Dynamic evaluation of the pulmonary protective effects of prone position ventilation via respiratory mechanics for patients with moderate to severe acute respiratory distress syndrome
Introduction
The global fatality rate of acute respiratory distress syndrome (ARDS) remains as high as 40–50% (1). There have been several major acute infectious diseases identified as epidemic (or pandemic) in recent years, including SARS, H5N1, H1N1, H7N9, and COVID-19, most of which are clinically characterized by severe pneumonia with ARDS (2,3). In addition to etiological treatment, lung-protective ventilating strategies (LPVS) are the main methods of life support. Early (within 33 hours after tracheal intubation) and standard (with the duration of 12–18 hours) prone position ventilation (PPV) has been shown to be particularly effective, and it may be beneficial to improve refractory hypoxemia and lung-protective ventilation, thus improving the prognosis of ARDS patients. However, the post-PPV improvement of oxygenation has been shown to be unrelated to the patient prognosis (4,5), and some patients also suffer from an elevated PaCO2 (6).
The fully prone position for a long time (more than 12 hours) is not a very routine position which may cause discomfort to the patients, and currently it is unclear whether long-term local compression, such as abdominal compression, affects diaphragmatic movement. In particular, the effects of the increased elastic resistance of the anterior chest wall on the transpulmonary pressure and whether the improvement of ventilation is due to changes in the gravity-dependent region over time both remain unknown.
Conventional monitoring of the tidal volume (Vt), respiration rate (RR), respiratory compliance (Cre), blood gas analyses and even evaluations of the mechanical energy can not reflect the actual lung protection provided by different treatments in patients under invasive ventilation (7). Monitoring should be conducted in combination with assessments of the respiratory mechanical parameters, such as the value of esophageal pressure swings (ΔPes), end-inspiratory transpulmonary pressure (end-PLei) or end-expiratory transpulmonary pressure (end-PLee), diaphragmatic electromyogram (EMGdi), etc. (8,9). Additionally, although they have been used empirically in some of these deeply-sedated patients, the impact of neuromuscular blocking agents (NMBAs) during PPV on the respiratory mechanics above of ARDS patients is unclear.
It is necessary to carry out more in-depth dynamic monitoring throughout PPV. For conventional respiratory mechanics monitoring, an esophageal balloon catheter must be implanted to the patients each time, it can affect daily tube feeding and increasing the medical workload. What’s worse, the stimulation above may interfere with the measurement of respiratory mechanics data in patients. In our study, we improved the problems above with the use of integrated four-channel multifunctional gastric tubes and it have obtained the appearance patent in China (Patent No. CN307246718S). Our catheter is composed of four sub-pipes for gastric feeding, detecting EMGdi, measuring esophageal pressure and gastric pressure. When severe patients in intensive care unit (ICU) need indwelling gastric tube for therapeutic purposes, the integrated four-channel multifunctional gastric tubes can be used instead of the conventional gastric tube, with which we can continuously monitor the respiratory mechanics data of patients without repeated indwelling measurement catheters. We hope to make a more accurate assessment of continuous lung protection in patients with moderate to severe ARDS undergoing PPV. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-291/rc).
Methods
Research subjects
Inclusion criteria: this study enrolled patients with moderate/severe ARDS [PaO2/FiO2 ratio (PFR)] ≤150 mmHg (1 mmHg =0.133 kPa) who met the 2012 Berlin Definition (10), the patients all had undergone invasive mechanical ventilation (IMV) through an artificial airway less than 36 h after being diagnosed with moderate/severe ARDS, and exhibited hemodynamic stability characterized by a mean arterial pressure (MAP) >65 mmHg, heart rate (HR) ≤120 beats/min, norepinephrine ≤0.4 µg·kg−1·min−1, or dopamine ≤10 µg·kg−1·min−1.
