Small cell lung cancer in young patients: trends in sociodemographic factors, diagnosis, treatment, and survival
Introduction
Lung cancer is the leading cause of cancer death in both men and women (1). Small cell lung cancer (SCLC) is an aggressive malignancy of neuroendocrine origin and accounts for ~14% of all lung cancers. While the pace of mortality reduction for lung cancer has doubled over the past decade with improved treatment and reduced smoking, the prognosis for SCLC remains dismal, with a two-year survival at 15% (2). In patients with limited-stage small cell lung cancer (LS-SCLC), combined modality therapy with chemotherapy and thoracic radiotherapy, with or without prophylactic cranial irradiation (PCI), has improved survival (3,4). For patients with extensive-stage (ES)-SCLC, multidisciplinary care with chemotherapy and immunotherapy, with or without thoracic radiation, provides a survival benefit (5-9).
Lung cancer is primarily a disease of the older population, with about 10% of patients <55 years at diagnosis (10). Several studies have revealed that younger patients with non-small cell lung cancer (NSCLC) represent a distinct clinico-biologic entity (11-20). Compared to older adults, young NSCLC patients are more likely to be women, Asians, or Pacific Islanders, have adenocarcinoma histology, and present with metastases (11-13,16-19). NSCLC patients <50 are significantly more likely to harbor driver mutations suitable for treatment with a growing array of targeted therapies (13,20). In the absence of a targetable mutation, patients under 40 had comparably poor survival as patients 70 years or older, suggesting that the NSCLC in the youngest patients may be particularly biologically aggressive (20).
SCLC in young patients is much less understood, with dated analyses available for review (21-25). Previous studies suggest that SCLC portends poor prognosis, irrespective of age (24,25). This retrospective review aimed to evaluate SCLC patient demographics, treatment patterns, and survival rates by age to expand understanding of the younger subset and the factors that impact their disease course. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-210/rc).
Methods
Data source
The National Cancer Database (NCDB) is a hospital-based nationwide clinical oncology database jointly operated by the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society. Over 1,500 CoC-accredited facilities report new cancer cases to the NCDB, representing >70% of all newly diagnosed cancer cases in the United States (26).
Patient selection
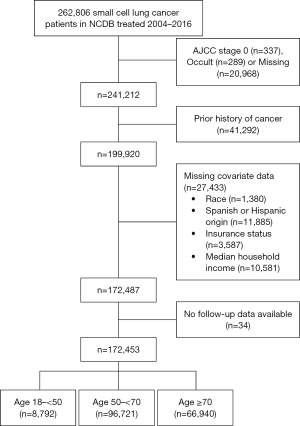
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The NCDB was queried for patients with a diagnosis of SCLC between 2004–2016. Using the International Classification of Diseases for Oncology (third edition), 262,806 patients were preliminarily identified. Patients were excluded if they had AJCC stage 0 disease, missing clinical stage information, prior history of another cancer, or missing data on race, ethnicity, insurance status, or median household income. After excluding patients without follow-up data, a total of 172,453 patients were available for analysis (Figure 1).
Study variables
The patients were divided into three age categories: ≥18–<50, ≥50–<70, and ≥70 years. We defined patients as “young” if they were <50 years old. The following additional demographic variables were collected from the database: gender, race (White, Black, or Other), ethnicity (Hispanic or non-Hispanic origin), insurance status (private, Medicare, Medicaid, other government insurance or uninsured), median household income (<$30,000, $30,000–$34,999, $35,000–$45,999 or $46,000+), and Charlson-Deyo Comorbidity Score (CCS). The year of diagnosis (2004–2010, 2011–2016) and AJCC stage (I–IV) were obtained for disease characteristics.
For treatment-related factors, we analyzed initial treatment received, treatment at more than one facility, and receipt of guideline-concordant care (GCC). Treatment subtypes included chemotherapy, radiotherapy, chemoradiotherapy, surgery, surgery with chemotherapy, surgery with radiation, and surgery with chemoradiotherapy. For stages I–III, GCC was defined as either surgery and chemotherapy, chemotherapy and radiation, or surgery, chemotherapy, and radiation (3,4,27,28). For stage IV disease, GCC was defined as either chemotherapy, surgery plus chemotherapy, chemotherapy and radiation, or a combination of chemotherapy, surgery, and radiation (5-9). Of our study population, 34,163 patients either had missing information on treatment status, received treatment other than the mentioned modalities, or had no treatment. These patients were excluded in analyses of clinicopathologic characteristics, GCC receipt, and survival.
Statistical analysis
The Chi-square and Kruskal-Wallis tests were used to examine differences in categorical and continuous variables, respectively. The primary outcome of interest was overall survival (OS) determined by vital status (deceased, alive) and the number of months from diagnosis to the time of last contact or death. Median OS and 1- and 3-year survival rates were estimated using the Kaplan-Meier method, and the log-rank test was used to determine statistical significance. Cox proportional hazards regression modeling was used to compute crude and adjusted hazard ratios (HR) with 95% CI. Statistical computations were performed on SAS 9.4 system (SAS Institute, Cary, NC, USA). All tests were two-sided, and a P value of <0.05 was considered statistically significant.
Results
Patient population
Full clinicopathologic characteristics are described in Table 1. There were 8,792 (5.1%) patients between the ages of ≥18–<50, 96,721 (56.1%) patients between ≥50–<70, and 66,940 (38.8%) patients ≥70. The median age of the study population was 66 (IQR: 59–74) years, with more females (n=88,173; 51.1%) than males. Most patients were White (n=155,649; 90.3%), non-Hispanic (n=168,280; 97.6%), on government insurance (n=115,720; 67.1%), and had no comorbidities (n=94,100; 54.6%). Over 64% of patients (n=110,799) presented with stage IV. Patients were rarely treated at more than one facility (n=18,496; 10.7%). Sixty-eight percent of all patients (n=117,231) received GCC.
