
Empyema after image-guided percutaneous intercostal drainage of subdiaphragmatic collection: a case series
Introduction
Intraabdominal fluid collections may occur after abdominal surgery, infection or trauma and are routinely treated by image-guided percutaneous drainage. This treatment is mostly safe and efficient with lower morbidity and mortality compared to surgical drain placement (1,2). However, in the case of subdiaphragmatic collections, image-guided percutaneous drainage may be challenging due to the anatomic proximity to the pleural space. Intercostal placement of a subdiaphragmatic drain increases the risk of pleural space contamination (2,3). Compared to subcostal drain placement, the intercostal approach is associated with higher rates of pleural complications including pneumothorax, pleural effusions and empyema (3). Progressive pleural empyema is a potential severe complication with high mortality rates (4). The typical clinical course as well as characteristics of patients with postinterventional pleural empyema are unknown.
Here, we have assessed a series of patients with empyema and decortication after intercostal drainage. The aim of this case series was to describe characteristics and clinical consequences of empyema after intercostal drainage and discuss potential risk factors. A detailed description of the characteristics of these patients and their clinical course are important to identify similarities and potential pitfalls in management. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-272/rc).
Methods
Study design
This is a single center, descriptive, retrospective case series including adult patients with decortication of pleural empyema after intercostal drainage of a subdiaphragmatic collection.
Data collection
We identified patients by assessing electronic medical records of the Bern University Hospital between 01.01.2009 and 31.01.2021 for the terms decortication, drainage and subdiaphragmatic collection. Adult patients (≥18 years old) who underwent intercostal drainage of subdiaphragmatic collection with subsequent pleural empyema and decortication were included. Pleural empyemas were classified using stages according to the American Thoracic Society (ATS) guidelines: simple exudative (ATS stage I), fibrinopurulent (ATS stage II) and organizational with scar tissue formation (ATS stage III) (5).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The local Ethics Committee (Ethikkomission Bern) reviewed this project and decided no authorisation from the Committee is necessary for this study. Informed consent therefore was waived for this study.
Statistical analysis
Categorical variables are reported as numbers and percentages, continuous variables as means and standard deviation. Statistical analyses were done using SPSS Software.
Results
A total of ten patients (nine male, one female) with decortication for pleural empyema after intercostal drain insertion into subdiaphragmatic collection were included in this case series. The average age at drain placement was 56 (±21) years. Six patients suffered from subdiaphragmatic collection after abdominal surgery, one patient had infected necrosis after pancreatitis, two patients were treated for liver abscess and one for splenic abscess. Patient demographics and clinical characteristics of percutaneous image-guided intercostal drain placement are demonstrated in Table 1. The drain was inserted under computed tomography (CT)-guidance in eight patients and under ultrasound-guidance in two patients. The drain was left in place for an average of 15 (±8) days. Drain size differed from 8 to 12 French. Drains were mainly inserted in lateral position through the eighth or ninth intercostal space (ICS). The decortication was performed at a mean of 20 (±11) days after drain placement. Five patients underwent minimally invasive thoracoscopic decortication and remaining five patients underwent open decortication. Five of the ten patients needed intensive care unit (ICU) treatment at some point after surgery. Average length of stay (LOS) was 37 (±18) days. All patients received antibiotic treatment, on average for 6 (±1) weeks. The most frequent comorbidities in our cohort were malnutrition, diabetes and cancer (60%, 40% and 30% of patients). Clinical characteristics of empyema and decortication are shown in Table 2.
