
Management of perioperative bleeding risk in patients on antithrombotic medications undergoing cardiac surgery—a systematic review
Introduction
Antithrombotic medications are cornerstone therapies for patients with cardiovascular disease. In this review, we summarize the impact of active dual antiplatelet therapy (DAPT) or direct-acting oral anticoagulants (DOAC) on the perioperative bleeding risk of patients requiring non deferrable cardiac surgery and discuss potential solutions to this complex and fairly common scenario.
Antiplatelets
Aspirin (ASA) together with one of the three oral P2Y12 receptor inhibitors, ticagrelor, prasugrel, and clopidogrel, is commonly referred to as DAPT and represents a bedrock of treatment in patients with an acute coronary syndrome (ACS) and those undergoing percutaneous coronary intervention (PCI). Frequently, DAPT is administered very early as loading doses in ACS patients in anticipation of emergent or urgent PCI (1). A rapid growth in the number of patients on these medications worldwide is expected (2).
The new generation of P2Y12 inhibitors, ticagrelor and prasugrel, are more potent and predictable and as such are often recommended over clopidogrel in ACS patients (2). The only one of these three P2Y12 receptor antagonists that reversibly binds to platelets is ticagrelor (3). This fact makes it a very interesting target for drug reversal or drug removal strategies to address the serious problem of highly elevated risk of peri-operative bleeding complications. In contrast, due to the irreversible binding of the active metabolites of clopidogrel and prasugrel, there are no similar solutions on the horizon that might quickly re-establish hemostasis necessary during and immediately after major surgery. Platelet function of these patients is irreversibly impaired, while those on ticagrelor may benefit from the restoration of their platelet aggregation potential, thereby mitigating the risk of perioperative bleeding complications. Accordingly, our review, which also discusses potential solutions in this clinical setting, will only focus on antiplatelet therapy with ticagrelor.
DOACs
DOACs, otherwise known as non-vitamin-K antagonist oral anticoagulants (NOACs), are today represented by four drugs: the direct activated factor II (thrombin) inhibitor dabigatran and three activated factor X (FXa) inhibitors—apixaban, rivaroxaban, and edoxaban. Apixaban and rivaroxaban are by far the most frequently prescribed DOACs worldwide, mostly for the prevention of stroke in patients with non-valvular atrial fibrillation (AF) and have emerged as the preferred choice, particularly for those newly started on oral anticoagulant therapy (4).
Antithrombotics and bleeding complications
Numerous studies have shown the increased risk of bleeding complications both during and after various surgical procedures on patients under antithrombotic therapy. Major bleeding complications are associated with increased morbidity, mortality, and healthcare costs (5,6). The risk of severe bleeding escalates in major surgery such as open cardio-thoracic procedures, so specific guidelines have been developed and endorsed by medical societies in the cardiovascular field. These guidelines advise on drug discontinuation strategies for elective procedures, but the challenge of managing emergency or urgent patients on antithrombotics undergoing major surgery remains.
This review aims to highlight the problem of perioperative bleeding complications in patients under DAPT or DOAC therapy undergoing major surgery in the cardiovascular milieu and to present currently available potential solutions that target ticagrelor or any of the four DOACs—apixaban, rivaroxaban, edoxaban, and dabigatran. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-428/rc).
Methods
The scope of this literature search was to identify all relevant information and summarize the current knowledge in this evolving topic. The search terms were chosen by applying neutral search keywords in an attempt to perform a non-biased systematic search and retrieve both favorable as well as unfavorable data. A single literature source was chosen—the online database of the United States National Library of Medicine (PubMed) (available at http://www.ncbi.nlm.nih.gov/pubmed), due to the fact that it is a well-recognized meta-database for retrieval of high-quality scientific publications yielding a comprehensive view of any given topic. In the literature screening and selection process, we followed the principles derived from the PRISMA statement (7) with an intention of preserving an objective approach.
A search of PubMed was made on January 26th, 2022 using the following key search words: “bleeding complications” AND “cardiac surgery” AND “intraoperative” OR “post operative” AND “ticagrelor” OR “rivaroxaban” OR “apixaban” OR “edoxaban” OR “dabigatran”. Abstracts were considered for inclusion if they were in the English language and published in the last 10 years.
The search resulted with 147 hits in total. After duplicates were removed (n=52), the remaining abstracts were screened and additional 4 were excluded (details are given within the flowchart, Figure 1A). Further, 91 reports were assessed out of which 72 were deemed ineligible—45 as in the ‘low bleeding risk’ field of investigation, 22 deemed as not relevant for the objectives of this review, and 5 case reports. Of note, there were a few published trial designs. For one study that was evaluated as relevant, published results of the trial in question were also searched for and included instead.
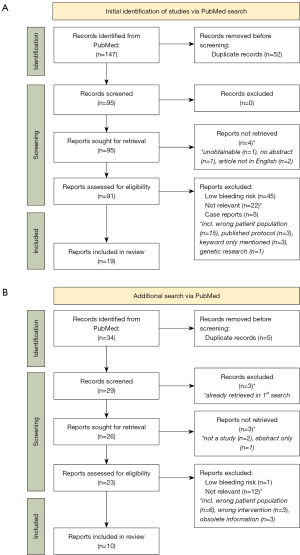
After full-text evaluation of the obtained 19 publications, we realized that the area of interest was poorly covered, so we performed an additional PubMed search on February 23rd, 2022, using the names of pharmacological or technological solutions known to the authors as keywords with the intention of retrieving reliable and scientific information that would allow a fair comparison between them.