Table 1
| Variables | All patients, N=172,453 | Age, years | P* | ||
|---|---|---|---|---|---|
| ≥18–<50, N=8,792 | ≥50–<70, N=96,721 | ≥70, N=66,940 | |||
| Age (years), median [IQR] | 66 [59–74] | 47 [44–48] | 62 [57–66] | 76 [72–80] | <0.0001 |
| Gender, n (%) | <0.0001 | ||||
| Males | 84,280 (48.9) | 4,349 (49.5) | 48,037 (49.7) | 31,894 (47.7) | |
| Females | 88,173 (51.1) | 4,443 (50.5) | 48,684 (50.3) | 35,046 (52.4) | |
| Race, n (%) | <0.0001 | ||||
| White | 155,649 (90.3) | 7,822 (89.0) | 86,854 (89.8) | 60,973 (91.1) | |
| Black | 13,670 (7.9) | 830 (9.4) | 8,257 (8.5) | 4,583 (6.9) | |
| Other | 3,134 (1.8) | 140 (1.6) | 1,610 (1.7) | 1,384 (2.1) | |
| Ethnicity, n (%) | 0.438 | ||||
| Non-Hispanic | 168,280 (97.6) | 8,579 (97.6) | 94,420 (97.6) | 65,281 (97.5) | |
| Hispanic | 4,173 (2.4) | 213 (2.4) | 2,301 (2.4) | 1,659 (2.5) | |
| Insurance status, n (%) | <0.0001 | ||||
| Private | 49,341 (28.6) | 4,342 (49.4) | 38,672 (40.0) | 6,327 (9.5) | |
| Medicare | 98,300 (57.0) | 836 (9.5) | 38,722 (40.0) | 58,742 (87.8) | |
| Medicaid | 14,551 (8.4) | 2,327 (26.5) | 11,325 (11.7) | 899 (1.3) | |
| Other government insurance | 2,869 (1.7) | 104 (1.2) | 2,123 (2.2) | 642 (0.96) | |
| Uninsured | 7,392 (4.3) | 1,183 (13.5) | 5,879 (6.1) | 330 (0.49) | |
| Median household income, n (%) | <0.0001 | ||||
| <$30,000 | 27,733 (16.1) | 1,634 (18.6) | 16,701 (17.3) | 9,398 (14.0) | |
| $30,000–$34,999 | 36,781 (21.3) | 1,980 (22.5) | 21,189 (21.9) | 13,612 (20.3) | |
| $35,000–$45,999 | 51,716 (30.0) | 2,707 (30.8) | 29,012 (30.0) | 19,997 (29.9) | |
| $46,000+ | 56,223 (32.6) | 2,471 (28.1) | 29,819 (30.8) | 23,933 (35.8) | |
| Charlson-Deyo comorbidity score, n (%) | <0.0001 | ||||
| 0 | 94,100 (54.6) | 6,126 (69.7) | 54,749 (56.6) | 33,225 (49.6) | |
| 1 | 50,665 (29.4) | 2,028 (23.1) | 27,989 (28.9) | 20,648 (30.9) | |
| 2 or more | 27,688 (16.1) | 638 (7.3) | 13,983 (14.5) | 13,067 (19.5) | |
| Year of diagnosis, n (%) | <0.0001 | ||||
| 2004–2010 | 86,167 (50.0) | 5,376 (61.2) | 47,491 (49.1) | 33,300 (49.8) | |
| 2011–2016 | 86,286 (50.0) | 3,416 (38.9) | 49,230 (50.9) | 33640 (50.3) | |
| AJCC stage, n (%) | <0.0001 | ||||
| I | 8,096 (4.7) | 291 (3.3) | 3,894 (4.0) | 3,911 (5.8) | |
| II | 6,560 (3.8) | 306 (3.5) | 3,500 (3.6) | 2,754 (4.1) | |
| III | 46,998 (27.3) | 2,624 (29.8) | 26,896 (27.8) | 17,478 (26.1) | |
| IV | 110,799 (64.3) | 5,571 (63.4) | 62,431 (64.5) | 42,797 (63.9) | |
| Treatment at >1 facility, n (%) | <0.0001 | ||||
| No | 153,957 (89.3) | 7,688 (87.4) | 85,448 (88.3) | 60,821 (90.9) | |
| Yes | 18,496 (10.7) | 1,104 (12.6) | 11,273 (11.7) | 6,119 (9.1) | |
| GCC*, n (%) | |||||
| GCC | 117,231 (68.0) | 7,256 (82.5) | 72,445 (74.9) | 37,530 (56.1) | <0.0001 |
| Non-GCC | 21,059 (12.2) | 710 (8.1) | 10,144 (10.5) | 10,205 (15.2) | |
| Missing/no/other treatment† | 34,163 (19.8) | 826 (9.4) | 14,132 (14.6) | 19,205 (28.7) | |
*, missing/no/other treatments were excluded from the P value calculation; †, includes 13,817 patients from 2004–2009 with missing information on treatment status, 14,124 patients from 2010–2016 with no treatment, 4,679 patients from 2010–2016 with treatment other than chemotherapy, radiation, and surgery, 130 patients from 2010–2016 on active surveillance and 1,413 patients from 2010–2016 with missing information on treatment. NCDB, National Cancer Database; AJCC, American Joint Committee on Cancer; GCC, guideline-concordant care.
