
Effect of 3A lymph node resection on survival in patients with right-sided NSCLC: a retrospective, multicentre, propensity-score matching study
Introduction
For decades, surgery has been the primary treatment in early-stage non-small-cell lung carcinoma (NSCLC) patients. It typically includes tumour resection with lymph node dissection (1). Unfortunately, the influence of lymph node dissection on patient survival is unclear. Furthermore, the optimal extent of lymph node dissection is still under discussion and generally depends on a surgeons’ experience. International medical societies related to lung cancer surgery [e.g., the European Society of Thoracic Surgeons (ESTS)] focus solely on the quantity of lymph nodes (e.g., a minimum of 6 resected) or anatomical location (i.e., N1 or N2 lymph node stations) without focusing on particular stations (e.g., 3A station) (2,3).Considering the on-going debate about which particular lymphadenectomy method is superior, additional studies in this field remain crucial
In most of the cases, only stations 2, 4, 7, 8, and 9 are commonly dissected in right-sided NSCLC, while station 3A is usually omitted. Station 3A nodes are the prevascular mediastinal lymph nodes, which are not adjacent to the trachea and lie within the fatty tissue of the anterior mediastinum (4). Aside from the technical difficulties of 3A dissection, station 3A is an uncommon place for lymphatic spread because of the phrenic nerve and superior vena cava blockage and generous communications between the stations superior 10R, 4R, and 2R (5). Also, these adjacent anatomical structures are potential sites of complications. According to a few other studies, metastasis of the 3A station is not rare and occurs in between 15.3% and 17.8% of cases (6,7). Hence, 3A resection might be an important aspect of lymph node dissection in NSCLC.
In this study, we aimed to assess the clinical significance of prevascular mediastinal lymph nodes (3A) and their impact on survival in patients with right-sided NSCLC. This was accomplished using a large multicentre database from the Polish Lung Cancer Study Group. We also used propensity score matching (PSM) to minimize the potential selection bias. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1957/rc).
Methods
The Prospective Polish Lung Cancer Study Group database (24 thoracic centres) was analysed retrospectively. We included 6,348 cases with right-sided NSCLC. Two subgroups were created: patients with 3A station resection (3ALN+) (n=221) and patients without 3A station resection (3ALN−) (n=6,127). For further analysis, we divided the 3ALN+ group into non-metastatic subgroup (N3ALN) with 204 patients and metastatic subgroup (M3ALN) with 17 patients.
Patients were staged accordingly to the 8th edition of TNM classification (8). Lymph nodes were assessed according to The International Association for the Study of Lung Cancer lymph node map (9). Around 57 per cent of patients underwent adjuvant chemotherapy or radiotherapy. Exact data regarding adjuvant treatment regimens are not available. However, cisplatin and vinorelbine were the most common ones. We performed PSM to reduce selection bias. In case of conversion from thoracoscopy to thoracotomy, the procedure was reported as thoracotomy in the database. The ethics committee of the National Research Institute of Chest Diseases, Warsaw, Poland approved our study (91/2020), and waived the requirement for informed consent for the retrospective analysis. We conducted our study in accordance with the Declaration of Helsinki (as revised in 2013).
New pN descriptors
IASLC’s new pN descriptors were used with the modifications proposed by Chen et al. Therefore, we were able to assess the impact of single or multiple status of pN2 station or the skip metastasis phenomenon. This allowed us to answer whether 3A station solely has an impact on survival. The definitions are as follows: N1a: single station N1; N1b multiple station N1; N2a1 single station N2 without N1 involvement (skip metastasis); N2a2: single station N2 with N1 involvement; N2b1: multiple station N2 without N1 involvement (skip metastasis); N2b2: multiple station N2 with N1 involvement (9,10).
Lymph node dissection
Mediastinal lymph node dissection (MLND) consists of nodal stations #2, #4, #7, #8, and #9 (2). In Poland, most thoracic surgeons use MLND as the technique of choice. Station 3A is not resected routinely. To our knowledge, there are two major reasons of 3A resection: intraoperative findings and/or surgeon personal experience (e.g., to increase lymph node retrieval yield). There were no preoperative findings in 3A as cN2-IIIA cases were excluded. Resection of 3A is possible via both thoracoscopy and thoracotomy.
Inclusion and exclusion criteria
In this study, we included patients with confirmed right-sided NSCLC after radical resection (R0) from 2005 to 2015 and after mediastinal lymph node dissection or lymph node sampling. The patients had to have minimum 6 resected lymph nodes from hilar and mediastinal stations according to the ESTS guidelines (2) and complete clinical data. We excluded patients if they had minor resection, non-radical resection (R1), pNx status, neoadjuvant therapy prior to surgery (IIIA-cN2), clinical information that was lost or recorded incompletely, or failure to complete the follow-up period. Later, we excluded right middle lobes (RML) from the analysis due to an insignificant number of cases in this subgroup.