This additional PubMed search with the similar criteria as the first one was conducted with the following keywords: “andexanet alfa” OR “idarucizumab” OR “bentracimab” OR “cytosorb” AND “bleeding” AND “cardiac surgery”. The search resulted in 34 hits, out of which 5 were excluded for being duplicates, and 3 as duplicates to publications already obtained in the first search. Additional 3 reports remained unretrieved and further 13 publications were excluded—1 for the low bleeding risk field of investigation, and 12 were deemed not relevant (reasons listed in the flowchart, Figure 1B). Overall 10 additional papers were retrieved. We included case reports in this additional search due to the lack of available clinical data.
Analysis of literature search results
The current PubMed search came with very few studies from the cardiac surgery setting assessing antithrombotic therapy-induced perioperative bleeding complications and potential solutions for this. Consequently, a second search was performed with keywords and criteria described in the methods section.
Antithrombotic therapy-related bleeding complications in cardiac surgery
Several studies included in our search reported on the correlation between bleeding complications and major procedures in patients under antithrombotic therapy. In a large multicenter prospective 3-cohort PAUSE trial, that investigated a simple standardized perioperative DOAC therapy interruption and resumption strategy, out of 3,007 patients with AF receiving either apixaban, rivaroxaban, or dabigatran, 1,007 had a high bleeding risk procedure (major cardiac surgery procedures in larger part). While this study validated the recommended 2 or more days DOAC discontinuation strategy for major elective surgery, it did not, however, help to answer what is best to do in emergency cases (8). Results from the randomized controlled RE-LY trial, that focused on comparison of the periprocedural use of dabigatran and warfarin, reported that dabigatran-associated major bleeding occurred in 18% of patients having urgent surgery, while this happened in only 3–4% of elective patients (9). A prospective Dresden NOAC registry found that major surgery was an independent risk factor for severe bleeding complications in patients receiving DOACs before a therapy interruption strategy was widely accepted (10). Moreover, Hassan et al. (11) reported on the consequences of a prolonged DOAC effect in open-heart surgery patients with renal insufficiency, therefore advising whenever possible to wait even longer than recommended by guidelines before performing surgery. This problem had already been addressed in the updated European Heart Rhythm Association (EHRA) practical guide on the use of NOACs from 2017. Specific recommendations of longer periods of drug wash-out before high-bleeding-risk elective surgery are given depending on a patient’s creatinine clearence (12).
Active therapy with ticagrelor during cardiopulmonary bypass graft (CABG) surgery leads to high blood loss, excessive red blood cell (RBC) transfusions, and a higher rate of re-thoracotomies (6,13,14). A more recent analysis of the SWEDEHEART registry from Hansson et al. (15) reported that the prevalence of major bleeding complications is significantly higher when ticagrelor is discontinued less than 2 days before CABG surgery—up to 41%. In a subsequent study the authors demonstrated that platelet function tests predict severe bleeding in cardiac surgery patients treated with ticagrelor less than 5 days before surgery. However, since the prognostic value of platelet function testing in this setting is yet unproven, the impact on clinical outcomes remains unclear (16). A review from the same group of authors emphasized the strong associations of bleeding complications or volume of transfused blood products with poor outcomes in cardiac surgery. They also pointed out the potential increased risk of thrombosis after discontinuation of antiplatelet therapy for several days before elective surgery (17). Furthermore, it has been shown that premature discontinuation of antiplatelet therapy is associated with an increase in ischemic complications and a reported mortality rate of up to 25–40% (18). Notwithstanding, because of poor patients’ condition or during an ongoing myocardial infarction (MI), waiting for the P2Y12 antagonist effect to cease is often not an acceptable option (6).
Conversely, one recent study by Diab et al. (18) supports continuation of DAPT with ticagrelor until 1 day before CABG, as the investigators did not find an increased incidence of major bleeding complications in the DAPT group compared to controls that received ASA only.
Patients suffering from acute type-A aortic dissection often present with similar symptoms to ACS, so a substantial proportion of them may be preloaded with ticagrelor prior to the diagnosis being confirmed. Chemtob et al. (19) from Denmark found that such patients had increased intraoperative bleeding rates by 45%, and that inadvertent preloading with the P2Y12 inhibitor happened in as many as 43% of patients. Correspondingly, transfusion requirements of RBC, fresh frozen plasma (FFP), and platelets were significantly increased, together with the need for reoperation due to hemorrhage.
Currently available potential solutions
There is high heterogeneity in the management of bleeding complications in patients treated with DOACs (20). The EHRA 2017 practical guide mentions reversal agents for dabigatran and FXa-inhibitors, at that time still under investigation, and administration of prothrombin complex concentrate (PCC) or activated PCC acknowledging, though, that efficacy and safety of this treatment for active bleeding has not been firmly established (12). A couple of case series (21,22) and a case report (23) together have described the use of the dabigatran antidote idarucizumab in the cardiac surgery setting, reporting optimal perioperative hemostasis being achieved in up to 93% of treated patients. The authors outlined these results as encouraging but highlighted the need for more studies to corroborate these observations, especially regarding cost and clinical effectiveness, and mortality benefit. In an expert review by Sodha and Sellke from 2016, idarucizumab was evaluated as an efficacious reversal agent for patients with significant bleeding while receiving dabigatran, or those needing urgent invasive procedures (24). Akhrass et al. (25) from Cleveland Clinic group presented very recently their assessment of the challenge concerning the observed increased use of DOACs and antiplatelet medications and bleeding management in emergency cardiac surgery. Among several techniques used to achieve resolution of the anticoagulant effect of antithrombotics, they specifically evaluated hemoadsorption treatment as promising for patients on antiplatelet medications. The authors further stated that specific reversal agent such as idarucizumab and andexanet alfa could be considered during operations with extensive tissue damage involvement, but also warn that this may lead to heparin resistance and anticoagulant rebound.