Cancer incidence trends
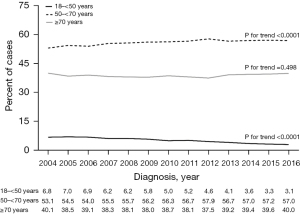
For ages ≥18–<50, a significant decrease in percent of cases each year was observed, P for trend <0.0001 (Figure 2). In contrast, there was a significant increase in total cases between 2004–2016 among patients aged ≥50–<70, from 53.1% in 2004 to 57% in 2016 (P for trend <0.0001). Cases among patients ≥70 remained steady without significant changes in trend.
Sociodemographic and clinical characteristics by age
There were more female patients than males across all age groups (P<0.0001). The youngest group of patients were more likely to be Black compared to ages ≥50–<70 and ages ≥70 (9.4% vs. 8.5% vs. 6.9%, P<0.0001) and healthier by CCS with a score of zero (69.7% vs. 56.6% vs. 49.6%, P<0.0001). The younger age group was more likely to be on Medicaid (26.5% vs. 11.7% vs. 1.3%, P<0.0001) or uninsured (13.5% vs. 6.1% vs. 0.49%, P<0.0001). They were also less likely to have a median household income greater than $46,000 (28.1% vs. 30.8% vs. 35.8%, P<0.0001). The majority (93.2%) of patients aged ≥18–<50 presented with stage III–IV disease, compared to 92.4% for ages ≥50–<70, and 90.0% in patients ≥70 (P<0.0001) (Table 1).
Treatment by age and stage
A greater proportion of older patients (28.7% in ages ≥70; 14.6% in ages ≥50–<70) were missing information on treatment, received no treatment, or treatment other than chemotherapy, radiation, or surgery, when compared to patients ≥18–<50 (9.4%) (Table 1). Patients ≥18–<50 years old were more likely to receive GCC (82.5% vs. 74.9% vs. 56.1%, P<0.0001) (Table 1), and this trend held for all stages of disease (Table 2). Regardless of age, patients with stage IV disease (91.2%) were more likely to receive GCC when compared to the patients with stage I (68.1%), II (77.4%), or III (74.9%) disease (Table 2).
Table 2
| Stage | All patients | Age, years | P | ||
|---|---|---|---|---|---|
| ≥18–<50 | ≥50–<70 | ≥70 | |||
| Stage I | N=6,875 | N=260 | N=3,446 | N=3,169 | <0.0001 |
| GCC | 4,681 (68.1) | 216 (83.1) | 2,576 (74.8) | 1,889 (59.6) | |
| Non-GCC | 2,194 (31.9) | 44 (16.9) | 870 (25.3) | 1,280 (40.4) | |
| Stage II | N=5,650 | N=284 | N=3,167 | N=2,199 | <0.0001 |
| GCC | 4,370 (77.4) | 243 (85.6) | 2,615 (82.6) | 1,512 (68.8) | |
| Non-GCC | 1,280 (22.7) | 41 (14.4) | 552 (17.4) | 687 (31.2) | |
| Stage III | N=40,086 | N=2,454 | N=24,129 | N=13,503 | <0.0001 |
| GCC | 30,024 (74.9) | 2,076 (84.6) | 19,256 (79.8) | 8,692 (64.4) | |
| Non-GCC | 10,062 (25.1) | 378 (15.4) | 4,873 (20.2) | 4,811 (35.6) | |
| Stage IV | N=85,679 | N=4,968 | N=51,847 | N=28,864 | <0.0001 |
| GCC | 78,156 (91.2) | 4,721 (95.0) | 47,998 (92.6) | 25,437 (88.1) | |
| Non-GCC | 7,523 (8.8) | 247 (5.0) | 3,849 (7.4) | 3,427 (11.9) | |
Data is presented as n (%). Patients with missing/no/other treatment were excluded from the analysis. NCDB, National Cancer Database; AJCC, American Joint Committee on Cancer; GCC, guideline-concordant care.
Overall survival
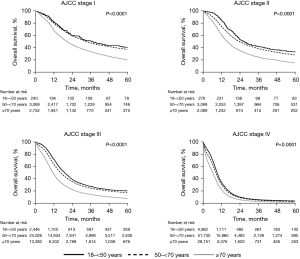
The median OS for the study population was 10.1 months, with a one-year survival rate of 41.8% and a three-year survival rate of 12.8% (Table 3). The median OS of patients between ages ≥18–<50 was 12 months compared to 10.8 months in patients ages ≥50–<70 and 8.5 months in patients ≥70 (P<0.0001). The survival rate at every disease stage was significantly lower with increasing age (Figure 3). The most significant difference in median OS between the youngest and oldest age group was observed in stage I disease (17.8 months), whereas the difference in OS between the same two groups was only 2.9 months in patients with stage IV disease (Table 3).
