Outcome of nonsurgical treatment for locally advanced thymic tumors
Introduction
Thymic epithelial tumor is an uncommon neoplasm originated from epithelial cells of the thymus. Its incidence is reported to be as low as 0.13/100,000 (1). Surgical resection remains the mainstay of treatment for patients with early-staged disease, with a 10-year-survival of 71% to 100% (2). By contrast, chemotherapy-based regimens are most commonly used in stage IV cases. As for locally advanced thymic tumors, surgical resections are usually pursued after neoadjuvant chemo- or radiotherapy (RT), because complete resection has been proved to be the most significant prognostic factor for survival (3). In clinical practice, however, there are situations in which surgery is inapplicable, either due to extensive tumor invasion into critical organs, or poor cardiopulmonary functions of the patients. By far, the optimal therapy for these inoperable patients has yet to be established. Reports aiming at comparing different nonsurgical treatment modalities in a short time period have been scanty, mainly because of the rarity of the disease. We thereby retrospectively studied 42 patients treated during a 10-year span at a single institution, trying to shed some light on this challenging clinical scenario.
Materials and methods
From October 2000 to December 2010, a total of 61 patients with thymic tumors were treated at the Department of Radiation Oncology, Shanghai Chest Hospital with definitive RT, sequential chemoradiation (SCRT) or concurrent chemoradiation (CCRT) plus consolidation chemotherapy. Among them, 42 were enrolled in the current study. The criteria for enrollment are as follows: (I) histology proven diagnosis as thymic tumor by pre-treatment biopsy; (II) invasive stage III upon radiological images; (III) stage IV with only adjacent pleural implant or lymph-node enlargement which could be covered by one radiation field along with the primary tumor; (IV) no metastasis to distant organs. This retrospective study was approved by the Institutional Review Board. All patients’ demographic information and tumor-related data were obtained by reviewing medical record.