Preoperative staging and follow up
In case of radiological imaging findings [chest radiograph, computed tomography (CT), magnetic resonance imaging, positron emission tomography (PET)], patients underwent invasive diagnostics (endobronchial ultrasound transbronchial needle aspiration, oesophageal ultrasound fine-needle aspiration, mediastinoscopy, and mediastinotomy).
After the surgery, the surgeon consulted patients within 2–3 weeks. Patients underwent follow-up diagnostics every three to five months for 5 years, which included chest radiographs, CT, or PETs in justified cases. Failure patterns were evaluated by imaging studies and data from invasive diagnostics similar to those in preoperative staging. Hilar and/or mediastinal failure was defined as a new or enlarging lymph node greater than 1 cm on the short axis in CT and/or hypermetabolism in PET imaging, in which the patient’s consecutive clinical follow-up was consistent with disease progression. Cancer recurrence was divided into two categories: locoregional and distant recurrence. Locoregional recurrence was within the ipsilateral hemithorax or mediastinum. Other anatomical locations were defined as distant metastasis. PET availability increased from 25% of cases (early study years) to nearly 75% of cases (late study years). In case of missing data or loss to follow-up, polish national personal identity databases were checked for missing data. But, we excluded 159 patients because of incomplete data or loss to follow-up.
Statistical analysis
We identified the mean with the standard deviation and the median with a range of values as continuous variables. The Mann-Whitney U-test was implemented to ascertain significant differences in continuous variables between groups. We summarised every group with categorical variables with a frequency and incidence. Every group with categorical variables is summarised with an incidence and frequency of the investigated population. We applied the Chi-squared test to conduct a statistical comparison between these groups. We approximated survival curves with the Kaplan-Meier method, and we used the log-rank test to contrast differences between groups. We used the Cox proportional hazards model in univariable and multivariable analysis to examine the influence on patients’ risk of death. We selected predictive variables based on univariable models (P<0.05). In accordance with the results, we established the following factors as important in the univariable analysis: age, sex, pT, pN, resection type, pathological stage, surgical approach, and comorbidities (kidney failure, insulin-dependent diabetes, chronic obstructive pulmonary disease (COPD), and cardiovascular diseases). The hazard ratio of the entire cohort of patients using subgroup analysis by pN was estimated in the Cox multivariable analysis. All tests were two-sided, and a P<0.05 was considered statistically significant. For pairwise comparison of more than 2 groups, the FDR adjustment was used. We conducted all analyses using survminer and survival packages in R-software.
Propensity score matching
Because of the disproportion in variables, we performed a case-matched analysis to acquire comparable subgroups. This allowed us to enhance the statistical power of the study and to confirm results found in unmatched cohorts. We used the PSM method in identical pN stages separately. Logistic regression was used to calculate propensity scores for cohort cases. We used the following variables in the PSM method: sex, age, smoking status, histopathology of cancer, stage, and pT descriptor. We reproduced Cox Multivariable analysis after the PSM.
Results
A total of 6,348 patients were included in the study (221 in the 3ALN+ group and 6,127 in the 3ALN− group). The patients’ flow chart is presented in Figure 1. After PSM, 221 vs. 221 patients were matched. Before PSM, groups differed significantly in terms of stage (P=0.0351), pN descriptors (P<0.001), new pN descriptors (P=0.00124), type of resection (P=0.0214), and surgical approach (P=0.0133). After PSM, all these variables were comparable (P>0.99), but the surgical approach remained significantly different (P=0.0433). Also, after PSM, new significant differences were found in terms of age (P=0.0101). In both groups, the incidence of mediastinoscopy was similar both before and after PSM (P>0.05). Detailed data about the included cases before and after PSM are listed in Table 1.