Table 3
| N | Events | Median survival (months) | Survival rate | Multivariate model* | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1-year | 3-year | P | Hazard ratio (95% CI) | P | |||||
| Age group | |||||||||
| All patients | 138,290 | 124,130 | 10.1 | 41.8% | 12.8% | ||||
| ≥18–<50 | 7,966 | 6,974 | 12.0 | 49.9% | 15.7% | <0.0001 | Ref | ||
| ≥50–<70 | 82,589 | 73,037 | 10.8 | 44.9% | 14.3% | 1.10 (1.07–1.13) | <0.0001 | ||
| ≥70 | 47,735 | 44,119 | 8.5 | 34.9% | 9.7% | 1.36 (1.32–1.39) | <0.0001 | ||
| Stage I | |||||||||
| ≥18–<50 | 260 | 156 | 39.7 | 82.0% | 52.7% | <0.0001 | Ref | ||
| ≥50–<70 | 3,446 | 2,273 | 34.5 | 80.5% | 48.7% | 1.19 (1.0–1.40) | 0.045 | ||
| ≥70 | 3,169 | 2,530 | 21.9 | 70.0% | 35.6% | 1.66 (1.40–1.97) | <0.0001 | ||
| Stage II | |||||||||
| ≥18–<50 | 284 | 187 | 27.5 | 85.7% | 43.5% | <0.0001 | Ref | ||
| ≥50–<70 | 3,167 | 2,301 | 23.9 | 75.3% | 38.7% | 1.32 (1.13–1.53) | 0.001 | ||
| ≥70 | 2,199 | 1,848 | 15.9 | 61.2% | 24.6% | 1.76 (1.50–2.07) | <0.0001 | ||
| Stage III | |||||||||
| ≥18–<50 | 2,454 | 1,962 | 19.2 | 71.9% | 27.6% | <0.0001 | Ref | ||
| ≥50–<70 | 24,129 | 19,865 | 16.5 | 63.8% | 25.0% | 1.14 (1.09–1.20) | <0.0001 | ||
| ≥70 | 13,503 | 12,180 | 11.4 | 47.6% | 14.7% | 1.45 (1.38–1.53) | <0.0001 | ||
| Stage IV | |||||||||
| ≥18–<50 | 4,968 | 4,669 | 9.4 | 35.3% | 6.3% | <0.0001 | Ref | ||
| ≥50–<70 | 51,847 | 48,598 | 8.5 | 31.8% | 5.4% | 1.06 (1.03–1.10) | 0.002 | ||
| ≥70 | 28,864 | 27,561 | 6.5 | 23.1% | 3.4% | 1.28 (1.24–1.33) | <0.0001 | ||
Patients with missing/no/other treatment were excluded from the analysis. *, multivariate model includes gender, race, Hispanic origin, insurance status, median household income, Charlson-Deyo comorbidity, year of diagnosis, treatment at >1 facility, GCC, and AJCC stage (except for analysis by AJCC stage). AJCC, American Joint Committee on Cancer; NCDB, National cancer database; GCC, guideline-concordant care.
Multivariate survival analysis
The results from multivariate survival analyses for each age group are presented in Table 4. After adjusting for patient and clinicopathologic characteristics, female patients, Hispanic ethnicity, later diagnosis in years 2011–2016, and treatment at >1 facility were associated with improved survival in all age groups. Blacks and Others, when compared to Whites, had improved survival across all ages. Increasing age was associated with worse outcomes in the ≥50–<70 and ≥70 age groups but not ≥18–<50 (HR: 1.01; 95% CI: 1.0–1.01, P=0.110). While no significant difference in survival was noted between stages I–III disease among patients aged ≥18–<50 (HR: 1.16; 95% CI: 0.94–1.44, P=0.170), stages III–IV were associated with worst survival for all three age groups.
Table 4
| Variables | ≥18–<50 years (N=7,966) | ≥50–<70 years (N=82,589) | ≥70 years (N=47,735) | |||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |||
| Age | 1.01 (1.0–1.01) | 0.110 | 1.01 (1.01–1.01) | <0.0001 | 1.02 (1.02–1.02) | <0.0001 | ||
| Gender | ||||||||
| Males | Ref | Ref | Ref | |||||
| Females | 0.79 (0.75–0.83) | <0.0001 | 0.84 (0.83–0.85) | <0.0001 | 0.86 (0.84–0.87) | <0.0001 | ||
| Race | ||||||||
| White | Ref | Ref | Ref | |||||
| Black | 0.88 (0.81–0.96) | 0.002 | 0.91 (0.88–0.93) | <0.0001 | 0.89 (0.85–0.92) | <0.0001 | ||
| Other | 0.76 (0.63–0.93) | 0.007 | 0.85 (0.80–0.90) | <0.0001 | 0.85 (0.80–0.91) | <0.0001 | ||
| Ethnicity | ||||||||
| Non-Hispanic | Ref | Ref | Ref | |||||
| Hispanic | 0.85 (0.72–1.0) | 0.047 | 0.81 (0.77–0.85) | <0.0001 | 0.84 (0.78–0.89) | <0.0001 | ||
| Insurance status | ||||||||
| Private | Ref | Ref | Ref | |||||
| Medicare | 1.41 (1.30–1.54) | <0.0001 | 1.14 (1.12–1.16) | <0.0001 | 1.0 (0.97–1.03) | 0.813 | ||
| Medicaid | 1.22 (1.15–1.29) | <0.0001 | 1.15 (1.13–1.18) | <0.0001 | 0.94 (0.86–1.03) | 0.206 | ||
| Other Government insurance | 1.14 (0.91–1.43) | 0.257 | 1.14 (1.09–1.20) | <0.0001 | 0.93 (0.85–1.03) | 0.169 | ||
| Uninsured | 1.26 (1.17–1.36) | <0.0001 | 1.18 (1.14–1.21) | <0.0001 | 1.15 (0.98–1.34) | 0.084 | ||
