shown to be more sensitive in HIV-infected persons (6,11)] would likely be more cost-effective in this sub-group. Third, as previously mentioned, due to the low sensitivity of smear microscopy, MGIT and Xpert, these tests are especially sensitive to increasing rates of empiric treatment. Fourth, one has to also consider implementation costs. In this respect a simple infrastructure needed to perform the ADA (a calorimetric test) already exists in most laboratories. However, IRISA-TB is also a low-cost assay requiring an ELISA plate reader which is already present in most basic laboratories in TB endemic settings (with the exception of microscopy centres).
There are several limitations to our findings; (I) we did not factor in the impact of the diagnostic test in reducing TB transmission (and hence secondary cases that may have been spawned due to onward transmission of disease). Although pleural TB is thought to be less transmissible than the pulmonary form of disease, detailed imaging using computerized tomography (CT) scanning has shown that pulmonary infiltrates are present in over 40% of cases (26-28), and sputum induction studies have shown culturable bacilli from the respiratory tract in at least 50% of cases (29,30). Thus, there is preliminary evidence that onward transmission can occur and is likely in patients with pleural TB. While transmission was not factored into this model (given the uncertainties around this estimate) its inclusion would favour tests with a higher sensitivity (as more TB cases would be prevented). As such, IRISA-TB would become even more cost-effective; (II) we did not use disability-adjusted life-years (DALYs) or quality-adjusted life-years (QALYs) as measures of outcome. While these metrics provide some level of standardization across health economic analyses of different disease interventions, they are crude and inherently biased measure of health (31,32). As such, we chose a cost-consequence model and used a more pragmatic cost-effectiveness outcome measure (cost per case diagnosed and initiated on treatment) which is more appropriate for the testing strategies being assessed based on availability of data and is easier to interpret by clinicians and health system managers; (III) the pleural TB test performance estimates are based, in some cases, on published studies rather than on empirical clinical or programmatic data. Further cost effectiveness analyses are warranted once more clinical data become available and the impact of these tests on clinical outcomes can be better assessed; (IV) we did not factor HIV and TB co-infection into the analysis. However, IRISA-TB has been shown to be more sensitive in HIV-infected patients (6,11) and thus would likely be more cost-effective in this group. Indeed, almost a third of patients in HIV endemic settings (like in Africa) have EPTB; (V) we only examined the cost effectiveness of single test strategies. There are no published data on the sensitivity and specificity of combined testing strategies, particularly for newer tests such as Xpert ULTRA and IRISA-TB. Furthermore, given the superior sensitivity (and similar specificity) of host biomarker tests (ADA, IRISA-TB) compared to microbiological tests (Xpert ULTRA, MGIT), it is highly likely that any cases detected by a combined test strategy would also be detected if using ADA or IRISA-TB alone. Subsequently, combination test strategies are likely to be less cost-effective (due to higher test costs but similar outcomes) than a single test strategy (IRISA or ADA alone); (VI) we did, not in our analysis, consider medical procedures such as thoracoscopy (which is more widely used in some settings). However, while such operative procedures can have a high diagnostic yield and may also aid in improving treatment outcomes (30), its implementation as a frontline diagnostic tool for pleural TB in many endemic settings is limited by a lack of relevant expertise, high costs and limited resources (particularly in Africa). Future setting-specific cost-effectiveness analyses should also include medical procedures such as thoracoscopy and their impact on diagnosis and management; (VII) IRISA-TB assay is not yet commercially available due to COVID-associated manufacturing delays (though it is planned to be available before the end of 2022) and thus the cost of the assay could only be estimated based on a range of values provided by Antrum Biotech Ltd., the developers of IRISA-TB. In order not to underestimate the cost of IRISA-TB, we chose to use the higher value of this range as the base case cost estimate in our analysis. IRISA-TB still remained the most cost-effective option, even when the test was further increased to $50 (Figure S2).
Conclusions
Amongst 5 diagnostic tests for pleural TB, IRISA-TB was found to be the most cost-effective strategy but was sensitive to changes in pleural TB prevalence, treatment costs, and empirical treatment rates, and could result in a cost saving of up to ~US$45 million per annum if IRISA-TB was implemented into the South African National TB Programme.
Acknowledgments
Funding: Antrum Biotech Ltd provided financial support for the research presented in this manuscript. However, the funder had no role in the identification, design, conduct or reporting of the analysis.
Footnote
Reporting Checklist: The authors have completed the CHEERS reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-106/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-106/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-106/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-106/coif). Philippa Randall and Rebeng Maine are employees of Antrum Biotech Ltd (developers of the IRISA-TB assay). Anil Pooran received financial support from Antrum Biotech to perform this study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. WHO global tuberculosis report 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf. Accessed February 15 2021.