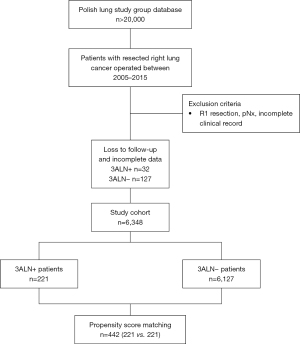
Table 1
| Variable | Entire cohort (N=6,348) | Propensity score matching (N=442) | |||||
|---|---|---|---|---|---|---|---|
| 3ALN− (N=6,127) | 3ALN+ (N=221) | P value (SMD) | 3ALN− (N=221) | 3ALN+ (N=221) | P value (SMD) | ||
| Age | 0.326 (0.058) | 0.0101 (0.214) | |||||
| Mean (SD) | 62.6 (8.25) | 63.1 (7.93) | 61.3 (8.55) | 63.1 (7.93) | |||
| Median [1Q, 3Q] | 62.0 [22.0, 87.0] | 63.0 [38.0, 81.0] | 61.0 [42.0, 83.0] | 63.0 [38.0, 81.0] | |||
| Sex | 1.0 (0) | 0.68 (0.049) | |||||
| Female | 1,942 (31.7) | 70 (31.7) | 65 (29.4) | 70 (31.7) | |||
| Male | 4,185 (68.3) | 151 (68.3) | 156 (70.6) | 151 (68.3) | |||
| Stage | 0.0351 (0.26) | 1.0 (0.019) | |||||
| IA1 | 59 (1.0) | 2 (0.9) | 2 (0.9) | 2 (0.9) | |||
| IA2 | 568 (9.3) | 16 (7.2) | 16 (7.2) | 16 (7.2) | |||
| IA3 | 544 (8.9) | 14 (6.3) | 14 (6.3) | 14 (6.3) | |||
| IB | 1,480 (24.2) | 45 (20.4) | 45 (20.4) | 45 (20.4) | |||
| IIA | 532 (8.7) | 13 (5.9) | 13 (5.9) | 13 (5.9) | |||
| IIB | 1,426 (23.3) | 55 (24.9) | 55 (24.9) | 55 (24.9) | |||
| IIIA | 1,244 (20.3) | 59 (26.7) | 60 (27.1) | 59 (26.7) | |||
| IIIB | 274 (4.5) | 17 (7.7) | 16 (7.2) | 17 (7.7) | |||
| Smoking | 4,265 (69.6) | 144 (65.2) | 0.181 (0.095) | 154 (69.7) | 144 (65.2) | 0.361 (0.097) | |
| Comorbidities | |||||||
| Diabetes I | 177 (2.9) | 3 (1.4) | 0.254 (0.106) | 3 (1.4) | 3 (1.4) | 1.0 (0.0) | |
| Myocardial infarction | 385 (6.3) | 10 (4.5) | 0.357 (0.078) | 17 (7.7) | 10 (4.5) | 0.233 (0.133) | |
| Nervous diseases | 49 (0.8) | 0 (0.0) | 0.345 (0.127) | 1 (0.5) | 0 (0.0) | 1 (0.095) | |
| Heart failure | 153 (2.5) | 20 (3.0) | 0.685 (0.04) | 13 (5.9) | 7 (3.2) | 0.253 (0.131) | |
| Kidney failure | 49 (0.8) | 7 (3.2) | 0.0508 (0.119) | 0 (0.0) | 5 (2.3) | 0.072 (0.215) | |
| COPD | 1,485 (24.2) | 52 (23.5) | 0.872 (0.017) | 48 (21.7) | 52 (23.5) | 0.733 (0.043) | |
| Hypertension | 2,342 (38.2) | 85 (38.5) | 0.999 (0.005) | 70 (31.7) | 85 (38.5) | 0.163 (0.143) | |
| Coronary disease | 400 (6.5) | 11 (5.0) | 0.435 (0.067) | 17 (7.7) | 11 (5.0) | 0.329 (0.112) | |
| pT | 0.64 (0.14) | 1.0 (0.015) | |||||
| 1a | 71 (1.2) | 2 (0.9) | 2 (0.9) | 2 (0.9) | |||
| 1b | 668 (10.9) | 23 (10.4) | 23 (10.4) | 23 (10.4) | |||
| 1c | 721 (11.8) | 23 (10.4) | 23 (10.4) | 23 (10.4) | |||
| 2a | 2,124 (34.7) | 67 (30.3) | 68 (30.8) | 67 (30.3) | |||
| 2b | 818 (13.4) | 31 (14.0) | 31 (14.0) | 31 (14.0) | |||
| 3 | 1,113 (18.2) | 48 (21.7) | 48 (21.7) | 48 (21.7) | |||
| 4 | 612 (10.0) | 27 (12.2) | 26 (11.8) | 27 (12.2) | |||
| cN | 0.797 (0.029) | 0.445 (0.059) | |||||
| 0 | 4,599 (75.1) | 164 (74.2) | 152 (68.8) | 164 (74.2) | |||
| 1 | 839 (13.7) | 29 (13.1) | 36 (16.3) | 29 (13.1) | |||
| pN | <0.001 (0.239) | 1.0 (0.0) | |||||
| 0 | 4,267 (69.6) | 131 (59.3) | 131 (59.3) | 131 (59.3) | |||
| 1 | 1,065 (17.4) | 44 (19.9) | 44 (19.9) | 44 (19.9) | |||
| 2 | 795 (13.0) | 46 (20.8) | 46 (20.8) | 46 (20.8) | |||
| New pN | 0.00124 (0.272) | 1.0 (0.0) | |||||
| N0 | 4,267 (69.6) | 131 (59.3) | 131 (59.3) | 131 (59.3) | |||
| N1a | 917 (15.0) | 38 (17.2) | 38 (17.2) | 38 (17.2) | |||