| Median household income | ||||||||
| <$30,000 | Ref | Ref | Ref | |||||
| $30,000–$34,999 | 0.92 (0.85–0.99) | 0.023 | 0.99 (0.96–1.01) | 0.304 | 0.98 (0.95–1.01) | 0.185 | ||
| $35,000–$45,999 | 0.91 (0.85–0.98) | 0.013 | 0.96 (0.94–0.99) | 0.001 | 0.99 (0.96–1.02) | 0.367 | ||
| $46,000+ | 0.91 (0.85–0.98) | 0.014 | 0.93 (0.91–0.96) | <0.0001 | 0.94 (0.91–0.97) | 0.0001 | ||
| Charlson-Deyo comorbidity score | ||||||||
| 0 | Ref | Ref | Ref | |||||
| 1 | 1.09 (1.03–1.15) | 0.005 | 1.12 (1.10–1.14) | <0.0001 | 1.15 (1.12–1.17) | <0.0001 | ||
| 2 or more | 1.40 (1.27–1.53) | <0.0001 | 1.29 (1.26–1.32) | <0.0001 | 1.29 (1.26–1.32) | <0.0001 | ||
| Year of diagnosis | ||||||||
| 2004–2010 | Ref | Ref | Ref | |||||
| 2011–2016 | 0.90 (0.85–0.94) | <0.0001 | 0.90 (0.89–0.91) | <0.0001 | 0.89 (0.87–0.91) | <0.0001 | ||
| AJCC stage | ||||||||
| I | Ref | Ref | Ref | |||||
| II | 1.16 (0.94–1.44) | 0.170 | 1.42 (1.34–1.50) | <0.0001 | 1.46 (1.37–1.55) | <0.0001 | ||
| III | 1.84 (1.56–2.17) | <0.0001 | 1.96 (1.88–2.05) | <0.0001 | 1.98 (1.89–2.06) | <0.0001 | ||
| IV | 4.89 (4.16–5.75) | <0.0001 | 4.82 (4.61–5.03) | <0.0001 | 4.47 (4.28–4.67) | <0.0001 | ||
| Treatment at >1 facility | ||||||||
| No | Ref | Ref | Ref | |||||
| Yes | 0.82 (0.77–0.88) | <0.0001 | 0.76 (0.74–0.78) | <0.0001 | 0.77 (0.74–0.79) | <0.0001 | ||
| GCC | ||||||||
| GCC | Ref | Ref | Ref | |||||
| Non-GCC | 1.96 (1.80–2.14) | <0.0001 | 2.06 (2.01–2.10) | <0.0001 | 2.02 (1.97–2.08) | <0.0001 | ||
Patients with missing/no/other treatment were excluded from the analysis. CI, confidence interval; AJCC, American Joint Committee on Cancer; GCC, guideline-concordant care.
In patients ≥18–<50 years old, increasing income was associated with improved survival, whereas having medicare (HR: 1.41; 95% CI: 1.30–1.54, P<0.0001), medicaid (HR: 1.22; 95% CI: 1.15–1.29, P<0.0001), or no insurance (HR: 1.26; 95% CI: 1.17–1.36, P<0.0001) was associated with worse survival (Table 4). In patients ≥50–<70, non-private insurance and lack of insurance were associated with worse survival, while in patients ≥70, insurance status did not have a clinically meaningful impact on the outcome as a majority (87.8%) had Medicare, who had comparable survival to privately insured patients (HR: 1.0; 95% CI: 0.97–1.03, P=0.813).
Compared to patients who received GCC, those who received non-GCC had significantly worse survival across all age groups (ages ≥18–<50, HR: 1.96, P<0.0001; ages ≥50–<70, HR: 2.06, P<0.0001; ages ≥70, HR: 2.02, P<0.0001; Table 4). The association of non-GCC with worse survival was applicable for all disease stages (stage I, HR: 1.39, P<0.0001; stage II, HR: 1.91, P<0.0001; stage III, HR: 1.98, P<0.0001; stage IV, HR: 2.44, P<0.0001; Table 5). When stratified by age group, similar results were observed except younger patients with stage I disease (HR: 1.09, P=0.716). Complete results are presented in Table 5.
Table 5
| GCC | Overall | ≥18–<50 years | ≥50–<70 years | ≥70 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||||
| Stage I | |||||||||||
| GCC | Ref | Ref | Ref | Ref | |||||||
| Non-GCC | 1.39 (1.31–1.47) | <0.0001 | 1.09 (0.70–1.69) | 0.716 | 1.47 (1.34–1.61) | <0.0001 | 1.32 (1.22–1.44) | <0.0001 | |||
| Stage II | |||||||||||
| GCC | Ref | Ref | Ref | Ref | |||||||
| Non-GCC | 1.91 (1.78–2.04) | <0.0001 | 1.56 (1.05–2.33) | 0.029 | 1.86 (1.67–2.06) | <0.0001 | 2.01 (1.82–2.23) | <0.0001 | |||
| Stage III | |||||||||||
| GCC | Ref | Ref | Ref | Ref | |||||||
| Non-GCC | 1.98 (1.93–2.03) | <0.0001 | 1.75 (1.55–1.97) | <0.0001 | 2.05 (1.98–2.12) | <0.0001 | 1.89 (1.81–1.96) | <0.0001 | |||
| Stage IV | |||||||||||
| GCC | Ref | Ref | Ref | Ref | |||||||
| Non-GCC | 2.44 (2.38–2.50) | <0.0001 | 2.73 (2.39–3.11) | <0.0001 | 2.32 (2.25–2.40) | <0.0001 | 2.49 (2.40–2.58) | <0.0001 | |||
Patients with missing/no/other treatment were excluded from the analysis. CI, confidence interval; GCC, guideline-concordant care; AJCC, American Joint Committee on Cancer.