Exclusion criteria: the following patients were excluded from the study: those with (I) contraindications for PPV (11,12), including hemodynamic instability, intracranial hypertension, acute active bleeding, spinal injury, orthopedic surgery, recent abdominal surgery, severe pneumothorax, or pregnancy; (II) contraindications for gastric intubation: patients with gastric and esophageal lesions and deformities, severe coagulation dysfunction, or severe gastric retention with transpyloric feeding; (III) factors affecting pressure measurement: pneumothorax, thoracic drainage tube, abdominal infection, intra-abdominal hypertension, age >80 or <18 years, or known chest deformity; (IV) refusal by the family members of the patients.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the First Affiliated Hospital of Guangzhou Medical University (No. 2017-034). Signed informed consent forms (ICF) were obtained from family members of patients with moderate/severe ARDS eligible to receive PPV and an indwelling integrated four-channel multifunctional gastric tube (designed and manufacture by Guangzhou Institute of Respiratory Health, Patent No. CN307246718S).
Monitoring indicators
Localization and connection to LabChart 8.0 after placement of an indwelling integrated four-channel multifunctional gastric tube.
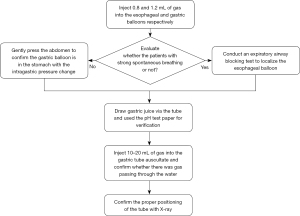
The patients stayed in the supine position and the head of the bed raised about 30°. After placing an indwelling integrated four-channel multifunctional gastric tube, several methods were combined for localization and fixation; First, 0.8 and 1.2 mL of gas were injected into the esophageal and gastric balloons respectively to monitor the waveform changes in the airway pressure, esophageal pressure and gastric pressure (13). Second, for patients with strong spontaneous breathing, an expiratory airway blocking test was conducted to localize the esophageal balloon (14-18). Third, for patients without spontaneous breathing, the abdomen was gently pressed, and the intragastric pressure could be observed to rise, confirming that the gastric balloon was in the stomach (8). Fourth, drew gastric juice via the tube and used the pH test paper for verification. Fifth, 10–20 mL of gas was injected into the gastric tube and the clinical operators determined whether the gastric tube was in the patient’s stomach by hearing the sound of gas passing through the gastric juice. Sixth, the clinical operators could further confirm the location of the gastric tube by bedside X-ray inspection. Figure 1 showed a flow chart of the operation above.
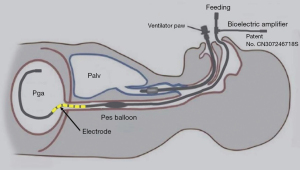
The pressure extension tube of the esophageal balloon and gastric balloon was connected with a U.S. Validyne DP15 pressure sensor, while the Y-type tube of the ventilator circuit was connected with a Validyne DP15 pressure sensor. The three-channel pressure signal was amplified by a Validyne CD280 pressure amplifier. The EMGdi signal was output to the bioelectric amplifier through a 10-channel aviation connector. After notch and gain adjustment, the pressure and EMG signals were input to Powerlab, and then output to LabChart 8.0 for data acquisition. Figure 2 provided a schematic diagram of the indwelling integrated four-channel multifunctional gastric tube.
Ventilator settings for data recording
Obvious spontaneous breathing was not observed when some deep-sedated patients were treated with a NMBA. The Drager Evita XL ventilator was adjusted to VC-IPPV and autoflow turned off. The clinicians referred to ARDSnet lung protective strategy to set ventilator parameters. And the patients underwent the treatment of fluid resuscitation and adjustment of acid-base balance according to clinical condition. The “Inspiration Hold” button on the ventilator was pressed for 1.5 s during an end-inspiratory pause to measure the plateau pressure (Pplat) and inspiratory esophageal pressure (Pesei) at this moment. The “Expiration Hold” button on the EVITA XL ventilator was pressed for 1.5 s during an end-expiratory pause to measure the total positive end expiratory pressure (PEEP) and expiratory esophageal pressure (Pesee) at this moment. When the esophageal pressure and intragastric pressure were recorded, the Vt, RR, PEEP and dynamic compliance displayed on the ventilator were exported in Excel through Ventview. The airway driving pressure (DP), Pesei, Pesee, ΔPes, PLei, PLee and transpulmonary driving pressure (DPL) were calculated according to Eqs. [1-5] (17,19):
Experimental procedures
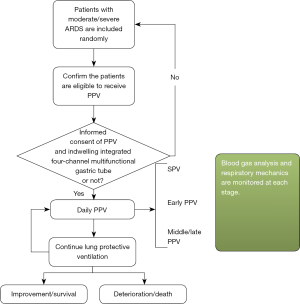
A prospective cohort study was conducted on patients with moderate/severe ARDS who underwent PPV in the ICU of the First Affiliated Hospital of Guangzhou Medical University from 2016 to 2021. The study subjects’ basic information was collected before PPV, including their name, gender, age, height (cm), body weight (kg), body mass index (BMI), admission diagnosis, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, Richmond Agitation-Sedation Scale (RASS), etc.