Table 1
| Case | Sex | Age (years) | Intercostal drainage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Guidance | Duration (days) | ICS | Size (Fr) | Position | Quality | Culture | |||
| 1 | M | 43 | Splenic abscess | US | 21 | 9th | 10 | Posterolateral | Bloody | β-hemolytic streptococcus group B |
| 2 | M | 57 | Perihepatic abscess | US | 24 | 8th | 9 | Lateral | Bloody | E. faecium |
| 3 | M | 40 | Liver abscess | CT | 6 | 8th | – | Lateral | Not available | – |
| 4 | M | 64 | Pancreatic Leakage |
CT | 27 | 8th | 8 | Lateral | Bloody | No growth |
| 5 | M | 37 | Necrotic pancreatic collection | CT | 22 | 9th | 12 | Posterolateral | Brownish | Coagulase negative staphylococcus |
| 6 | F | 76 | Subdiaphragmatic abscess | CT | 14 | 8th | 10 | Lateral | Putrid | Mixed anaerobic flora |
| 7 | M | 21 | Liver abscess | CT | 4 | 7th, 8th | 10 | Ventral, lateral | Putrid | S. milleri |
| 8 | M | 86 | Subdiaphragmatic abscess | CT | 7 | 8th | 8 | Lateral | Putrid | E. cloacae |
| 9 | M | 65 | Subdiaphragmatic abscess | CT | 12 | 6th | 8 | Lateral | Putrid | E. coli |
| 10 | M | 79 | Liver abscess | CT | 20 | 9th | 8, 10 | Posterolateral | Putrid | E. coli |
ICS, intercostal space; Fr, French; M, male; F, female; US, ultrasound; CT, computed tomography; E. faecium, Enterococcus faecium; S. milleri, Streptococcus milleri; E. cloacae, Enterobacter cloacae; E. coli, Escherichia coli.
Table 2
| Case | Empyema | LOS (days) | LOS after decortication (days) | ||||
|---|---|---|---|---|---|---|---|
| ATS Stage | Time to decortication (days) | Surgery | Potential risk factors | Culture | |||
| 1 | III | 35 | Thoracoscopic | Diabetes, malnutrition | – | 44 | 15 |
| 2 | III | 29 | Open | Diabetes, cancer | No growth | 44 | 10 |
| 3 | II | 12 | Thoracoscopic | None | No growth | 27 | 4 |
| 4 | II | 27 | Thoracoscopic | Diabetes, cancer malnutrition | E. faecium | 57 | 15 |
| 5 | III | 35 | Open | Diabetes, malnutrition | No growth | 71 | 27 |
| 6 | III | 19 | Open | Cancer, malnutrition | Actinomyces | 25 | 4 |
| 7 | III | 9 | Open | None | No growth | 31 | 13 |
| 8 | II | 7 | Thoracoscopic | Malnutrition | No growth | 18 | 10 |
| 9 | II | 5 | Thoracoscopic | None | No growth | 14 | 7 |
| 10 | III | 20 | Open | Malnutrition | E. coli | 46 | 25 |
ATS, American Thoracic Society; LOS, length of stay; E. faecium, Enterococcus faecium; E. coli, Escherichia coli.
Case 1
A 43-year-old male patient presented with splenic abscess caused by septic emboli originating from beta-hemolytic streptococcus group B aortic valve endocarditis. He had no previous illness except type II diabetes. The abscess drain was placed, ultrasound-guided, in the posterolateral position through the ninth ICS. The quality of drain fluid was bloody, microbiological testing showed the presence of β-hemolytic streptococcus group B.
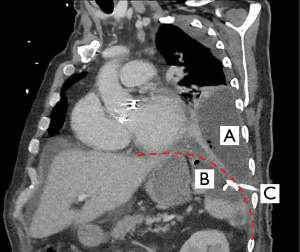
Six days after drain placement the patient suffered from fever and a severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) swab was positive. Treatment with antibiotics was combined with steroids. Twenty-one days after drain placement imaging demonstrated a decrease of the splenic collection and the drain was removed. Another two weeks later the auscultation sounds and saturation levels were decreased, imaging suggested pleural empyema on the left side (Figure 1). Thoracoscopic decortication revealed stage III empyema. In the same procedure, the splenic abscess had to be drained laparoscopically due to progression under conservative management. No complications after the surgical treatment were reported, total LOS was 44 days with discharge 15 days after surgery.