Discussion
Young patients with SCLC represent a distinct sociodemographic and clinical entity. The existing data on young individuals with SCLC are limited to older data registry or single-institution retrospective studies. In a prospective study of 3,560 new patients with lung cancer by the Edinburgh Lung Cancer Group between 1981–1986, only 48 patients were <45 years old, of whom 16 had SCLC. The median survival was eight months among those young patients with SCLC (21). A retrospective review of 174 patients with LS-SCLC between 1991–1999 revealed that 32% were <65 years old; younger patients were more likely to receive chemoradiotherapy (CRT), intensive chemotherapy, and PCI (23). With advancing age, survival rates were significantly lower, with a median survival of 17, 12, and 7 months among patients <65, 65–74, and 75 or older (23). In a 2017 analysis of 22,863 SCLC patients diagnosed between 1998–2012 in the California Cancer Registry, 975 (4.2%) were <50 years of age (29). Age <50 years was associated with significantly better cause specific survival (CSS) than those ≥50. Among those <50 years, female sex, rural residence, and Asian/Pacific Islander race were associated with significantly improved CSS while advanced stage at diagnosis was associated with worse CSS (29). Other single-institution retrospective studies of SCLC patients under the age of 40 found a higher proportion of never-smokers (52%) with limited-stage (67% vs. 33% ES-SCLC) disease at time of diagnosis (22), and prognosis was significantly worse compared to those presenting with adenocarcinoma of any stage (13). To our knowledge, we provide the largest and most contemporaneous study to date examining the characteristics and outcomes of 172,453 patients with SCLC, of which 8,792 patients were between the ages of 18 and 49.
Since 2004, the number of young patients annually diagnosed with SCLC has been declining, which largely correlates to the decreasing tobacco use rates among youths and adults (30). Interestingly, our analysis showed that SCLC among patients 50 to 69 years has steadily increased. This rising incidence may be related to stricter enforcement of lung cancer screening, which the U.S. Preventive Services Task Force first recommended in 2013 (31).
Across all age groups, we found that SCLC patients were more likely to be non-Hispanic, White females. Men had worse outcomes than women, a finding consistent with prior publications of SCLC patients (29,32).
There was a higher percentage of Black patients among those <50 years old. This is consistent with previous reports that the age-adjusted incidence rates of lung cancer among individuals between 45–54 years were significantly higher in Blacks vs. Whites (14.2% vs. 8.2%) (33). Black individuals are more likely to start smoking later in life, and those diagnosed with lung cancer are more likely to be intermittent or light smokers than Caucasians (34,35). Although Black youths and young adults have a significantly lower prevalence of cigarette smoking than Caucasians or Hispanics, they are more likely to be exposed to second-hand smoke than any other racial or ethnic group (36,37). Such observed racial disparity in SCLC incidence in young patients warrants further investigation of inherent host and modifiable risk factors.
In our cohort, patients under 50 years had the highest percentage on Medicaid or were uninsured compared to the older age groups. In previous studies, Medicaid and Medicare coverage were independently associated with shorter survival in patients with SCLC, and Medicaid coverage did not provide a survival advantage compared to those who were uninsured (38,39). Consistent with the aforementioned data, Medicare, Medicaid, or uninsured status were significantly associated with worse survival in young patients.
Young patients were more likely to have lower median household incomes. Our finding that lower income was associated with worse survival is supported by existing literature on lung cancer (40-42). In a systematic review and meta-analysis by Forrest et al. (43), patients in more socioeconomically deprived circumstances were less likely to receive any treatment, surgery, or chemotherapy than the least deprived groups. In another NCDB cohort study of 69,168 patients with stage I NSCLC, patients had increased odds of receiving no therapy or non-standard treatments with an increasing number of socioeconomic status risk factors (44).
While stage III or IV disease was the most common presentation among all ages, younger patients had a higher proportion of advanced disease than the older groups. This is contradictory to one single institution study from China of 103 patients <40 (22). Only 33% presented with extensive stage. The difference may be attributable to environmental, geographic, and behavioral health factors that may not be captured within the scope of either study and the small size of the Chinese study.
Younger age was associated with improved OS at all stages, but the survival benefit was most appreciable than patients older than 70 years in stage I–IIII disease. For example, the median survival in Stage I patients <50 was 37.1 months compared to 20.9 months in the oldest group (≥70). However, when presenting with stage IV disease, the difference in median survival between the youngest and oldest group was only 2.9 months, despite 95% of patients <50 receiving GCC compared to 88.1% in the latter. This speaks to the aggressive clinical course of SCLC, regardless of age, especially when presenting with advanced-stage disease.
Though there is a paucity of data on GCC for patients with SCLC, several studies on NSCLC have demonstrated a significant survival benefit in older patients and patients with either node-positive or locally advanced and unresectable disease with the receipt of GCC (45-47). Presumably, this principle may apply to younger patients and patients with SCLC. Notably, 68% of patients in our cohort received GCC, which was associated with improved overall survival across all ages and stages of the disease. Across all age groups, patients with stage IV disease were more likely to receive GCC when compared to those with stage I–IIII disease.