| N1b | 148 (2.4) | 6 (2.7) | 6 (2.7) | 6 (2.7) | |||
| N2a1 | 277 (4.5) | 14 (6.3) | 14 (6.3) | 14 (6.3) | |||
| N2a2 | 294 (4.8) | 12 (5.4) | 12 (5.4) | 12 (5.4) | |||
| N2b1 | 66 (1.1) | 6 (2.7) | 6 (2.7) | 6 (2.7) | |||
| N2b2 | 158 (2.6) | 14 (6.3) | 14 (6.3) | 14 (6.3) | |||
| Extent of resection | 0.0214 (0.185) | 0.993 (0.011) | |||||
| Lower lobectomy | 1,752 (28.6) | 50 (22.6) | 50 (22.6) | 50 (22.6) | |||
| Upper lobectomy | 3,418 (55.8) | 123 (55.7) | 124 (56.1) | 123 (55.7) | |||
| Pneumonectomy | 957 (15.6) | 48 (21.7) | 47 (21.6) | 48 (21.7) | |||
| Approach | 0.0133 (0.239) | 0.0433 (0.226) | |||||
| Thoracotomy | 5,886 (96.1) | 220 (99.5) | 213 (96.4) | 220 (99.5) | |||
| VATS | 241 (3.9) | 1 (0.5) | 8 (3.6) | 1 (0.5) | |||
| Histopathology | 0.435 (0.058) | 0.923 (0.018) | |||||
| Adenocarcinoma | 3,288 (53.7) | 125 (56.6) | 127 (57.5) | 125 (56.6) | |||
| Squamous | 2,839 (46.3) | 96 (43.4) | 94 (42.5) | 96 (43.4) | |||
| Mediastinoscopy | 702 (11.5) | 26 (11.8) | 0.973 (0.01) | 24 (10.9) | 26 (11.8) | 0.881 (0.029) | |
3ALN+, patients with prevascular node resection; 3ALN−, patients without prevascular node resection; COPD, chronic obstructive pulmonary disease; SMD, standardized mean difference; VATS, video-assisted thoracic surgery.
Incidence of metastasis in 3A lymph node station
In the entire cohort, the incidence of patients with 3A nodes removed was 3.5%. In 3ALN group, the mean incidence of observed M3ALN was 8% (Table 2). The incidence of diagnosed 3A nodal metastases depended on the type of resection, tumour size, and the presence of mediastinal nodal metastases. The highest incidence of M3ALN was found among patients undergoing pneumonectomy (10%) and in the N2b1 and N2b2 subgroups (33% and 64%). These subgroups also had higher incidence of 3A nodes retrieved (4.8%, 8.3%, and 8.1%, respectively). There was no difference in the M3ALN incidence between the right upper lobe (RUL) and right lower lobe (RLL) (7% and 6%). Regarding tumour size, a notable increase in the metastatic 3ALN incidence was found between pT1c and pT2a (from 4% to 9%), and for pT4, it was 15%.
Table 2
| Variable | n | Positive ratea | Examined (%) |
|---|---|---|---|
| Overall | 6,348 | 8% | 221 (3.5%) |
| New pN | |||
| N0 | 4,398 | 0% | 131 (3.0%) |
| N1a | 955 | 0% | 38 (4.0%) |
| N1b | 154 | 0% | 6 (3.9%) |
| N2a1 | 291 | 21% | 14 (4.8%) |
| N2a2 | 306 | 25% | 12 (3.9%) |
| N2b1 | 72 | 33% | 6 (8.3%) |
| N2b2 | 172 | 64% | 14 (8.1%) |
| pT | |||
| 1a | 73 | 0% | 2 (2.7%) |
| 1b | 691 | 4% | 23 (3.3%) |
| 1c | 744 | 4% | 23 (3.1%) |
| 2a | 2,191 | 9% | 67 (3.1%) |
| 2b | 849 | 10% | 31 (3.7%) |
| 3 | 1,161 | 4% | 48 (4.1%) |
| 4 | 639 | 15% | 27 (4.2%) |
a, % positive if lymph nodes station was examined. n, number of patients.
Incidence of M3ALN (8%) was lower than metastasis rate in station 4R (10%), but higher than metastasis rate in stations 2R (5%), 7 (7%), 8 (4%) and 9 (2%). Comparison of 3A resection and metastasis rate in comparison to routine MLND lymph node stations (depending on surgery type) is presented in Table 3.