Following implantation and positioning, the airtightness of the esophageal balloon and gastric balloon were checked to ensure that they were not leaky or plicate. The three-channel pressure sensor was calibrated and clinician reconfirmed patient’s body position and made sure that the head of the bed was raised to 30°. All of the patients were ventilated with the Drager Evita XL ventilator in therapeutic mode VC-IPPV. Autoflow was enabled, and the flow-trigger was 1 L/min. PPV was performed continuously every day, and the duration was determined by the clinician, with the duration generally being 16 h. The tube feeding rate was determined by the chief physician.
The respiratory mechanics were measured at the moment before the patients began PPV [supine position ventilation (SPV) stage], within 4 hours after the patients had been started on PPV (early PPV), and after the patients had been receiving PPV for more than 6 h (middle/late PPV) every day.
Every patient was placed in the prone position after recording during SPV. In brief, with the aid of 3–4 medical workers, the patient’s head was tilted to one side to protect the face against compression damage. The arms were straightened upward and put on both sides of the head and a soft pillow was placed under the shoulders and pelvis. It was confirmed that the abdomen was not compressed to avoid effects on the venous drainage. The endotracheal tube, ventilator circuit, central and peripheral catheter and other drainage tubes were kept clear throughout the process. Attention was paid to the occurrence of pressure sores, etc. after PPV. The PPV lasted 16 hours each time as planned, followed by the next round.
Standards for terminating PPV (20): PPV was terminated if the patient experienced cardiac arrest, large airway obstruction, artificial airway extrusion or displacement, artificial airway obstruction, severe arrhythmia, facial injury, hemodynamic instability, pressure sores, hemoptysis, MAP <60 mmHg or systolic pressure drop >30 mmHg, a significant rise or decrease in HR, or when the PFR continuously and stably exceeded 200 mmHg. The clinician made the final decision whether to terminate PPV. Figure 3 showed the flow chart of the experimental procedure.
Statistical analysis
All available data were used without imputation for missing values. Descriptive data are presented as the median (interquartile range) or n (%). The relationships of the supine and prone position (SPV, PPV early stage, PPV middle/late stage) with repeated respiratory mechanics [Pesei, Pesee, ΔPes, PLei, PLee, DPL, DP, PEEP, peak airway pressure (Ppeak), Cre, Vt] and blood gas analysis (pH, PaCO2, PaO2) were investigated using generalized estimating equations (GEE) of linear regression to account for intra-individual correlations over time. The correlates of respiratory mechanics and blood gas indices were analyzed by multiple GEE models that included key factors (NMBAs use, BMI, age, gender) which differed among the three position groups. In these models, the evaluation of the respiratory mechanics and blood gas analysis were introduced as repeated measurements. Then, differences in the respiratory mechanics and blood gas analysis between the patients who survived and those who died were further assessed with a multiple GEE model of linear regression. In the GEE models, results with a two-sided P<0.05 were considered significant. Data are expressed as marginal mean, regression coefficients (B) and P values. The data analysis was performed using SPSS® 24.0 (IBM Corporation, Armonk, NY, USA) for Windows®.3. Manuscript figures were prepared with GraphPad Prism.