Case 2
A 57-year-old male patient underwent liver transplantation for treatment of hepatocellular carcinoma. Two weeks postoperatively a perihepatic hematoma was suspected by ultrasound. Antibiotic treatment was initiated and an 8-French drain was inserted, ultrasound-guided, into the collection entering the ninth lateral ICS. Enterococcus faecium was isolated. During the following weeks, a clinically relevant pleural effusion on the right side was treated twice by puncture followed by pleural drainage. The cultures were sterile. The pleural drain and intercostal drain in the perihepatic collection were removed on the same day, 24 days after first drain placement. Five days after drain removal a CT-scan demonstrated a pleural empyema on the right side as well as progressive perihepatic collection. The intercostal drain placement in the perihepatic collection was repeated and open decortication confirmed stage III empyema. The total LOS was 44 days. Two days after discharge the patient was rehospitalized for another two weeks due to a pneumonia on the apical left side, with good control of the disease under antibiotic treatment.
Case 3
A 40-year-old previously healthy patient developed a liver abscess in liver segment V after appendectomy for uncomplicated appendicitis. The collection was treated by CT-guided drainage through the eighth ICS in lateral position. After six days the drain was removed. Five days after removal the patient suffered from severe thoracic pain on the right side and inflammation parameters were increasing. CT-scan suggested pleural empyema on the right side. The diagnosis of stage II empyema was confirmed the following day by thoracoscopic decortication. Total length of hospital stay was 27 days with discharge four days after surgery. No complications after the decortication were reported.
Case 4
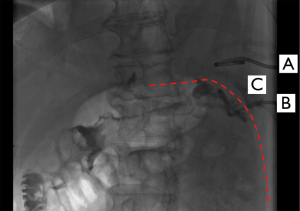
A 64-year-old patient underwent multivisceral resection including left pancreatic resection, splenectomy and partial gastrectomy for metastasized neuroendocrine tumor of the pancreas. Twelve days after surgery he developed a collection at the pancreatic resection margin as well as in the splenic bed. The collection was percutaneously drained through the eighth lateral ICS under CT-guidance. Drain fluid was bloody with evidence of increased amylase level confirming the suspected pancreatic leakage. After drain insertion, the patient suffered from pleural effusion as well as thoracic pain on the left side. Pleural effusion was treated with drainage. Enterococcus faecium was isolated and antibiotic treatment was adapted. Later the pleural drain was repositioned (Figure 2) and changed to irrigation drain due to decreasing output—with lack of success. In the following days the patient suffered from persistent thoracic pain and inflammatory parameters remained high despite antibiotics. The CT-scan suggested pleural empyema. 27 days after initial drain placement the patient underwent thoracoscopic decortication for stage II empyema and removal of the transdiaphragmatic drain. The further course after surgery was uneventful with discharge 15 days postoperative with a total LOS of 57 days.
Case 5
A 37-year-old male patient with acute necrotic collection as a result of severe necrotizing pancreatitis of biliary origin was treated with 12-French percutaneous CT-guided drainage through the ninth ICS. The drain fluid was brownish. Coagulase negative staphylococcus was isolated. After two and half weeks the drain was dislocated and replaced with a 10-French drain. Five days later the inflammation parameters remained high and video-assisted retroperitoneal necrosectomy was performed with removal of the transdiaphragmatic drain. Postoperative X-ray demonstrated new large pleural effusion on the left side. Upon sonography-guided insertion of a pleural pigtail drain, half a liter protein-rich fluid was drained, cultures were found to be sterile. The day after, breath sounds on the left side were still lowered. CT-scan demonstrated persistent effusion with septations. In the following days, signs of systemic inflammation persisted under antibiotic treatment. Open decortication revealed stage III empyema. The postoperative course was uneventful.
Case 6
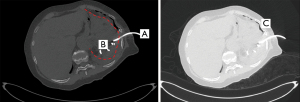
A 76-year-old female patient underwent partial gastrectomy and splenectomy for a gastrointestinal stromal tumor. The postoperative course was uneventful and the patient was discharged eight days after surgery. Two weeks later the patient presented with deterioration of her general condition. CT-scan showed a collection in the splenic bed and discreet pleural effusion on the left side. A 10-French drain was placed through the eighth ICS under CT-guidance (Figure 3). Drain fluid was putrid with isolation of mixed anaerobic flora. Seven days after drainage the patient suffered from pleuritic chest pain and signs of systemic inflammation. CT-scan demonstrated massively progressive left sided pleural effusion with partial septation. The patient was treated by chest tube insertion, the culture was sterile. The chest tube as well as the drain in subdiaphragmatic collection were removed eight days later due to decreasing flow rates and a clinically stable patient. In the following days, breath sounds were significantly lowered on the left side. CT-scan demonstrated persistent effusion with septation suggesting empyema. An open decortication revealed stage III empyema. Further hospitalization was uneventful with discharge of the patient four days after decortication.