In 2019, a French working group on perioperative hemostasis published a proposal on the management of antiplatelet therapy for non-elective invasive procedures focusing on platelet transfusion. They recommended neutralization of the antiplatelet agent with platelet transfusion at an adequate dose. Regarding ticagrelor specifically, having in mind its pharmacodynamic properties, they concluded that platelet transfusion would have been ineffective, particularly if less than 24 hours have passed since the last intake of the drug (26).
Due to its reversable nature of binding to platelets, a specific reversal agent for ticagrelor named bentracimab has been developed and is currently being formally evaluated in a phase 3 clinical trial. A recent survey among Canadian cardiac surgeons identified ticagrelor-related bleeding as a major concern and the significant unmet need that a ticagrelor reversal agent could address was recognized (27).
A potential solution for the problem described in this review with the highest number of publications retrieved in our literature search is the CytoSorb® or DrugSorb-ATRTM device. CytoSorb has already been used in Europe as a blood purification treatment based on the process of hemoadsorption to remove ticagrelor and/or rivaroxaban from a patient’s blood during cardiopulmonary bypass (CPB) (28-31). A prospective, multi-center, double-blind, randomized controlled trial (RCT) has been designed to evaluate its safety and effectiveness in the reduction of postoperative bleeding by removal of ticagrelor in patients undergoing cardio-thoracic surgery within the first two days of ticagrelor discontinuation under the name DrugSorb-ATR (32). Previously, two studies by Hassan et al. (28,29) from Hamburg, Germany reported significant improvements in several clinical outcomes with hemoadsorption on CPB in patients under either ticagrelor or rivaroxaban therapy requiring emergency cardiac surgery, including acute type-A aortic dissection repairs. The first study evaluated the results of intraoperative hemoadsorption in emergency predominantly isolated CABG patients treated with ticagrelor or rivaroxaban, comparing them to a non-randomized control group. Authors could show significantly shorter overall operation time in the hemoadsorption group (even though CPB duration was similar in both groups), lower drainage volumes, and less RBC and platelet transfusions. A significantly higher re-thoracotomy rate and significantly longer stays on the ICU and in the hospital were seen in the control group. Moreover, the authors observed better intraoperative control of the bleeding when the adsorber was added into the CPB circuit (28). The authors concluded that intraoperative hemoadsorption can significantly reduce bleeding complications and recommended routine use of this technique in emergent patients undergoing cardiac surgery pretreated with ticagrelor or rivaroxaban. In their recent published second analysis, this group evaluated the outcomes of patients presenting with acute aortic dissection on antithrombotic therapy. Even in this high-risk group of patients, the authors could validate their previous results. The operation time was again significantly shorter in the adsorber group and once again no re-thoracotomies had to be performed [compared to two re-thoracotomies (18.9%) among patients without the adsorber use]. Moreover, patients without intraoperative hemoadsorption required significantly more platelet transfusions. Therefore, the authors concluded that the intraoperative use of hemoadsorption in patients on antithrombotics presenting with acute aortic dissection is beneficial and could improve outcomes (29).
In addition, two case reports described CytoSorb hemoadsorption use in patients under triple therapy (DAPT + DOAC). The more recent case reported on a patient with an acute type-A aortic dissection who was on rivaroxaban and DAPT with clopidogrel and ASA (30). The older case report described a patient under rivaroxaban and DAPT with ticagrelor undergoing off-pump coronary artery bypass (OPCAB) (31). Both patients were treated with hemoadsorption utilizing a hemodialysis platform. Rivaroxaban removal was demonstrated in the first case by a rapid decrease in anti-FXa activity. Despite an extremely high risk of severe bleeding complications, both patients survived without any consequences. A very recent review by Jackson et al. (33) from Canada specifically evaluated hemoadsorption for removal of ticagrelor and DOACs in cardiac surgery. Authors comprehensively assessed all aspects of CytoSorb therapy, including its demonstrated ability to also remove excessive levels of cytokines and other inflammatory mediators or metabolites. Moreover, they argued that despite advances in the development of reversal agents for ticagrelor and DOACs, great interest remains in more economic means of mitigating bleeding risk, especially since in vitro investigations and some case reports imply that CytoSorb may offer the same solution for all DOACs and not only rivaroxaban. In conclusion, the authors state that hemoadsorption of ticagrelor and DOACs with CytoSorb is a feasible, safe, and effective solution for reducing bleeding complications in cardiac surgery, and that it has the potential to change clinical practice guidelines and enhance high-standard patient care.
Only one publication retrieved in our search reported on cost-effectiveness. A United Kingdom (UK)-based cost-utility analysis by Javanbakht et al. (34) evaluated CytoSorb for the intraoperative removal of ticagrelor versus usual care among patients requiring emergent or urgent cardiac surgery in the UK. Their analysis revealed that the treatment with CytoSorb could save almost £4,000 over a 30-day time horizon due to consumption of less blood products, fewer re-thoracotomies, and shorter length of stay in the emergency surgical setting, with the price of the device taken into account. In addition, the authors determined that this treatment may be a cost-saving solution even in urgent cardiac surgery. No publication was identified in the literature search with a budget-impact comparison between the solutions reviewed in our paper, but some prices are reported in the above expert review from Canada (33). The authors declared that the CytoSorb column costs approximately US$1,500, the DOAC reversal agents idarucizumab and andexanet alfa cost around US$3,482 and US$24,750 per treatment, respectively, while the cost of a single PCC dose is about US$5,670.
Discussion
Perioperative bleeding complications related to antithrombotic medications are a serious and growing problem based on the increasing popularity of these agents. A real unmet need remains for safe and effective solutions.
The most extensively investigated method for mitigating the risk of major bleeding and bleeding complications during and after cardiac surgery was allowing enough time before the procedure for the physiological wash-out of the drug. The importance of the medication-tailored time delay after antiplatelet discontinuation and perioperative clinical outcomes was first reported in the post-hoc analysis of the PLATO trial that evaluated efficacy and safety of ticagrelor and clopidogrel in patients with ACS undergoing CABG and have remained consistent in subsequent literature (35-37).