- Gounden S, Perumal R, Magula NP. Extrapulmonary tuberculosis in the setting of HIV hyperendemicity at a tertiary hospital in Durban, South Africa. Southern African Journal of Infectious Diseases 2018;33:57-64. [Crossref]
- Karstaedt AS. Extrapulmonary tuberculosis among adults: experience at Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa. S Afr Med J 2013;104:22-4. [Crossref] [PubMed]
- Zhai K, Lu Y, Shi HZ. Tuberculous pleural effusion. J Thorac Dis 2016;8:E486-94. [Crossref] [PubMed]
- Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology 2019;24:962-71. [Crossref] [PubMed]
- Meldau R, Randall P, Pooran A, et al. Same-Day Tools, Including Xpert Ultra and IRISA-TB, for Rapid Diagnosis of Pleural Tuberculosis: a Prospective Observational Study. J Clin Microbiol 2019;57:e00614-19. [Crossref] [PubMed]
- Wang G, Wang S, Yang X, et al. Accuracy of Xpert MTB/RIF Ultra for the Diagnosis of Pleural TB in a Multicenter Cohort Study. Chest 2020;157:268-75. [Crossref] [PubMed]
- Friedrich SO, von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol 2011;49:4341-2. [Crossref] [PubMed]
- Maurya AK, Kant S, Kushwaha RA, et al. The advantage of using IS6110-PCR vs. BACTEC culture for rapid detection of Mycobacterium tuberculosis from pleural fluid in northern India. Biosci Trends 2011;5:159-64. [Crossref] [PubMed]
- Gui X, Xiao H. Diagnosis of tuberculosis pleurisy with adenosine deaminase (ADA): a systematic review and meta-analysis. Int J Clin Exp Med 2014;7:3126-35. [PubMed]
- Meldau R, Peter J, Theron G, et al. Comparison of same day diagnostic tools including Gene Xpert and unstimulated IFN-γ for the evaluation of pleural tuberculosis: a prospective cohort study. BMC Pulm Med 2014;14:58. [Crossref] [PubMed]
- Sharma SK, Banga A. Pleural fluid interferon-gamma and adenosine deaminase levels in tuberculosis pleural effusion: a cost-effectiveness analysis. J Clin Lab Anal 2005;19:40-6. [Crossref] [PubMed]
- Pooran A, Pieterson E, Davids M, et al. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One 2013;8:e54587. [Crossref] [PubMed]
- Meyer-Rath G, Schnippel K, Long L, et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS One 2012;7:e36966. [Crossref] [PubMed]
- Knight LK, Lehloenya RJ, Sinanovic E, et al. Cost of managing severe cutaneous adverse drug reactions to first-line tuberculosis therapy in South Africa. Trop Med Int Health 2019;24:994-1002. [Crossref] [PubMed]
- WHO. South Africa: Tuberculosis finance profile World Health Organization, Geneva, Switzerland 2015.
- Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014;383:424-35. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Dhooria S, et al. Comparative accuracy of pleural fluid unstimulated interferon-gamma and adenosine deaminase for diagnosing pleural tuberculosis: A systematic review and meta-analysis. PLoS One 2021;16:e0253525. [Crossref] [PubMed]
- Kumar P, Sen MK, Chauhan DS, et al. Assessment of the N-PCR assay in diagnosis of pleural tuberculosis: detection of M. tuberculosis in pleural fluid and sputum collected in tandem. PLoS One 2010;5:e10220. [Crossref] [PubMed]
- Kohli M, Schiller I, Dendukuri N, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2021;1:CD012768. [PubMed]
- Bahr NC, Meintjes G, Boulware DR. Inadequate diagnostics: the case to move beyond the bacilli for detection of meningitis due to Mycobacterium tuberculosis. J Med Microbiol 2019;68:755-60. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Dhooria S, et al. Unstimulated Pleural Fluid Interferon Gamma for Diagnosis of Tuberculous Pleural Effusion: a Systematic Review and Meta-analysis. J Clin Microbiol 2021;59:e02112-20. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Sehgal IS, et al. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PLoS One 2019;14:e0213728. [Crossref] [PubMed]
- Maartens G, Bateman ED. Tuberculous pleural effusions: increased culture yield with bedside inoculation of pleural fluid and poor diagnostic value of adenosine deaminase. Thorax 1991;46:96-9. [Crossref] [PubMed]
- Mohammed H, Assefa N, Mengistie B. Prevalence of extrapulmonary tuberculosis among people living with HIV/AIDS in sub-Saharan Africa: a systemic review and meta-analysis. HIV AIDS (Auckl) 2018;10:225-37. [Crossref] [PubMed]
- Ko JM, Park HJ, Kim CH. Pulmonary changes of pleural TB: up-to-date CT imaging. Chest 2014;146:1604-11. [Crossref] [PubMed]
- Kim HJ, Lee HJ, Kwon SY, et al. The prevalence of pulmonary parenchymal tuberculosis in patients with tuberculous pleuritis. Chest 2006;129:1253-8. [Crossref] [PubMed]
- Seiscento M, Vargas FS, Bombarda S, et al. Pulmonary involvement in pleural tuberculosis: how often does it mean disease activity? Respir Med 2011;105:1079-83. [Crossref] [PubMed]
- Conde MB, Loivos AC, Rezende VM, et al. Yield of sputum induction in the diagnosis of pleural tuberculosis. Am J Respir Crit Care Med 2003;167:723-5. [Crossref] [PubMed]
- Vorster MJ, Allwood BW, Diacon AH, et al. Tuberculous pleural effusions: advances and controversies. J Thorac Dis 2015;7:981-91. [PubMed]
- Nord E, Daniels N, Kamlet M. QALYs: some challenges. Value Health 2009;12:S10-5. [Crossref] [PubMed]
- Dolan P, Kahneman D. Interpretations of Utility And Their Implications For The Valuation Of Health. The Economic Journal 2008;118:215-34. [Crossref]