Between 2000 and 2006, all RT was delivered by three-dimension conformal radiation (3-D CRT). After that, it was replaced by intensity modulated radiation (IMRT). Chemotherapy was used as initial treatment in SCRT group, followed by RT at a time point when tumors shrunk to a stable size or tumors showed no response to chemotherapy. In the CCRT group, chemotherapy and RT were started simultaneously on the first day, and the cycles of consolidation chemotherapy varied based on patients’ status and oncologist’s judgment. According to medical record, 16 patients did not receive chemotherapy mainly due to medical reasons (compromised renal function, old age, Parkinson disease, etc.).
Evaluation of response and toxicity
The radiographic response was evaluated according to a new Response Evaluation Criteria in Solid Tumors (RECIST) guideline proposed by International Thymic Malignancy Interest Group (ITMIG) (4). Toxicity associated with treatment was assessed by Common Terminology Criteria for Adverse Events (CTCAE 4.0).
Statistical analysis
The χ2 or Fisher’s exact tests were used to compare categorical data when appropriate. Survival was estimated by Kaplan-Meier curves, and differences among groups were compared by log-rank test. A Cox regression model was used to calculate hazard ratio (HR) and its 95% confidence interval (CI). Statistical analysis was performed using SPSS version 16.0 software. All tests were two-sided, with P<0.05 deemed as statistically significant.
Results
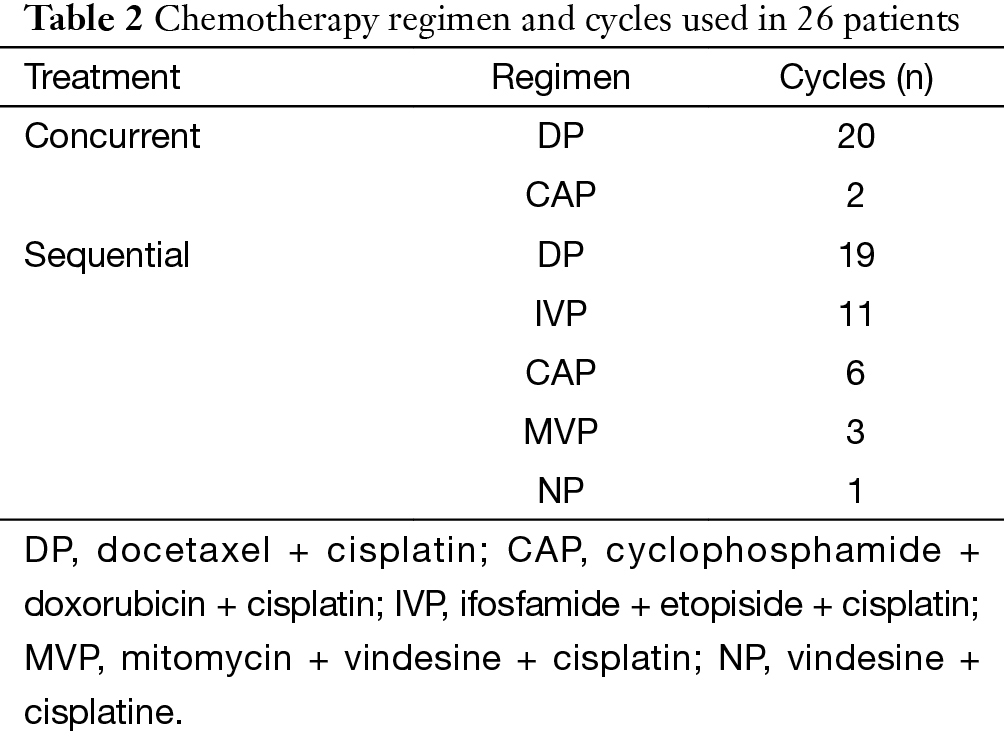
There were 42 patients included in this study. The characteristics of these patients are summarized in Table 1. Of these patients, 16 were treated by RT alone, 10 by SCRT and 16 by CCRT. The median dose of RT was 60 Gy (range, 34–70 Gy). Chemotherapy regimens varied during a 10-year period, but docetaxol and cisplatin (DP) was most frequently used. The details of regimens are shown in Table 2.
Full table
Full table
Response data and survival analysis
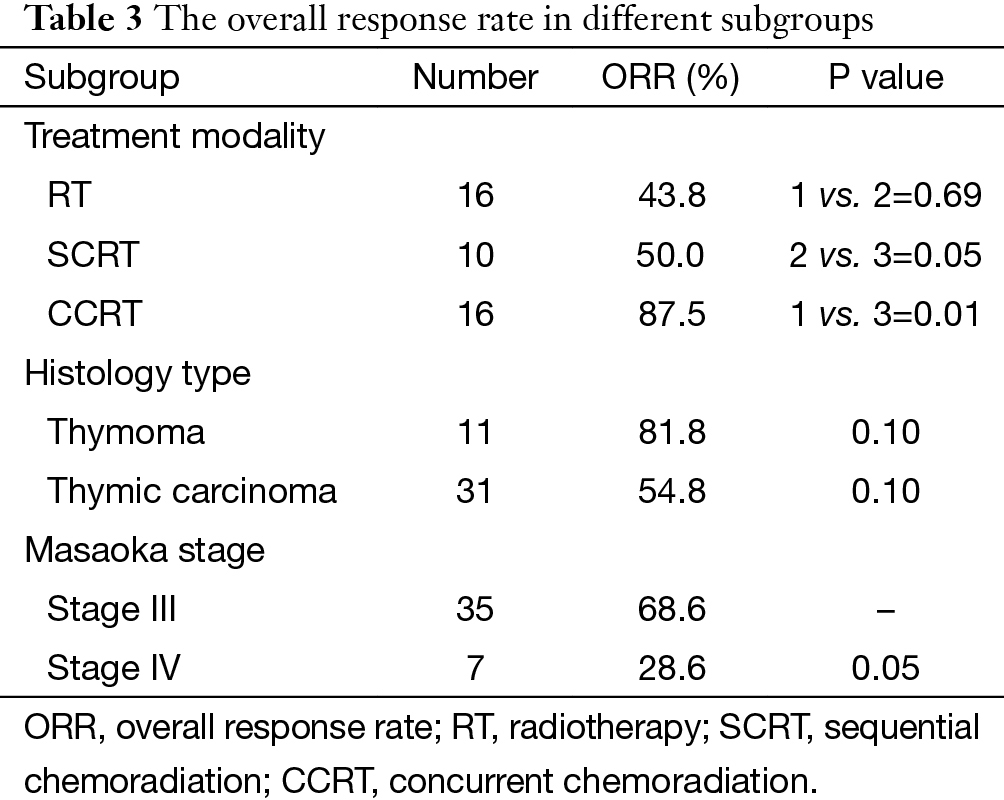
The overall objective response rate (ORR) in all 42 patients was 61.9% (26/42). The ORR in different subgroups was listed in Table 3, and CCRT rendered higher ORR than RT (87.5% vs. 43.8%, P=0.009) and SCRT (50%, P=0.051). In SCRT group, a sub-group comparison was made between DP regimen and non-DP regimen, and the result showed no significant difference (75% vs. 50%, P=0.571).