The European Association for Cardio-Thoracic Surgery (EACTS), in collaboration with the European Association of Cardiothoracic Anaesthesiology (EACTA) issued the 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery (38), which recommended postponement of surgery for at least 3 days after discontinuation of ticagrelor and at least 2 days after DOAC cessation in patients undergoing non-emergent cardiac surgery. For DOACs especially, it is stated that an even longer interval may be necessary for patients with impaired renal function. The same recommendations were given by European Society of Cardiology (ESC) (2) and the EHRA (4). Similarly, a 3- or 2-day interruption strategy for DAPT or DOAC therapy, respectively, has been endorsed by the United States of America (USA)-based respective medical societies (39-41).
However, patients that require urgent, non-deferrable surgery are not in a position to wait for antithrombotics to be naturally metabolized or washed-out. Moreover, in a substantial group of urgent ACS patients there is the risk for thromboembolic events (stent thrombosis and MI) during therapy discontinuation that is not negligible. In such cases, bridging therapies with cangrelor or glycoprotein IIb/IIIa inhibitors are often used, adding complexity and significant costs in the management of these patients (42). Alternatively, utilization of reversal agents for some of the DOACs can be considered (4).
Preloading with DAPT in ACS remains a common strategy and frequently this regiment is initiated while patients are being transported or prepared for a primary catheterization even before the underlying diagnosis is confirmed (6). Since approximately 10% of all patients presenting with ACS ultimately need urgent CABG surgery, the early administration of DAPT results in excess perioperative bleeding risk. The population at risk is even greater when one also considers the number of patients with a failed PCI or those requiring urgent/emergent cardiothoracic procedure other than CABG (e.g., proximal aortic dissections, thoracic aortic aneurysms, open valve repair/replacement procedures, combined operations, etc.). Adding the above categories, it is clear that there is a substantial number of such patients encountered in everyday practice.
Results from our literature search largely support the concern about the impact of impaired hemostasis caused by DAPT or DOAC therapy in patients undergoing non-elective major cardiac surgery. Even with the established drug discontinuation protocol for elective surgery with several days of delay for its physiological elimination from the body, a realistic threat of thrombotic adverse events exists for the patient. Two specific patient populations that may be the most vulnerable in this setting are those who present with acute type-A aortic dissection and urgent cardiac surgery patients with renal insufficiency under DOAC therapy. Symptoms of the former are not infrequently mistaken for ACS, when they are preloaded with ticagrelor or other P2Y12 inhibitor, which dramatically increases their already high mortality risk, while the latter require longer wash-out periods of the respective DOAC medication, which potentially puts them at higher thromboembolic risk.
Since traditional treatment options, such as transfusions of blood products, administration of PCCs or antifibrinolytic agents have shown to have limited influence or even lack of effect on bleeding during and after major surgery on patients under ticagrelor or DOAC therapy, potentially increasing the risk of thrombosis in turn, an important unmet need has been recognized and several “remedies” offered to the interested medical community (25).
Reversal agents for DOACs
There are currently two reversal agents available for DOACs—idarucizumab, a monoclonal antibody fragment developed to reverse the anticoagulant effect of dabigatran (43), and andexanet alfa, a modified recombinant inactive form of human factor Xa developed for reversal of factor Xa inhibitors (44).
Idarucizumab (Praxbind®, Boehringer Ingelheim, Germany) is indicated in adult patients treated with dabigatran etexilate (Pradaxa®) when rapid reversal of its anticoagulant effects is required—for emergency surgery/urgent procedures or in life-threatening or uncontrolled bleeding (45). A multicenter retrospective observational study described the use of idarucizumab in 53 heart transplant patients and showed effective hemostasis in most patients, although 4 patients required re-operation in the immediate postoperative period to control bleeding and 2/3 of patients needed transfusion of blood products (22). A small case series from USA, (13 patients, where 1 underwent CABG) reported that optimal hemostasis was achieved in almost 3/4 of patients with major bleeding and that there were no thrombotic complications or side effects that could be attributed to idarucizumab (21). The REVERSE-AD trial, a multicenter, prospective, open-label one-arm study that aimed to determine whether idarucizumab would be able to reverse the anticoagulant effect of dabigatran in patients who had uncontrolled bleeding or were about to undergo an urgent procedure, demonstrated fast and effective reversal of dabigatran with idarucizumab, resulting in shortening the time for patients to be deemed eligible for surgery to 1.6 hours, while periprocedural hemostasis was assessed as normal in 93.4% of patients (43). The incidence of thrombotic events among 202 patients on dabigatran in the surgical group (not exclusively cardiovascular) was 5%, but there was no control group to compare this outcome with. Based on these data, idarucizumab is recommended in several guidelines to prevent perioperative bleeding in patients on dabigatran (4,38,40,41).
Andexanet alfa (Ondexxya® or Andexxa®, Alexion Pharmaceuticals, USA) is approved for adult patients on direct FXa inhibitors apixaban (Eliquis®) or rivaroxaban (Xarelto®) when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. This antidote was not initially tested in a surgical environment but only in patients who had acute major bleeding, predominately intracranial, within 18 hours after administration of a factor Xa inhibitor (44). An optimal hemostatic effect was achieved in 82% of treated patients with this antidote and the incidence of thrombotic events was 10% in a 30-day follow-up period. Interestingly, the Society of Thoracic Surgeons (STS) in their recent Update to the Clinical Practice Guidelines on Patient Blood Management recommended administration of andexanet alfa in patients in need of emergent cardiac surgery with recent ingestion of either apixaban or rivaroxaban, or laboratory evidence of a DOAC effect (40). This recommendation is given contrary to Direct Healthcare Professional Communication letter issued by the European Medicines Agency that advises against the use of this reversal agent prior to heparinization since it causes unresponsiveness to the anticoagulant effects of heparin (46). The use of andexanet alfa for FXa reversal before urgent surgery has not been evaluated and the fact that it seems to neutralize the anticoagulant effect of heparin makes it incompatible with the vast majority of CPB-assisted cardiothoracic procedures.