Table 3
| Lymph node station | Right lower lobectomy (n=1,802) | Right upper lobectomy (n=3,541) | Pneumonectomy (n=1,005) | Overall (n=6,348) |
P value |
|---|---|---|---|---|---|
| 2R | |||||
| Examined (%) | 521 (28.9) | 1,381 (39.0) | 360 (35.8) | 2,262 (35.6) | 0.0101 |
| % positive* | 5 | 5 | 6 | 5 | 0.436 |
| 3A | |||||
| Examined (%) | 50 (2.8) | 123 (3.5) | 48 (4.8) | 221 (3.5) | 0.0214 |
| % positive* | 6 | 7 | 10 | 8 | 0.695 |
| 4R | |||||
| Examined (%) | 1,179 (65.4) | 2,827 (79.8) | 755 (75.1) | 4,761 (75.0) | <0.001 |
| % positive* | 5 | 10 | 14 | 10 | <0.001 |
| 7 | |||||
| Examined (%) | 1,478 (82.0) | 2,795 (78.9) | 898 (89.4) | 5,171 (81.5) | <0.001 |
| % positive* | 11 | 3 | 16 | 7 | <0.001 |
| 8 | |||||
| Examined (%) | 582 (32.3) | 972 (27.4) | 401 (39.9) | 1,955 (30.8) | <0.001 |
| % positive* | 8 | 1 | 5 | 4 | <0.001 |
| 9 | |||||
| Examined (%) | 737 (40.9) | 1,041 (29.4) | 379 (37.7) | 2,157 (34.0) | <0.001 |
| % positive* | 2 | 1 | 3 | 2 | <0.001 |
*, positive if lymph node station was examined. n, number of patients.
Univariable survival analysis
The median follow-up time in the entire study group was 1,913 days. There was no statistical difference in survival between 3ALN+ and 3ALN− before PSM (51% vs. 51%, P=0.74) and after PSM (51% vs. 48%, P=0.58). With regard to the type of resection in the whole group, after the PSM the lowest survival rate was found in patients after pneumonectomy (37%), while no differences were found between RUL and RLL (54% vs. 52%). For comparison, survival in the M3ALN subgroup was 9% and was significantly lower than in both 3ALN− and N3ALN (P<0.0001) (Figure 2).
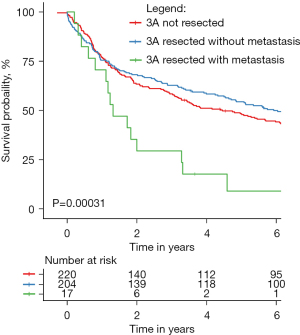
In the subgroup analysis of the 3ALN+ and 3ALN− survival rates, no significant differences were found in terms of extent of resection, pT, pN, and new pN (Table 4). Also, in the analysis with the M3ALN subgroup, no differences were found in subgroups N2a1, N2a2, N2b1, and N2b2. Survival rates of 3ALN+ and 3ALN− as a function of new pN subgroups are presented in Figure S1.
Table 4
| Subgroup | 3ALN+ | 3ALN− | P value |
|---|---|---|---|
| Overall | 51% | 48% | 0.58 |
| Extent of pulmonary resection | |||
| Right lower lobectomy | 56% | 48% | 0.35 |
| Right upper lobectomy | 54% | 54% | 0.69 |
| Pneumonectomy | 40% | 34% | 0.35 |
| pN descriptor | |||
| pN0 | 64% | 60% | 0.69 |
| pN1 | 45% | 39% | 0.46 |
| pN2 | 19% | 24% | 0.96 |
| New classification of pN descriptor | |||
| pN0 | 64% | 60% | 0.84 |
| pN1a | 45% | 34% | 0.39 |
| pN1b | 50% | 67% | 0.81 |
| pN2a1 | 36% | 21% | 0.39 |
| pN2a2 | 17% | 42% | 0.26 |
| pN2b1 | 17% | 17% | 0.62 |
| pN2b2 | 0% | 14% | 0.77 |
| pT descriptor | |||
| pT1a | 50% | 50% | 0.81 |
| pT1b | 78% | 74% | 0.67 |
| pT1c | 57% | 52% | 0.48 |
| pT2a | 54% | 59% | 0.22 |
| pT2b | 48% | 42% | 0.36 |
| pT3 | 50% | 39% | 0.58 |
| pT4 | 22% | 19% | 0.43 |
3ALN+, patients with prevascular node resection; 3ALN−, patients without prevascular node resection.
Multivariable survival analysis
In the entire cohort after PSM, the multivariable analysis showed that the independent prognostic risk factors were pneumonectomy (HR =1.73, 95% CI: 1.16–2.57, P=0.007), heart failure (HR =1.96, 95% CI: 1.13–3.46, P=0.017), and coronary disease (HR =2.06, 95% CI: 1.33–3.20, P=0.001). In contrast, removal of the 3A lymph nodes as well as the presence of 3A nodal metastases were not shown to be independent prognostic factors (HR =0.91, 95% CI: 0.71–1.18, P=0.50 and HR =1.08, 95% CI: 0.60–1.93, P=0.79) (Table 5). Also, the multivariable analysis after PSM in the subgroups of patients with mediastinal metastases (subgroups pN2a1, pN2a2, pN2b1, and pN2b2) did not show that removal of 3A nodes or 3A nodal metastases is an independent prognostic factor in any given group for any of the stages. Detailed results of multivariable analysis of pN2 subgroups are presented in Table S1.