Full table
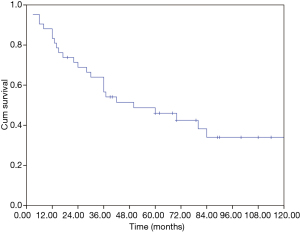
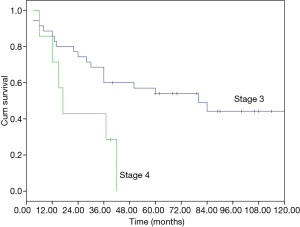
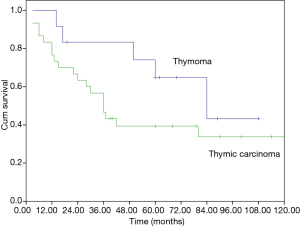
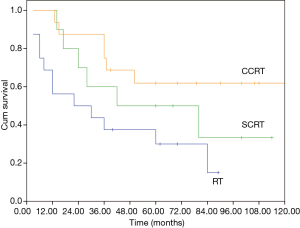
The median overall survival (OS) of the whole cohort was 41 months (95% CI, 40.5–64.5), with a 5-year OS of 46% (Figure 1). The survival curves of different groups are shown in Figures 2,3,4.
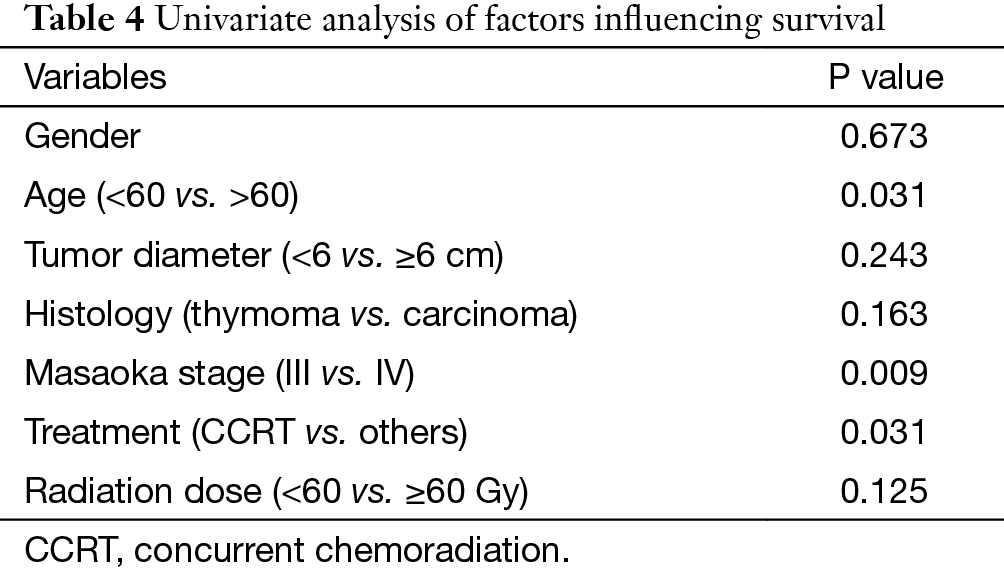
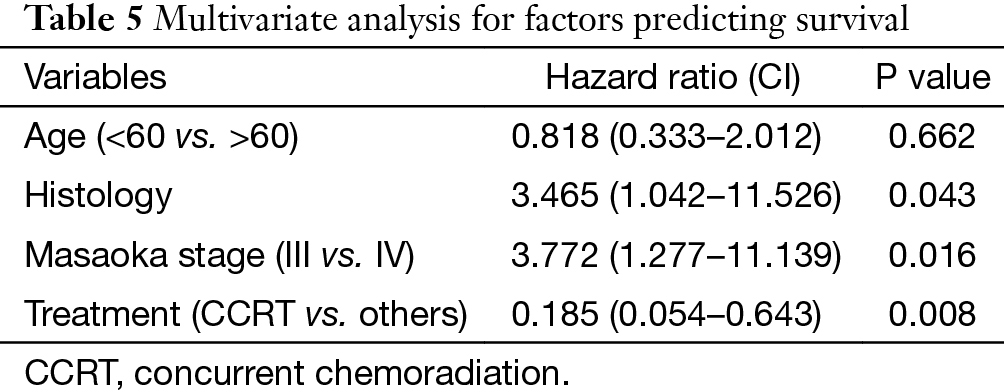
In univariate analysis (Table 4), age (P=0.031), Masaoka stage (P=0.009) and treatment modality (P=0.031) were significant variables affecting OS, with CCRT providing the best OS. In Cox regression, Masaoka stage, treatment modality and histology type were independent predictors for OS (Table 5).