It seems that idarucizumab is becoming an accepted solution with respect to perioperative bleeding risk in patients on dabigatran. Andexanet alfa, on the other hand, may cause serious harm to heparinized patients undergoing cardiac surgery with CPB, therefore such off-label use should be discouraged. Moreover, a reported thrombotic event incidence of 10% appears rather high, so the significance of this observation should be further assessed. Noteworthy, these agents are costly (33) and have a short plasma half-life that may lead to a rebound effect once the infusion is ceased (25).
Reversal agent for ticagrelor
Ticagrelor (Brilique® or Brilinta®) is the only reversibly binding oral P2Y12 inhibitor. The antidote for ticagrelor bentracimab (a monoclonal antibody fragment) is currently being investigated in a single-arm study (ClinicalTrials.gov, NCT04286438) that aims to confirm its reversal efficacy and show achievement of effective hemostasis in patients who require urgent surgery or have major hemorrhages. An interim analysis of the phase 3 data has recently been published after 150 enrolled patients, almost all of whom were surgical. The authors reported rapid and sustainable ticagrelor reversal and adjudicated hemostasis achieved for more than 90% of patients, while approximately 5% of patients experienced thrombotic events (47). The single arm design of the study did not allow for a comprehensive evaluation of the safety of this approach. The lack of a control group, particularly for surgical patients who receive this investigational treatment as a preventive measure, represents a serious limitation of this study.
Hemoadsorption therapy
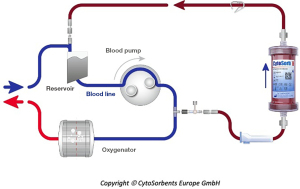
Hemoadsorption therapy with CytoSorb® (CytoSorbents Corporation, Monmouth Junction, USA) reduces excessive levels of inflammatory mediators with the aim to modulate the overshooting immune response, mitigate the cytotoxic effects of elevated cytokine levels and increase the chances for recovery. Moreover, the adsorber is CE-approved to remove ticagrelor and rivaroxaban intraoperatively during CPB-assisted surgery (48). The cartridge, which can be easily integrated into various extracorporeal circuits, such as e.g., continuous renal replacement therapy (CRRT), extracorporeal membrane oxygenation (ECMO) and CPB (Figure 2), is filled with biocompatible, porous polymer beads covered with a divinylbenzene coating. Each polymer bead is between 300 and 800 µm in size and has pores and channels, giving it a large (over 40,000 m2) effective surface area for binding hydrophobic small- and middle-sized molecules (Figure 3).
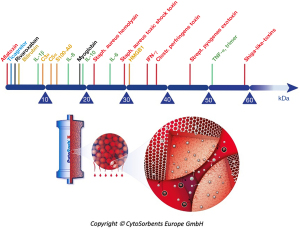
A strong signal towards significantly improved clinical outcomes in emergency cardiac surgery (including type-A aortic dissections) in patients on ticagrelor or rivaroxaban has already been presented in the analysis of literature results, together with the cost effectiveness. The first reports about CytoSorb’s ability to remove ticagrelor and rivaroxaban came out in 2017 with two independent in vitro studies (49,50), demonstrating the very fast decrease in plasma concentrations of these drugs in experimental conditions. Various in vitro investigations since reported that CytoSorb also removes apixaban (51), edoxaban (52), and dabigatran (53), the latter even concomitantly with ticagrelor (54). A recent case report by Mendes et al. (55) presented a patient on apixaban requiring complex cardiac surgery. The authors reported a decrease of 50% in anti-FXa activity after 100 min of CPB with hemoadsorption. Apixaban and rivaroxaban removal by hemoadsorption is the aim of a “sister”-trial to the already mentioned RCT investigating ticagrelor removal (32). The “family name” of these investigations is Safe and Timely Antithrombotic Removal (56) of ticagrelor (T) or rivaroxaban and apixaban (D; standing for DOACs), hence the names STAR-T (ClinicalTrials.gov, NCT04976530) and STAR-D (Clinical.Trials.gov, NCT05093504). A “close cousin” under the same family name is STAR Registry (Clinical.Trials.gov, NCT05077124), capturing real-world clinical use patterns and associated clinical outcomes with the use of CytoSorb for the removal of antithrombotic agents. These studies are in the early phase of recruitment.
Interestingly, to date, no clinical study reported on the results of platelet function or viscoelastic testing in the context of antithrombotic removal by hemoadsorption. Rotational thromboelastometry (ROTEM)-guided perioperative hemorrhage management has been shown to be useful in predicting bleeding complications and choosing the right hemostatic strategy (57). Recent investigations showed that ROTEM might also be an excellent tool to guide DOAC reversal treatments (58), so potentially its role will be evaluated together with hemoadsorption soon, as well.
Future considerations
Approximately 2% of Europeans have AF. As a result of a steep increase in the prevalence of AF with age and the continuously aging population, the projected number of people with AF in Europe is 17.9 million by the year 2060 (59). In the last 10 years in the USA, the proportion of patients taking DOACs has increased 9-fold to more than 2/3 of anticoagulated patients with AF (60). A rapid increase in ticagrelor prescription rates has been reported as well (61,62). Within such a landscape, there will likely be the need for all of the aforementioned solutions to mitigate the risk of major bleeding complications in patients taking antithrombotic agents. While administration of antidotes is easy and does not require any special circumstances or additional equipment, it carries important risks from potential thrombotic adverse events, and costs are substantial. Hemoadsorption, however, is safe, represents an “all-in-one” solution for multiple antithrombotics, and has already shown significant clinical benefits. In the future, it is expected that evidence will begin emerging for the removal of antithrombotic medication outside of cardiac surgery (e.g., trauma or neurosurgery). Pros and cons for all of the specific pharmacological or technological solutions presented in this review are given in Table 1.