Case 7
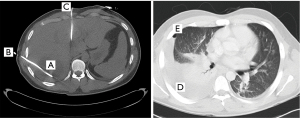
A 21-year-old male patient presented with fever and deterioration of the general condition and was diagnosed with two abscesses in liver segment IV respectively VI/VII. The abscesses were treated with percutaneous CT-guided placement of two 10-French drains (Figure 4). One drain was placed laterally through the eighth ICS and one through the ventral seventh ICS. The fluid was putrid with isolation of Streptococcus milleri. Four days after drainage the patient suffered from fever and thoracic pain on the right basal site. A pleural effusion was diagnosed by sonography. During subsequent puncture, an iatrogenic pneumothorax developed and a thoracic drain was inserted. In the further course of treatment, the patient suffered from respiratory distress, imaging demonstrated progressive effusion and atelectasis on the left side. Open decortication was carried out nine days after intercostal drain placement and demonstrated stage III empyema. The further course was uneventful. The cause for the liver abscesses remained unclear.
Case 8
A family doctor referred an 86-year-old asymptomatic male patient due to elevated inflammatory parameters. A CT-scan revealed an extensive subdiaphragmatic collection on the right side. The patient had a cholecystectomy 14 years previously and re-laparotomy due to a subdiaphragmatic abscess caused by a biliary concrement eight years before presentation. In the meantime, the patient was in a good condition and asymptomatic. Upon CT-guided insertion of an 8-French drain through the eighth ICS drain fluid was putrid with isolation of Enterobacter cloacae. A week after drain placement inflammatory parameters were rising. Imaging demonstrated complete drainage of the subdiaphragmatic abscess but the possibility of pleural empyema on the right side. The thoracoscopic decortication showed stage II empyema. Further hospitalization was beside a postoperative urinary retention uneventful.
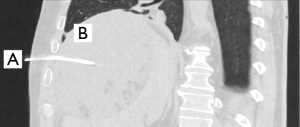
Case 9
A 65-year-old patient underwent laparoscopic segmental resection of colon transversum for treatment of endoscopically unresectable recurrence of polyps. The postoperative course was uneventful with hospital discharge after seven days. Two days later the patient was rehospitalized for a week of intravenous antibiotic treatment for a microinsufficiency of the anastomosis. Two and a half weeks after discharge, the family doctor referred the patient with increased inflammation parameters under antibiotic treatment. CT-scan demonstrated huge subdiaphragmatic abscess on the right side. The abscess was treated with an intercostal drain placement under CT-guidance through the sixth ICS (Figure 5), drain fluid was putrid with isolation of Escherichia coli. On the same day, the patient suffered from respiratory distress but X-ray demonstrated no abnormalities. Four days after drainage the inflammatory parameters were rising and a CT-scan showed suspected empyema. In the decortication on the following day a pleural stage II empyema was found. Four weeks after discharge the patient was rehospitalized for surgical treatment of abscess due to progression under conservative management.
Case 10
A 79-year-old septic patient was treated for liver abscess in segment VI/VII with a 10-French drain through the ninth ICS under CT-guidance. Drain fluid was putrid with isolation of Escherichia coli. Four days after drainage, the patient suffered from respiratory distress. X-ray demonstrated a new pleural effusion on the right side. Due to persisting symptoms under conservative management a pleural drain was inserted with isolation of Escherichia coli. In the further course repetitive X-rays demonstrated persistent partially septated effusion. Open decortication with simultaneous removal of the transdiaphragmatic drain revealed a stage III empyema.
Postoperative inflammation parameters remained high and a CT-scan suggested pneumonic infiltrates on both sides as well as recurrence of the liver abscess. The abscess was treated by an intercostal 8-French drain through the ninth ICS in posterolateral position. Culture was sterile. One and a half week after drainage placement the patient suffered from a sudden massive respiratory decompensation resulting in carbon dioxide narcosis with temporary cardiac arrest. After temporary circulatory stabilization, a renewed clinical deterioration occurred and the patient died after initiation of comfort care.