Table 5
| Variable | Hazard risk (95% CI) | P value |
|---|---|---|
| Comorbidities | Reference = lack of disease | |
| Coronary disease | 2.06 (1.33–3.20) | 0.001 |
| Heart failure | 1.96 (1.13–3.46) | 0.017 |
| cN | ||
| cN0 | Reference | |
| cN1 | 0.99 (0.69–1.42) | 0.951 |
| pN | ||
| pN0 | Reference | |
| pN1a | 0.78 (0.37–1.61) | 0.496 |
| pN1b | 0.51 (0.18–1.44) | 0.202 |
| pN2a1 | 0.57 (0.11–2.93) | 0.497 |
| pN2a2 | 0.53 (0.10–2.69) | 0.444 |
| pN2b1 | 0.46 (0.09–2.24) | 0.338 |
| pN2b2 | 0.70 (0.16–3.18) | 0.649 |
| Mediastinoscopy (reference = lack of mediastinoscopy) | 1.16 (0.79–1.68) | 0.452 |
| Surgery | ||
| Lower lobectomy | Reference | |
| Upper lobectomy | 1.08 (0.78–1.51) | 0.629 |
| Pneumonectomy | 1.73 (1.16–2.57) | 0.007 |
| Approach | ||
| Thoracotomy | Reference | |
| VATS | 0.19 (0.03–1.38) | 0.100 |
| pT | ||
| pT1a | Reference | |
| pT1b | 1.41 (0.34–5.94) | 0.639 |
| pT1c | 1.97 (0.46–8.44) | 0.360 |
| pT2a | 1.76 (0.47–6.55) | 0.399 |
| pT2b | 2.54 (0.69–9.35) | 0.162 |
| pT3 | 1.10 (0.58–2.09) | 0.767 |
| Stage | ||
| IA1 | Reference | |
| IA2 | 0.25 (0.03–2.10) | 0.204 |
| IA3 | 0.29 (0.03–2.38) | 0.249 |
| IB | 0.30 (0.04–2.17) | 0.235 |
| IIA | 0.25 (0.03–1.90) | 0.182 |
| IIB | 0.64 (0.13–3.19) | 0.589 |
| IIIA | 1.56 (0.36–6.81) | 0.551 |
| IIIB | 4.53 (0.65–31.75) | 0.129 |
| 3A station | ||
| Not-resected | Reference | |
| Resected and non-metastatic | 0.91 (0.71–1.18) | 0.497 |
| Resected and metastatic | 1.08 (0.60–1.93) | 0.793 |
CI, confidence interval; VATS, video-assisted thoracic surgery.
Discussion
Lymphadenectomy of mediastinum is an integral part of the surgical treatment of NSCLC. However, as shown by clinical data, biopsy or resection of the lymph nodes is not performed in some cases. Osarogiagbon and Yu showed that the incidence of these cases reaches 15%, which results in inadequate staging of patients. Thus, survival is worse and more similar to pN1 rather than pN0 (3). In turn, Little et al. showed that only 57.8% of patients underwent mediastinal lymph node sampling (MLNS) or MLND (11). In a recently published study by Ray et al., this incidence was 10%, and 75% of cases did not even meet the sampling criteria (12). Many authors have proposed liberalizing the extent of lymph node resection. In a prospective and randomized study, Darling et al. showed that there is no statistical difference between MLNS and MLND (13). In recent years, the number of supporters of lobe-specific lymph nodes dissection (LS-LND) has increased, especially in the early stages of lung cancer (14,15). However, a large group of opponents still support MLND as the standard of care in the surgical treatment of lung cancer. Riquet et al. showed that the incidence of metastases in the upper mediastinum in the case of lower lobectomies is similar to that of upper lobectomies. This supports systemic resection of all nodal groups (16). Also, Maniwa et al. showed that in the LS-LND group, the incidence of local and distant recurrences was higher than in the MLND group (P=0.005) (17).
In many studies, lymph nodes of the 3A station are not routinely included in both the MLNS and MLND groups (18). Therefore, when analysing this nodal group, it is not surprising that the incidence of patients in whom it is removed is usually small. In our study, only 3.5% of patients had 3A nodes removed. Also, the current international recommendations do not take this node station into consideration (2). Therefore, in practice, 3A nodes are rarely removed. Only a few who are devoted to lymphadenectomy in lung cancer include this nodal station in their analyses or daily practice.