Table 1
| A specific solution | Pros | Cons |
|---|---|---|
| Idarucizumab | Approved for emergency surgery/urgent procedures or in life-threatening or uncontrolled bleeding | Target: dabigatran only |
| Fast and effective reversal | Costly ($3,482) | |
| Potential rebound effect | ||
| Humanized monoclonal antibody fragment indicated in adult patients treated with dabigatran when reversal of the anticoagulant effect is needed for emergency surgery/urgent procedures, or in the event of life-threatening or uncontrolled bleeding; no contraindications; precautions to be taken in patients with hypersensitivity, hereditary fructose intolerance, and increased thromboembolic risk (45) | ||
| Andexanet alfa | Approved for life-threatening or uncontrolled bleeding | Not approved for emergency surgery/urgent procedures |
| Fast and effective reversal | Incompatible with heparin (e.g., during CPB) | |
| Target: rivaroxaban or apixaban (off-label use possible for edoxaban) | Potentially high risk of thrombotic events (10%) | |
| Costly ($24,750) | ||
| Potential rebound effect | ||
| A recombinant form of human factor Xa protein indicated in adult patients treated with apixaban or rivaroxaban when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding; contraindicated in known hypersensitivity; precautions to be taken prior urgent surgery, with heparin, higher risk of thrombosis for patients receiving the higher dose (46) | ||
| Bentracimab* | Rapid and sustainable reversal (preliminary data!) | Target: ticagrelor only |
| Potentially costly (estimation based upon prices of other monoclonal antibody technologies present in the market) | ||
| A neutralizing monoclonal antibody fragment that binds ticagrelor | ||
| Hemoadsorber | Fast and effective removal | Approved for intraoperative use on CPB only (Europe) |
| Target: ticagrelor and rivaroxaban (off-label use possible for apixaban, edoxaban, dabigatran) | ||
| Durable effect | ||
| Proven safety (no adverse events reported) | ||
| Cost-effective | ||
| Potential to expand outside of CPB-assisted cardiac surgery to different clinical settings with various platforms (CRRT, ECMO, etc., currently off-label) | ||
| A polymer based adsorption system designed in the area of extracorporeal therapies, indicated for use in conditions where elevated levels of cytokines and/or bilirubin and/or myoglobin exist and for use intraoperatively during CPB surgery for the removal of ticagrelor and/or rivaroxaban; contraindicated in HIT positive patients when citrate regional anticoagulation is unavailable and in patients with very low platelet counts (<20,000/μL); relative contraindications include pregnancy, acute sickle cell crisis, concurrent immunosuppressive therapy, with the exception of corticosteroids, profound immunosuppression (e.g., CD4 <200 or neutropenia with ANC <1,000/μL); general precautions to be taken when handling extracorporeal blood purification techniques and discretion should be used when treating a patient weighing less than 45 kg (48) | ||
*, not approved, phase 3 clinical trial in progress NCT04286438. CPB, cardiopulmonary bypass; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; HIT, heparin-induced thrombocytopenia; ANC, absolute neutrophil count.
Limitations
This analysis summarizes the current knowledge of all published articles in the investigated area so far. Many of the published articles do not refer directly to cardiac surgery. Therefore, a bias by removing publications from cardiology over cardiac surgery may exist. Given that there is a lack of studies in this research area, the overall number of patients being presented might be small. And finally, the general limitation of a review per se applies, that statistically combining data in this way seems to be impossible. To counteract this specific limitation, it is necessary that a review is conducted in an explicit, rigorous, and reproducible fashion.
Conclusions
The incidence of bleeding complications in patients on DAPT or DOACs undergoing urgent cardiac surgery is very high. As the proportion of surgical patients under antithrombotics rapidly grow, there is an increasing unmet clinical need. Reversal agents for DOACs have been launched in recent years, but their usefulness in the non-elective cardio-thoracic surgery setting remains very limited due to high cost or incompatibility with heparin-based anticoagulation for CPB. The reversal agent for ticagrelor is under development, preliminary results look promising, however, once approved, expected high price might limit its availability. In contrast, it seems that intraoperative hemoadsorption strategy represents universal and cost-effective method to mitigate perioperative bleeding risk and improve clinical outcomes. It is still not approved by the USA Food and Drug Administration, so, currently, two multicenter RCTs are being conducted in the USA evaluating the effectiveness and safety of antithrombotic removal. Contemporary evidence suggests hemoadsorption as a method of choice in the management of perioperative bleeding risk in patients on antithrombotic medications undergoing CPB-assisted cardiac surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-428/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-428/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-428/coif). MMS is a full-time consultant to CytoSorbents. KH, SG and MT received honoraria for lectures by CytoSorbents. MT and RFS are the investigators in the STAR Registry (Clinical.Trials.gov, No. NCT05077124), sponsored by CytoSorbents. RFS and MS received honoraria for lectures and consultancy fees by CytoSorbents. HA, END, and DW are full-time employees of CytoSorbents. DW serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2022 to March 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213-60. [Crossref] [PubMed]
- European Medicines Agency. Brilique Epar product information. Annex 1 Summary of Product Characteristics. 2022.
- Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330-93. [Crossref] [PubMed]
- Hansson EC, Jidéus L, Åberg B, et al. Coronary artery bypass grafting-related bleeding complications in patients treated with ticagrelor or clopidogrel: a nationwide study. Eur Heart J 2016;37:189-97. [Crossref] [PubMed]
- Schlachtenberger G, Deppe AC, Gerfer S, et al. Major Bleeding after Surgical Revascularization with Dual Antiplatelet Therapy. Thorac Cardiovasc Surg 2020;68:714-22. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern Med 2019;179:1469-78. [Crossref] [PubMed]
- Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012;126:343-8. [Crossref] [PubMed]
- Beyer-Westendorf J, Gelbricht V, Förster K, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J 2014;35:1888-96. [Crossref] [PubMed]
- Hassan K, Bayer N, Schlingloff F, et al. Bleeding Complications After Use of Novel Oral Anticoagulants in Patients Undergoing Cardiac Surgery. Ann Thorac Surg 2018;105:702-8. [Crossref] [PubMed]
- Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. Eur Heart J 2017;38:2137-49. [PubMed]
- Schaefer A, Sill B, Schoenebeck J, et al. Preoperative Ticagrelor administration leads to a higher risk of bleeding during and after coronary bypass surgery in a case-matched analysis. Interact Cardiovasc Thorac Surg 2016;22:136-40. [Crossref] [PubMed]
- Tomšič A, Schotborgh MA, Manshanden JS, et al. Coronary artery bypass grafting-related bleeding complications in patients treated with dual antiplatelet treatment. Eur J Cardiothorac Surg 2016;50:849-56. [Crossref] [PubMed]
- Hansson EC, Rexius H, Dellborg M, et al. Coronary artery bypass grafting-related bleeding complications in real-life acute coronary syndrome patients treated with clopidogrel or ticagrelor. Eur J Cardiothorac Surg 2014;46:699-705; discussion 705. [Crossref] [PubMed]
- Malm CJ, Hansson EC, Åkesson J, et al. Preoperative platelet function predicts perioperative bleeding complications in ticagrelor-treated cardiac surgery patients: a prospective observational study. Br J Anaesth 2016;117:309-15. [Crossref] [PubMed]
- Hansson EC, Jeppsson A. Platelet inhibition and bleeding complications in cardiac surgery: A review. Scand Cardiovasc J 2016;50:349-54. [Crossref] [PubMed]
- Diab S, Arazi M, Sternik L, et al. Coronary artery bypass graft surgery in patients on ticagrelor therapy is not associated with adverse perioperative outcomes. J Cardiothorac Surg 2021;16:139. [Crossref] [PubMed]
- Chemtob RA, Moeller-Soerensen H, Holmvang L, et al. Outcome After Surgery for Acute Aortic Dissection: Influence of Preoperative Antiplatelet Therapy on Prognosis. J Cardiothorac Vasc Anesth 2017;31:569-74. [Crossref] [PubMed]
- Testa S, Ageno W, Antonucci E, et al. Management of major bleeding and outcomes in patients treated with direct oral anticoagulants: results from the START-Event registry. Intern Emerg Med 2018;13:1051-8. [Crossref] [PubMed]
- Sheikh-Taha M. Idarucizumab for Reversal of Dabigatran: Single-Center Real-World Experience. Am J Cardiovasc Drugs 2019;19:59-64. [Crossref] [PubMed]
- Crespo-Leiro MG, López-Vilella R, López Granados A, et al. Use of Idarucizumab to reverse the anticoagulant effect of dabigatran in cardiac transplant surgery. A multicentric experience in Spain. Clin Transplant 2019;33:e13748. [Crossref] [PubMed]
- Herrera-Escandón Á, Castaño-Cifuentes O, Plata-Mosquera CA. Use of Idarucizumab to Revert the Anticoagulant Effect of Dabigatran in Heart Transplant Surgery: An Institutional Experience. Case Rep Cardiol 2020;2020:6927423. [Crossref] [PubMed]
- Sodha NR, Sellke FW. Reversal of Dabigatran with Idarucizumab. Expert Rev Cardiovasc Ther 2016;14:889-93. [Crossref] [PubMed]
- Akhrass R, Gillinov M, Bakaeen F, et al. Emergency cardiac surgery in patients on oral anticoagulants and antiplatelet medications. J Card Surg 2022;37:214-22. [Crossref] [PubMed]
- Godier A, Garrigue D, Lasne D, et al. Management of antiplatelet therapy for non-elective invasive procedures or bleeding complications: Proposals from the French Working Group on Perioperative Haemostasis (GIHP) and the French Study Group on Thrombosis and Haemostasis (GFHT), in collaboration with the French Society for Anaesthesia and Intensive Care (SFAR). Arch Cardiovasc Dis 2019;112:199-216. [Crossref] [PubMed]
- Makhdoum A, Dhingra NK, Kirubaharan A, et al. Ticagrelor use and practice patterns among Canadian cardiac surgeons. J Card Surg 2021;36:2793-801. [Crossref] [PubMed]
- Hassan K, Kannmacher J, Wohlmuth P, et al. Cytosorb Adsorption During Emergency Cardiac Operations in Patients at High Risk of Bleeding. Ann Thorac Surg 2019;108:45-51. [Crossref] [PubMed]
- Hassan K, Brüning T, Caspary M, et al. Hemoadsorption of Rivaroxaban and Ticagrelor during Acute Type A Aortic Dissection Operations. Ann Thorac Cardiovasc Surg 2022;28:186-92. [Crossref] [PubMed]
- Krüger B, Renner T, Van Hemelrijck M, et al. The effect of hemoadsorption on rivaroxaban blood plasma concentration in emergency cardiac surgery. Indian J Thorac Cardiovasc Surg 2021;37:680-3. [Crossref] [PubMed]
- Mair H, Jilek C, Haas B, et al. Ticagrelor and Rivaroxaban Elimination With CytoSorb Adsorber Before Urgent Off-Pump Coronary Bypass. Ann Thorac Surg 2020;110:e369-70. [Crossref] [PubMed]