Full table
Full table
Toxicity
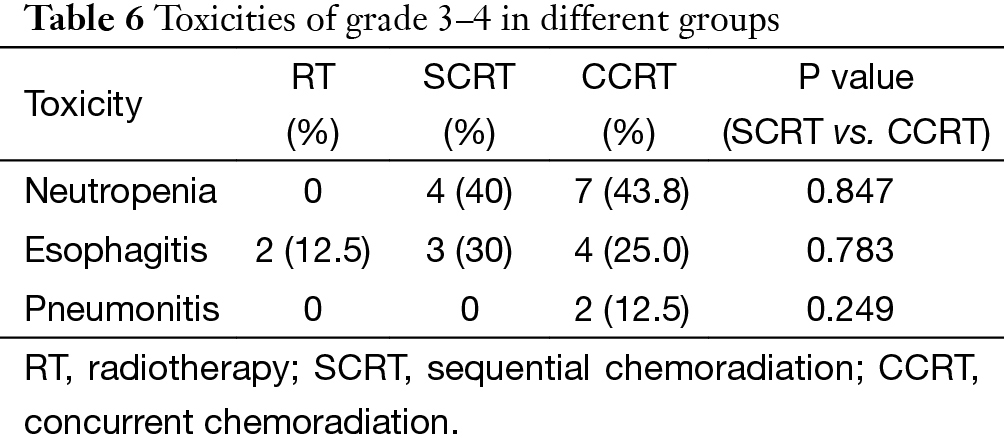
There was no treatment related death in this cohort. The major toxicity was grade 3–4 neutropenia, which was observed in 11 patients. Other adverse events are listed in Table 6. The overall complication rate was similar in SCRT and CCRT group (70% vs. 80.3%), both higher than that in RT group (12.5%).
Full table
Discussion
For well-encapsulated, noninvasive thymic epithelial tumors, complete resection is usually curative, with a risk of local recurrence of less than 2% (2). However, there is no standard approach to advanced thymic tumors apart from surgery. Non-surgical modalities, such as RT, chemotherapy, or their combinations, are randomly adopted based on individual oncologist’s experience or preference. To the best of our knowledge, this is the first report comparing three different non-surgical treatments for inoperable thymic tumors. Our results showed that CCRT achieved more favorable outcomes than SCRT and RT alone in both tumor response and long time survival.
Apart from complete resection, WHO classification and Masaoka stage are generally accepted as the most important prognostic factors for thymic malignancies (2,5). In the current study for unresectable tumors, we found that in addition to the above factors, treatment modality also had important influence on OS in multivariate analysis as shown in Table 5. And CCRT also showed significant superiority over the other two treatment methods in ORR (CCRT vs. SCRT: 87.5% vs. 50%, P=0.051). When chemotherapy and radiation are applied simultaneously, interaction between the two modalities often shows a synergistic effect, and eliminates the tumor to the maximum extent. The advantages of CCRT have been explained through the following mechanisms: (I) chemotherapy agents and radiation can cover different tumor components in a heterogeneous tumor; (II) they also have spatial coordination effect; (III) tumor cells in different cell cycle phase show different sensitivity to chemotherapy and radiation, the concomitant use of the two allows a maximum decrease in tumor; (IV) some chemotherapy agents can act as radiation sensitizers and enhance the anti-tumor effect of RT (6-9). By far, there are no large-scale reports regarding CCRT on locally advanced thymic tumors. Chen and his colleagues (10) conducted CCRT on 16 patients with unresectable thymic carcinomas. The 5-year OS was 67.7%, which is similar to our result (61.9%). These results are even better when compared with some treatments involving surgery (11-13), of which the 5-year OS were around 35%. Wright (14) and Korst (15) have both tried CCRT on locally invasive thymic tumors as preoperative induction therapy. After surgical resection, they reported an R0 resection rate of 80% and complete pathologic response rate of 20%, which was better than other inductive modalities (16-18). Therefore, the role of CCRT as an induction therapy for potentially resectable invasive thymic tumors should definitely be studied on a large scale.
Due to its retrospective nature, chemotherapy regimens varied a lot in our study. But it should be notified that in the CCRT group, the most often used chemotherapy regimens (91%) was DP. In Chen’s study (10), the ORR was only 50%, much lower than the 87.5% ORR in our CCRT group. Looking into details, the median radiation dose was almost the same in the two groups (60 Gy). But the chemotherapy regimen in Chen’s study (5-FU + cisplatin) was different from ours (DP). The ORRs in Korst’s (15) and Wright’s (14) trials were both around 45%, also lower than the ORR in the current study. Of course the radiation dose in these two trials in an inductive setting (45 Gy) was lower than that in the current study as a definitive therapy (60 Gy). But there was also difference in chemotherapy agents (EP vs. DP). Watanabe et al. also reported (19) that docetaxol was an active agent against thymic carcinoma, with an ORR of 31%. In our SCRT group, we compared the ORRs between DP regimen and non-DP regimen and found no significant difference. However, there is still the possibility that their roles might be different in CCRT. At least from the current study, concomitant use of DP and radiation showed the highest activity in reducing tumor volume. Therefore, the efficacy of DP in CCRT should be further tested in prospective trials.