Discussion
Management of intraabdominal collections by image-guided drainage is mostly efficient and associated with low morbidity (1,6). Drainage of collections in the upper abdomen are more challenging since subcostal access is often limited and intercostal drain placement is correlated with higher rates of pleural complications. To our knowledge, the study of Preece et al. is the most comprehensive study comparing an intercostal to subcostal approach for drainage of subdiaphragmal collection by assessing 258 patients (3). They describe significantly higher rates of pleural complications for the intercostal compared to the subcostal approach (26% vs. 8%) (3). Most of the complications were minor, however, the rate of potential life-threatening empyema was higher in the intercostal group (1.4% vs. 0.5%) (3). Although empyema after intercostal drainage seems rare, the complication is potential lethal and to our knowledge there is no study describing clinical course of this delicate patient population. We present the first series describing the course of ten patients with empyema after intercostal drainage. We further demonstrate similarities and discuss possible risk factors for development of the complication.
Patients with intercostal drainage are at risk for pleural complications since the drain might create a permanent communication between pleural space and subdiaphragmatic collection with increased risk of pleural contamination (3). The costodiaphragmatic recesses run between the neck of the twelfth rib posteriorly and the seventh costal cartilage anteriorly. The pleural lines cross the tenth rib on midaxillary line (7). In an older study from 1984 needle insertion into the ninth ICS of cadavers lead to dissection of the costodiaphragmatic recess in all cases. By anterior insertion between cartilage of the ninth and tenth rib it was possible to insert a needle transdiaphragmally without pleural dissection (8). In the retrospective study of Preece et al., the posterior approach was associated with lowest complication rates (3). Anterolateral drainage had the highest total complication rate and lateral approach the highest rate of serious complications although there were no statistically significant differences (3). In our series the majority of drains were placed in the lateral position. The drains were inserted through the sixth to ninth ICS and therefore likely traversed the pleura according to known physiological anatomy of pleural reflections.
Risk factors for progressive pleural empyema after intercostal drainage are unknown.
There are several commonly known patient-related risk factors for pleural empyema, however not all of them are applicable on our patients since the etiology differs fundamentally from patients with parapneumonic pleural empyema (9) that is the most common cause for pleural empyema. Particularly, factors increasing the risk for pleural empyema due to increased risk of aspiration such as alcoholism, intravenous drug abuse or stroke presumably are not involved in risk of empyema after intercostal drainage (10,11).
In our series four out of ten patients suffered from diabetes. Diabetic patients had higher risk of pleural empyema in a recent study in absence of other comorbidities (12). Hyperglycemic state in diabetes is associated with immune dysfunction and higher infection rates (13,14). Six out of ten patients were diagnosed with malnutrition, a well-known risk factor for any infection including pleural empyema (10,15). Patients with cancer might have an elevated risk for development of pleural empyema (10). In our series, three patients suffered from cancer. Overall, most of the patients in our series had at least one priorly established (10,12-15) patient-related potential risk factor for pleural empyema.
Potential contribution to development of empyema by transdiaphragmatic translocation from intraabdominal infection cannot be excluded (16,17). However, in all of the ten cases described in this series no relevant effusion nor beginning empyema was visible prior to drain placement.
We assumed that a long duration and large size of the drain might be associated with increased risk of pleural contamination. However, there were only few similarities between the cases, duration of drainage varied from four to 27 days and diameter of drains between 8 and 12 French. This is in agreement with the results of Preece et al., reporting similar complication rates for catheter size ranging from 8 to 16 French (3).
The culture of the abdominal drain was positive in eight of ten cases, while most of the pleural culture were sterile. This is most likely the result of the treatment with broad-spectrum antibiotics, which all patients were receiving. The average duration of antibiotic treatment was six weeks. The patients in our series had long duration of hospitalization and half of the patients needed ICU treatment. In several cases thoracic symptoms or increasing inflammatory parameters were observed for several days without change of treatment. The clinical course after decortication was in the majority of cases uneventful with hospital discharge at a mean of two weeks after surgery. This study is limited by the design as case series with low patient number and missing comparison group and therefore has limited generalizability to larger populations of patients.