The highest rates of GCC were observed among patients under 50 years old (82.5%), and the lowest rates were observed among the oldest group (56.1%), consistent with an NCDB cohort study which reported lower odds of receiving GCC with advancing age (48). The reasons why older patients did not receive GCC are likely multifactorial, including factors such as performance status, personal goals of care, and social support that are not captured by the NCDB. A higher proportion of the older patients had a CCS of 2 or more (19.5% in age ≥70 years vs. 7.3% in ages 18–59 years, P<0.0001), which may have contributed to decisions regarding treatment in this study. Our group previously reported that in patients with ES-SCLC, older age (≥80 years) and higher comorbidity scores (CCS score ≥2) were two of several factors associated with lower odds of receiving chemotherapy (49). A study of 4,142 patients with SCLC from the Netherlands Cancer Registry in Eindhoven examined the trends in comorbidities among SCLC patients (50). The study found that SCLC patients with two or more concomitant diseases increased from 23% in 1995–1998 to 51% in 2011–2012, and the prevalence of multimorbidity increased with age (50). In patients with LS-SCLC, increasing comorbidities were associated with worse survival, independent of treatment received. In those with ES-SCLC, survival was worse for patients with multiple comorbidities, but this effect disappeared when adjusted for the type of treatment received (50). Our study found increasing comorbidities to be a negative prognostic factor for survival as well. Perhaps the development of less toxic treatments could improve our ability to offer GCC, regardless of patient age and comorbidities.
To our knowledge, we present the results of the largest retrospective analysis of SCLC in young patients. This study has several limitations. NCDB is a retrospective database that lacks longitudinal treatment data and does not capture progression-free survival, patient-reported complications or outcomes, overall response rate, or cause of death. The NCDB does not capture several other potentially important patient and treatment attributes. These include, but are not limited to, performance status, tobacco dependence, fertility issues, patient’s social history or cultural background that might impact attitude towards disease and treatment, compliance, clinical trial participation, and treatment delays. While 262,806 patients who were diagnosed with SCLC between 2004–2016 were initially identified, 90,353 (34.4%) were excluded from analysis due to missing key data, which may result in unpredictable bias. An additional 38,163 excluded from survival and treatment analyses as well. Additionally, the generalizability of the data is limited since NCDB is hospital-based rather than population-based.
Conclusions
Our study suggests that younger patients with SCLC may be an economically disadvantaged group needing expedient multidisciplinary care, given a typical presentation with advanced disease. Despite being healthier than the older age groups and being offered GCC, survival in young patients remains dismal, especially at the more advanced stages. Further investigations are warranted to determine patient- and treatment-related factors to achieve health equity and improve outcomes among young patients with SCLC. An examination of the genomic alterations in SCLC and how they pertain to age may facilitate our understanding of disease tempo, treatment response and resistance. Updated data, including patients treated with chemoimmunotherapy as front-line therapy for ES-SCLC, will provide valuable insight into survival. Finally, additional efforts to bridge the gaps in health produced by insurance or income status must be made within the U.S. healthcare system as a whole.
Acknowledgments
We thank the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society for providing access to the National Cancer Database (NCDB) as a resource for de-identified, high-quality, oncologic data. The CoC and the American Cancer Society have not verified the statistical analyses and conclusions drawn from the authors of this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-210/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-210/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-210/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505-27. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [Crossref] [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Agra Y, Pelayo M, Sacristan M, et al. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev 2003;CD001990. [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. [Crossref] [PubMed]
- de Groot PM, Wu CC, Carter BW, et al. The epidemiology of lung cancer. Transl Lung Cancer Res 2018;7:220-33. [Crossref] [PubMed]
- Kuo CW, Chen YM, Chao JY, et al. Non-small cell lung cancer in very young and very old patients. Chest 2000;117:354-7. [Crossref] [PubMed]
- Rich AL, Khakwani A, Free CM, et al. Non-small cell lung cancer in young adults: presentation and survival in the English National Lung Cancer Audit. QJM 2015;108:891-7. [Crossref] [PubMed]
- Liu B, Quan X, Xu C, et al. Lung cancer in young adults aged 35 years or younger: A full-scale analysis and review. J Cancer 2019;10:3553-9. [Crossref] [PubMed]
- Garrana SH, Dagogo-Jack I, Cobb R, et al. Clinical and Imaging Features of Non-Small-Cell Lung Cancer in Young Patients. Clin Lung Cancer 2021;22:23-31. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Rosen JE, et al. Lung Cancer in the Very Young: Treatment and Survival in the National Cancer Data Base. J Thorac Oncol 2016;11:1121-31. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Mauri D, Pentheroudakis G, Bafaloukos D, et al. Non-small cell lung cancer in the young: a retrospective analysis of diagnosis, management and outcome data. Anticancer Res 2006;26:3175-81. [PubMed]