- Gibson CM, Mack MJ, Lee VT, et al. Rationale and design of the safe and timely antithrombotic removal - ticagrelor (STAR-T) trial: A prospective, multi-center, double-blind, randomized controlled trial evaluating reductions in postoperative bleeding with intraoperative removal of ticagrelor by the drugsorb™-ATR device in patients undergoing cardiothoracic surgery within 48 hours from last ticagrelor dose. Am Heart J 2022;245:19-28. [Crossref] [PubMed]
- Jackson R, Trus RM, El-Diasty M. Hemadsorption for removal of ticagrelor and direct oral anticoagulants in cardiac surgery. Expert Rev Cardiovasc Ther 2022;20:141-50. [Crossref] [PubMed]
- Javanbakht M, Trevor M, Rezaei Hemami M, et al. Ticagrelor Removal by CytoSorb® in Patients Requiring Emergent or Urgent Cardiac Surgery: A UK-Based Cost-Utility Analysis. Pharmacoecon Open 2020;4:307-19. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- Held C, Asenblad N, Bassand JP, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol 2011;57:672-84. [Crossref] [PubMed]
- Varenhorst C, Alström U, Scirica BM, et al. Factors contributing to the lower mortality with ticagrelor compared with clopidogrel in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol 2012;60:1623-30. [Crossref] [PubMed]
- Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth 2018;32:88-120. [Crossref] [PubMed]
- Writing Committee Members. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e21-129. [Crossref] [PubMed]
- Tibi P, McClure RS, Huang J, et al. STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management. Ann Thorac Surg 2021;112:981-1004. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104-32. [Crossref] [PubMed]
- Sousa-Uva M, Head SJ, Milojevic M, et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:5-33. [Crossref] [PubMed]
- Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J Med 2017;377:431-41. [Crossref] [PubMed]
- Connolly SJ, Crowther M, Eikelboom JW, et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N Engl J Med 2019;380:1326-35. [Crossref] [PubMed]
- European Medicines Agency. Praxbind product information. Annex 1 Summary of Product Characteristics. 2015:1-32.
- European Medicines Agency. Direct Healthcase Professional Communication; Ondexxya (andexanet alfa): Avoid use of andexanet prior to heparinization. 2020:1-3.
- Bhatt DL, Pollack CV Jr., Mazer CD, et al. Bentracimab for Ticagrelor Reversal in Patients Undergoing Urgent Surgery. NEJM Evid 2021;1:EVIDoa2100047.
- CytoSorbents. CytoSorb 300mL Device. Instructions for use. 2021. Available online: www.cytosorb.com
- Angheloiu GO, Gugiu GB, Ruse C, et al. Ticagrelor Removal From Human Blood. JACC Basic Transl Sci 2017;2:135-45. [Crossref] [PubMed]
- Koertge A, Wasserkort R, Wild T, et al. Extracorporeal Hemoperfusion as a Potential Therapeutic Option for Critical Accumulation of Rivaroxaban. Blood Purif 2018;45:126-8. [Crossref] [PubMed]
- Røed-Undlien H, Schultz NH, Lunnan A, et al. In Vitro Apixaban Removal By CytoSorb Whole Blood Adsorber: An Experimental Study. J Cardiothorac Vasc Anesth 2022;36:1636-44. [Crossref] [PubMed]
- Angheloiu AA, Tan Y, Ruse C, et al. In-Vitro Sorbent-Mediated Removal of Edoxaban from Human Plasma and Albumin Solution. Drugs R D 2020;20:217-23. [Crossref] [PubMed]
- Angheloiu GO, van Ryn J, Goss AM. Removal of Dabigatran Using Sorbent Hemoadsorption. AHA/ASA Scientific Sessions. Orlando, FL, USA: Circulation, 2015:A12901.
- Angheloiu AA, Angheloiu GO. Removal of dabigatran using sorbent hemadsorption. Int J Cardiol 2019;293:73-5. [Crossref] [PubMed]
- Mendes V, Colombier S, Verdy F, et al. Cytosorb® hemoadsorption of apixaban during emergent cardio-pulmonary bypass: a case report. Perfusion 2021;36:873-5. [Crossref] [PubMed]
- Barton H, Zechendorf E, Ostareck D, et al. Prognostic Value of GDF-15 in Predicting Prolonged Intensive Care Stay following Cardiac Surgery: A Pilot Study. Dis Markers 2021;2021:5564334. [Crossref] [PubMed]
- Görlinger K, Pérez-Ferrer A, Dirkmann D, et al. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J Anesthesiol 2019;72:297-322. [Crossref] [PubMed]
- Pavoni V, Gianesello L, Conti D, et al. "In Less than No Time": Feasibility of Rotational Thromboelastometry to Detect Anticoagulant Drugs Activity and to Guide Reversal Therapy. J Clin Med 2022;11:1407. [Crossref] [PubMed]
- Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J 2019;4:198-223. [Crossref] [PubMed]
- Bolliger D, Mauermann E, Erdoes G. A New Tool in the Management of Direct-Acting Oral Anticoagulants in Emergency Cardiac Surgery. J Cardiothorac Vasc Anesth 2022;36:1645-7. [Crossref] [PubMed]
- Basra SS, Wang TY, Simon DN, et al. Ticagrelor Use in Acute Myocardial Infarction: Insights From the National Cardiovascular Data Registry. J Am Heart Assoc 2018;7:008125. [Crossref] [PubMed]
- Faridi KF, Garratt KN, Kennedy KF, et al. Physician and Hospital Utilization of P2Y12 Inhibitors in ST-Segment-Elevation Myocardial Infarction in the United States: A Study From the National Cardiovascular Data Registry's Research to Practice Initiative. Circ Cardiovasc Qual Outcomes 2020;13:e006275. [Crossref] [PubMed]