In the work of Liang et al., the incidence of removed 3A nodes for RUL, RML, and RLL were 3.2%, 5.4%, and 1.8%, respectively. At the same time, the incidence of 3A node metastases were 12.2%, 20%, and 7.4%, respectively (19). In other study by Liu et al., the incidence of 3A nodes removed was 29.9%, and 15.3% had metastases. Also, the authors showed that the metastasis rate of station 3A was second only to that of stations 4 and 3A was significantly higher than those of stations 8 and 9 (6). In a study by Yang et al., the incidence of removed and metastatic 3A nodes were 24% and 11.4% (RUL), 28.2% and 12.1% (RML), and 23.7% and 5% (RLL), respectively (20). In our study, the incidence of positive 3A nodes in the entire group was 8%. The incidence of removed and metastatic nodes were 2.8% and 6% for RUL and 3.5% and 7% for RLL, respectively. Furthermore, there were no statistical differences in the incidence of positive nodes between both types of lobectomy.
Our study also showed that the incidence of 3A nodal metastases may increase with the size of the tumour. There was a difference between pT1c and pT2a where the incidence of positive nodes doubled (4% vs. 9%). A similar observation between the tumour size and the incidence of positive 3A nodes was demonstrated by Zheng et al. However, it was not confirmed by the multivariable analysis (7).
Earlier analyses of 3A nodes have shown that survival in the group with positive 3A nodes was worse than in other pN2 patients. In the study by Zheng et al., the 3-year survival in the group with positive 3A nodes was lower than in the other cases and amounted to 22% and 63%, respectively (P<0.001). Moreover, there were differences between single and multistation nodal involvement (72% vs. 33%, P<0.001) (7). On the other hand, Liu et al. showed differences in 5-year survival between 3ALN+ and 3ALN− (58.8% vs. 48.7%, P=0.007), finding better survival in stages II and III. Notably, the multivariable analysis showed that 3A nodal removal is an independent prognostic factor for survival (HR =0.76, 95% CI: 0.64–0.90, P=0.001) (6). But in our study, both univariable and multivariable analyses (after PSM) for 3ALN+ and 3ALN− showed no significant differences in survival. Also, the multivariable analysis after PSM in the subgroups of pN2 patients with single/multistation 3A or with/without pN1 involvement (N2a1, N2a2, N2b1, and N2b2) did not show the presence of 3A metastases as an independent prognostic factor. However, other factors such as age, sex, comorbidities, and the severity of the disease had a significant influence on the long-term results.
Nevertheless, we believe that, 3A station resection has significant clinical importance. First, the incidence of 3A metastases is the second highest after 4R nodes, which is true for both upper and lower lobe tumours (Table 3). This is especially important in LS-LND, for which removal of the upper mediastinal nodes is not recommended in inferior lobectomy. Also, 3A metastasis rate is higher overall than other routine MLND stations: 2R, 7, 8, 9. This is even more apparent in upper lobe cases (Table 3) Moreover, even in the nodes of the 3A station that are 5–10 mm in size, the incidence of metastatic changes reaches 22.2% (7). In the context of the arguments presented above, an important reservation is raised by the low incidence of patients with 3A nodes removed, which amounts to 3.5%. This result is also comparable with the data of some of the previously cited study (19).
Several reasons could explain why 3A resection would potentially enhance patients survival. Undiscovered metastasis during nodal staging obviously lowers the survival due to patients not being eligible for beneficial adjuvant therapy. In our study pN0 and pN1 cases had better survival within the 3ALN+ subgroup compared to the 3ALN−, but it was without statistical significance. This might suggest there were undetected 3A metastases in 3ALN− subgroups. The second important reason is micrometastasis, which cannot be detected macroscopically or during image diagnostics. Thus, a patient would not undergo neoadjuvant therapy or would not be disqualified from surgery (21). And as we stated before some surgeons resect 3A based on intraoperative findings. Thus, micrometastatic 3A would not draw an attention of these surgeons. Thirdly, residual disease, which is a presence of single metastatic cells, is another explanation. This phenomenon could be missed even by experienced pathologists and result in future recurrence (22).