- Jin X, Zhao X, Liu X, et al. Non-Small Cell Lung Cancer in Young Patients: An Analysis of Clinical, Pathologic and TNM Stage Characteristics Compared to the Elderly. Risk Manag Healthc Policy 2020;13:1301-7. [Crossref] [PubMed]
- Thomas A, Chen Y, Yu T, et al. Trends and Characteristics of Young Non-Small Cell Lung Cancer Patients in the United States. Front Oncol 2015;5:113. [Crossref] [PubMed]
- Sacher AG, Dahlberg SE, Heng J, et al. Association Between Younger Age and Targetable Genomic Alterations and Prognosis in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:313-20. [Crossref] [PubMed]
- Capewell S, Wathen CG, Sankaran R, et al. Lung cancer in young patients. Respir Med 1992;86:499-502. [Crossref] [PubMed]
- Jiang S, Hao X, Li J, et al. Small cell lung cancer in the young: Characteristics, diagnosis and outcome data. Clin Respir J 2019;13:98-104. [Crossref] [PubMed]
- Ludbrook JJ, Truong PT, MacNeil MV, et al. Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? a community-based population analysis. Int J Radiat Oncol Biol Phys 2003;55:1321-30. [Crossref] [PubMed]
- Brueckl WM, Herbst L, Lechler A, et al. Predictive and prognostic factors in small cell lung carcinoma (SCLC)--analysis from routine clinical practice. Anticancer Res 2006;26:4825-32. [PubMed]
- Gaspar LE, McNamara EJ, Gay EG, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clin Lung Cancer 2012;13:115-22. [Crossref] [PubMed]
- Mallin K, Browner A, Palis B, et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012-2014. Ann Surg Oncol 2019;26:1604-12. [Crossref] [PubMed]
- Moreno AC, Lin SH. The optimal treatment approaches for stage I small cell lung cancer. Transl Lung Cancer Res 2019;8:88-96. [Crossref] [PubMed]
- Higgins KA, Gorgens S, Sudmeier LJ, et al. Recent developments in limited stage small cell lung cancer. Transl Lung Cancer Res 2019;8:S147-52. [Crossref] [PubMed]
- Lara JD, Brunson A, Riess JW, et al. Clinical predictors of survival in young patients with small cell lung cancer: Results from the California Cancer Registry. Lung Cancer 2017;112:165-8. [Crossref] [PubMed]
- Meza R, Jimenez-Mendoza E, Levy DT. Trends in Tobacco Use Among Adolescents by Grade, Sex, and Race, 1991-2019. JAMA Netw Open 2020;3:e2027465. [Crossref] [PubMed]
- Jonas DE, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021;325:971-87. [Crossref] [PubMed]
- Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 2017;7:1339. [Crossref] [PubMed]
- Annangi S, Nutalapati S, Foreman MG, et al. Potential Racial Disparities Using Current Lung Cancer Screening Guidelines. J Racial Ethn Health Disparities 2019;6:22-6. [Crossref] [PubMed]
- Holford TR, Levy DT, Meza R. Comparison of Smoking History Patterns Among African American and White Cohorts in the United States Born 1890 to 1990. Nicotine Tob Res 2016;18:S16-29. [Crossref] [PubMed]
- Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med 2006;354:333-42. [Crossref] [PubMed]
- Tsai J, Homa DM, Gentzke AS, et al. Exposure to Secondhand Smoke Among Nonsmokers - United States, 1988-2014. MMWR Morb Mortal Wkly Rep 2018;67:1342-6. [Crossref] [PubMed]
- Arrazola RA, Singh T, Corey CG, et al. Tobacco use among middle and high school students - United States, 2011-2014. MMWR Morb Mortal Wkly Rep 2015;64:381-5. [PubMed]
- Pezzi TA, Schwartz DL, Mohamed ASR, et al. Barriers to Combined-Modality Therapy for Limited-Stage Small Cell Lung Cancer. JAMA Oncol 2018;4:e174504. [Crossref] [PubMed]
- Pezzi TA, Schwartz DL, Pisters KMW, et al. Association of Medicaid Insurance With Survival Among Patients With Small Cell Lung Cancer. JAMA Netw Open 2020;3:e203277. [Crossref] [PubMed]
- Finke I, Behrens G, Weisser L, et al. Socioeconomic Differences and Lung Cancer Survival-Systematic Review and Meta-Analysis. Front Oncol 2018;8:536. [Crossref] [PubMed]
- Ou SH, Zell JA, Ziogas A, et al. Low socioeconomic status is a poor prognostic factor for survival in stage I nonsmall cell lung cancer and is independent of surgical treatment, race, and marital status. Cancer 2008;112:2011-20. [Crossref] [PubMed]
- Greenwald HP, Polissar NL, Borgatta EF, et al. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health 1998;88:1681-4. [Crossref] [PubMed]
- Forrest LF, Adams J, Wareham H, et al. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med 2013;10:e1001376. [Crossref] [PubMed]
- Ebner PJ, Ding L, Kim AW, et al. The Effect of Socioeconomic Status on Treatment and Mortality in Non-Small Cell Lung Cancer Patients. Ann Thorac Surg 2020;109:225-32. [Crossref] [PubMed]
- Farrow NE, An SJ, Speicher PJ, et al. Disparities in guideline-concordant treatment for node-positive, non-small cell lung cancer following surgery. J Thorac Cardiovasc Surg 2020;160:261-271.e1. [Crossref] [PubMed]
- Nadpara P, Madhavan SS, Tworek C. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: A population-based study. Cancer Epidemiol 2015;39:1136-44. [Crossref] [PubMed]
- Ahmed HZ, Liu Y, O'Connell K, et al. Guideline-concordant Care Improves Overall Survival for Locally Advanced Non-Small-cell Lung Carcinoma Patients: A National Cancer Database Analysis. Clin Lung Cancer 2017;18:706-18. [Crossref] [PubMed]
- Blom EF, Ten Haaf K, Arenberg DA, et al. Disparities in Receiving Guideline-Concordant Treatment for Lung Cancer in the United States. Ann Am Thorac Soc 2020;17:186-94. [Crossref] [PubMed]
- Tapan U, Furtado VF, Qureshi MM, et al. Racial and Other Healthcare Disparities in Patients With Extensive-Stage SCLC. JTO Clin Res Rep 2021;2:100109. [Crossref] [PubMed]
- Aarts MJ, Aerts JG, van den Borne BE, et al. Comorbidity in Patients With Small-Cell Lung Cancer: Trends and Prognostic Impact. Clin Lung Cancer 2015;16:282-91. [Crossref] [PubMed]