Our case series describes patient characteristics and illustrates the clinical course of pleural empyema after intercostal drain placement. In our cohort drain insertion above ninth ICS as well as lateral position of the drain was the most common drain position. A potential correlation of those factors with postinterventional empyema should be addressed in further prospective studies. In this series the complication was potentially life-threatening and the patients suffered from protracted treatment. Awareness and early recognition of potential pleural complications after intercostal path of drain placement is essential for prompt and correct treatment of affected patients.
Acknowledgments
The authors thank Deborah Stroka and Adrian Keogh for giving their valuable input on the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-272/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-272/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-272/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Politano AD, Hranjec T, Rosenberger LH, et al. Differences in morbidity and mortality with percutaneous versus open surgical drainage of postoperative intra-abdominal infections: a review of 686 cases. Am Surg 2011;77:862-7. [Crossref] [PubMed]
- Avella DM, Toth JW, Reed MF, et al. Pleural space infections after image-guided percutaneous drainage of infected intraabdominal fluid collections: a retrospective single institution analysis. BMC Surg 2015;15:42. [Crossref] [PubMed]
- Preece SR, Nelson RC, Bashir MR, et al. Safety of an intercostal approach for imaging-guided percutaneous drainage of subdiaphragmatic abscesses. AJR Am J Roentgenol 2014;202:1349-54. [Crossref] [PubMed]
- Semenkovich TR, Olsen MA, Puri V, et al. Current State of Empyema Management. Ann Thorac Surg 2018;105:1589-96. [Crossref] [PubMed]
- Reichert M, Hecker M, Witte B, et al. Stage-directed therapy of pleural empyema. Langenbecks Arch Surg 2017;402:15-26. [Crossref] [PubMed]
- Stan-Ilie M, Plotogea OM, Rinja E, et al. Ultrasound-Guided Percutaneous Drainage of Abdominal Collections—An Analysis over 5 Years. Gastroenterol Insights 2021;12:366-75. [Crossref]
- Bertin F, Deslauriers J. Anatomy of the pleura: reflection lines and recesses. Thorac Surg Clin 2011;21:165-71. vii. [Crossref] [PubMed]
- Nichols DM, Cooperberg PL, Golding RH, et al. The safe intercostal approach? Pleural complications in abdominal interventional radiology. AJR Am J Roentgenol 1984;142:1013-8. [Crossref] [PubMed]
- Chalmers JD, Singanayagam A, Murray MP, et al. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 2009;64:592-7. [Crossref] [PubMed]
- Shen TC, Lin CY, Lin CL, et al. Risk of developing pleural empyema in patients with stroke: a propensity-matched cohort study. Intern Emerg Med 2017;12:1131-8. [Crossref] [PubMed]
- Mandell LA, Niederman MS. Aspiration Pneumonia. N Engl J Med 2019;380:651-63. [Crossref] [PubMed]
- Lai SW, Lin CL, Liao KF. Population-based cohort study investigating the correlation of diabetes mellitus with pleural empyema in adults in Taiwan. Medicine (Baltimore) 2017;96:e7763. [Crossref] [PubMed]
- Berbudi A, Rahmadika N, Tjahjadi AI, et al. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev 2020;16:442-9. [Crossref] [PubMed]
- Daryabor G, Atashzar MR, Kabelitz D, et al. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol 2020;11:1582. [Crossref] [PubMed]
- Bourke CD, Berkley JA, Prendergast AJ. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol 2016;37:386-98. [Crossref] [PubMed]
- AlGhamdi ZM, Boumarah DN, Alshammary S, et al. Pleural Empyema as a Complication of Pyogenic Liver Abscess: Can the Minimum Achieve the Optimal? A Comparison of 3 Approaches. Am J Case Rep 2021;22:e935169. [Crossref] [PubMed]
- Kim DH. Empyema caused by transdiaphragmatic extension of pyogenic liver abscess. Clin Case Rep 2019;7:240-1. [Crossref] [PubMed]