In case of toxicities, no fatal events occurred in our patients. Neutropenia and esophagitis were the two major side effects, but most of them were moderate and manageable. It should be specified that almost all of the esophagitis happened before 2006, when 3-D conformal RT was dominant and opposed anteroposterior fields were frequently used at the time. After upgrading the radiation technique to IMRT, severe esophagitis was no longer observed. The overall toxicity rate was similar in the SCRT group and CCRT group. However, the two cases of grade-3 pneumonitis were both found in the CCRT group, suggesting that potential risk of pulmonary damage should not be neglected when definitive CCRT is applied.
There are several limitations to our study due to its retrospective nature with limited number of patients. Treatment was not carried out by unified protocol but based on physician’s own experience. And there was lack of consistency in chemotherapy regimen. Nevertheless, we found significantly improved results in response rate and survival with DP based CCRT in our patients. Thus we believe this management modality should be further tested by prospective trials.
In conclusions, when used to treat locally advanced thymic tumors unsuitable for surgical resection, CCRT performed more favorably than RT alone or SCRT in both tumor response and long time survival, but probably with increased risk of radiation pneumonitis. Based on these results, CCRT may offer the best chance of disease control for this group of patients. And the role of CCRT in induction setting for locally advanced thymic tumors should also be tested so as to increase the complete resection rate and to improve long-term outcome.
Acknowledgements
The authors want to thank Ms. Yuan Liu for her support in statistics.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Riely GJ, Huang J. Induction therapy for locally advanced thymoma. J Thorac Oncol 2010;5:S323-6. [Crossref] [PubMed]
- Benveniste MF, Korst RJ, Rajan A, et al. A practical guide from the International Thymic Malignancy Interest Group (ITMIG) regarding the radiographic assessment of treatment response of thymic epithelial tumors using modified RECIST criteria. J Thorac Oncol 2014;9:S119-24. [Crossref] [PubMed]
- Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg 2005;80:2002-7. [Crossref] [PubMed]
- Vokes EE, Weichselbaum RR. Concomitant chemoradiotherapy: rationale and clinical experience in patients with solid tumors. J Clin Oncol 1990;8:911-34. [PubMed]
- Trott KR. Radiobiological mechanisms for new strategies in radiochemotherapy. Strahlenther Onkol 1998;174:421-6. [Crossref] [PubMed]
- Bartelink H, Schellens JH, Verheij M. The combined use of radiotherapy and chemotherapy in the treatment of solid tumours. Eur J Cancer 2002;38:216-22. [Crossref] [PubMed]
- Slampa P. Overview of concomitant chemoradiotherapy of solid tumors. Cas Lek Cesk 2003;142:4-8. [PubMed]
- Chen YY, Huang CH, Tang Y, et al. Concurrent chemoradiotherapy for unresectable thymic carcinoma. Chang Gung Med J 2004;27:515-22. [PubMed]
- Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025-32. [Crossref] [PubMed]
- Liu HC, Hsu WH, Chen YJ, et al. Primary thymic carcinoma. Ann Thorac Surg 2002;73:1076-81. [Crossref] [PubMed]
- Ogawa K, Toita T, Uno T, et al. Treatment and prognosis of thymic carcinoma: a retrospective analysis of 40 cases. Cancer 2002;94:3115-9. [Crossref] [PubMed]
- Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44, 46.e1.
- Lucchi M, Ambrogi MC, Duranti L, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg 2005;79:1840-4. [Crossref] [PubMed]
- Rea F, Sartori F, Loy M, et al. Chemotherapy and operation for invasive thymoma. J Thorac Cardiovasc Surg 1993;106:543-9. [PubMed]
- Onuki T, Ishikawa S, Yamamoto T, et al. Pathologic radioresponse of preoperatively irradiated invasive thymomas. J Thorac Oncol 2008;3:270-6. [Crossref] [PubMed]
- Watanabe N, Umemura S, Niho S, et al. Docetaxel for platinum-refractory advanced thymic carcinoma. Jpn J Clin Oncol 2015;45:665-9. [Crossref] [PubMed]