Our study has several significant limitations. First of all, it is a retrospective study, which significantly affects the errors in the clinical evaluation of the issue. This effect was partially offset by the use of PSM in both univariable and multivariable analyses. The second important limitation was the very low incidence of patients with 3A nodes removed, despite observing data from several centres. Relatively high metastasis rate in 3A (in comparison to MLND lymph node stations) might be due to intraoperative findings, which affected surgeon decision. Important reservations can be made with regard to preoperative diagnostics, particularly the use of PET-CT, which was very low in the initial period of the study. Also, the incidence of mediastinoscopy performed in the 3ALN+ and 3ALN− groups is too low (11.8% and 11.5%, respectively), considering the very high pN2 incidence of 20.8% in the 3ALN+ group. From a statistical point of view, in the multivariable analysis concerning pN2 subgroups, the population of individual subgroups after PSM was too small, which may limit the statistical value of the presented results. Prospective studies are needed, especially as a low 3A resection rate makes retrospective studies (even with big cohorts) hard to analyse correctly. Also, due to incompleteness of our database some important variables could not be introduced in this analysis (e.g., relapse-free survival, cancer specific survival or detailed complication analysis)
To sum up, the incidence of 3A node metastases in the pN2 patient group is high and increases in advanced stages. Also, 3A metastasis rate is comparable to routine MLND lymph node stations. The location of the tumour in the inferior lobe does not exclude the presence of 3A node metastases. Removal of the 3A station may be recommended as a routine procedure in superior mediastinal lymphadenectomy in all cases of suspected mediastinal node metastases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1957/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1957/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1957/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the National Research Institute of Chest Diseases, Warsaw, Poland (91/2020) and the requirement for informed consent was waived for the retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Watanabe S, Asamura H. Lymph node dissection for lung cancer: significance, strategy, and technique. J Thorac Oncol 2009;4:652-7. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg 2013;96:1178-89. [Crossref] [PubMed]
- Zieliński M, Rami-Porta R. Proposals for changes in the Mountain and Dresler mediastinal and pulmonary lymph node map. J Thorac Oncol 2007;2:3-6. [Crossref] [PubMed]
- Riquet M, Arame A, Foucault C, et al. Prognostic classifications of lymph node involvement in lung cancer and current International Association for the Study of Lung Cancer descriptive classification in zones. Interact Cardiovasc Thorac Surg 2010;11:260-4. [Crossref] [PubMed]
- Liu C, Wei S, Guo C, et al. Clinical Significance of Station 3A Lymph Node Dissection in Patients with Right-Side Non-Small-Cell Lung Cancer: A Retrospective Propensity-Matched Analysis. Ann Surg Oncol 2021;28:194-202. [Crossref] [PubMed]
- Zheng H, Gao W, Fei K, et al. Prognostic role of station 3A mediastinal nodes for non-small-cell lung cancers. Interact Cardiovasc Thorac Surg 2013;17:447-54. [Crossref] [PubMed]
- Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours, 8th edition. Oxford, UK; Hoboken, NJ: John Wiley & Sons, Inc; 2017.
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Chen W, Zhang C, Wang G, et al. Feasibility of nodal classification for non-small cell lung cancer by merging current N categories with the number of involved lymph node stations. Thorac Cancer 2019;10:1533-43. [Crossref] [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [Crossref] [PubMed]
- Ray MA, Smeltzer MP, Faris NR, et al. Survival After Mediastinal Node Dissection, Systematic Sampling, or Neither for Early Stage NSCLC. J Thorac Oncol 2020;15:1670-81. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Deng HY, Qin CL, Li G, et al. Can lobe-specific lymph node dissection be an alternative to systematic lymph node dissection in treating early-stage non-small cell lung cancer: a comprehensive systematic review and meta-analysis? J Thorac Dis 2018;10:2857-65. [Crossref] [PubMed]
- Riquet M, Rivera C, Pricopi C, et al. Is the lymphatic drainage of lung cancer lobe-specific? A surgical appraisal. Eur J Cardiothorac Surg 2015;47:543-9. [Crossref] [PubMed]
- Maniwa T, Okumura T, Isaka M, et al. Recurrence of mediastinal node cancer after lobe-specific systematic nodal dissection for non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:e59-64. [Crossref] [PubMed]
- Fang L, Xu J, Ye B, et al. Is lobe specific lymph node dissection adequate for cN0-1 non-small cell lung cancer? J Cardiothorac Surg 2020;15:46. [Crossref] [PubMed]
- Liang RB, Yang J, Zeng TS, et al. Incidence and Distribution of Lobe-Specific Mediastinal Lymph Node Metastasis in Non-small Cell Lung Cancer: Data from 4511 Resected Cases. Ann Surg Oncol 2018;25:3300-7. [Crossref] [PubMed]
- Yang MZ, Hou X, Liang RB, et al. The incidence and distribution of mediastinal lymph node metastasis and its impact on survival in patients with non-small-cell lung cancers 3 cm or less: data from 2292 cases. Eur J Cardiothorac Surg 2019;56:159-66. [Crossref] [PubMed]
- Coello MC, Luketich JD, Litle VR, et al. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer 2004;5:214-25. [Crossref] [PubMed]
- Frisone D, Friedlaender A, Addeo A. The Role and Impact of Minimal Residual Disease in NSCLC. Curr Oncol Rep 2021;23:136. [Crossref] [PubMed]