Results
General patient information
A total of 22 patients were enrolled and 17 of them were male (77.27%). Their oxygenation index calculated based on blood gas analysis met the diagnostic criteria for ARDS. And 18 of the 22 patients had pulmonary ARDS, and 16 of the pulmonary ARDS were due to severe pneumonia. A total of 76 times of PPV were performed on these patients and all of them were under deep sedation, with a median RASS score of −4. In the middle/late stage group, the median data of collection time point was 12 hours after the patients accepted PPV (Table 1).
Table 1
| Characteristics | Value |
|---|---|
| Age (years) | 58 [45, 69] |
| Females, n [%] | 5 [23] |
| Height (cm) | 169.50 [161.50, 172.75] |
| BMI (kg/m2) | 23.00 [19.75, 25.25] |
| APACHE II | 18 [15, 20] |
| SOFA | 7.50 [6.00, 10.00] |
| RASS | −4 [−4, −3] |
| Cause of respiratory failure, n [%] | |
| Pulmonary, infectious | 16 [73] |
| Pulmonary, non-infectious | 2 [9] |
| Extrapulmonary | 4 [18] |
| The duration of the overall patient from hospital admission to ICU admission (days) | 0.50 [0, 11.25] |
| The duration of the overall patient intubated before PPV start (days) | 5 [1, 8.5] |
| PFR before PPV (mmHg) | 131 [105, 163] |
| PaCO2 before PPV (mmHg) | 56 [50, 60] |
| FiO2 during PPV | 0.65 [0.60, 0.75] |
| Times of each patient accept PPV | 2 [2, 4] |
| Data collection time after the patients accept PPV (hours) | |
| Middle/late stage of PPV | 12 [11, 13] |
| NMBAs used, cases (%) | 133 (38.66) |
| ICU outcome, death (%) | 10 (45.45) |
Descriptive data are presented as the median [interquartile range], if not otherwise specified. BMI, body mass index; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; RASS, Richmond Agitation-Sedation Scale; ICU, intensive care unit; PPV, prone position ventilation; PFR, PaO2/FiO2 ratio; NMBAs, neuromuscular blocking agents.
The changes in respiratory mechanics based on the treatment position
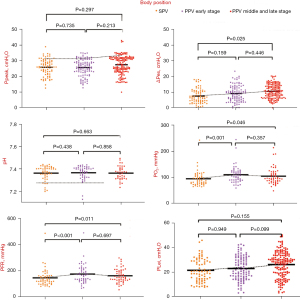
Due to the differences in body position, The PaO2 and PFR during PPV early stage and middle/late stage were significantly higher than those in SPV (SPV 95.35 vs. early PPV 109.46 vs. middle/late PPV 106.96 mmHg PaO2 marginal mean; both PSPVvs. early PPV and PSPVvs. middle/late PPV<0.05), (SPV 147.86 vs. early PPV 171.56 vs. middle/late PPV 169.78 mmHg PFR marginal mean; both PSPVvs. early PPV and PSPVvs. middle/late PPV<0.05). The ΔPes were significantly higher during PPV middle/late stage (SPV 7.46 vs. middle/late PPV 8.30 mmHg ΔPes marginal mean; PSPVvs. middle/late PPV=0.025<0.05). Although the ΔPes during PPV higher, it still fluctuated within a normal range (Table 2 and Figure 4).
Table 2
| Indices | SPV [1] | Early PPV [2] | Middle/late PPV [3] | B (coefficient) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 vs. 3 | 2 vs. 3 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |||||
| Gas exchange | |||||||||
| pH | 7.359 | 7.364 | 7.367 | −0.008 | −0.003 | 0.438 | 0.663 | 0.858 | |
| PaO2 (mmHg) | 95.345 | 109.464 | 106.956 | −11.610 | 2.509 | 0.001 | 0.046 | 0.357 | |
| PaCO2 (mmHg) | 56.090 | 55.576 | 56.432 | −0.341 | −0.856 | 0.642 | 0.887 | 0.680 | |
| PFR (mmHg) | 147.860 | 171.560 | 169.780 | −21.928 | 1.778 | <0.000 | 0.011 | 0.697 | |
| Respiratory mechanics | |||||||||
| Pesei (cmH2O) | 3.604 | 3.516 | 3.137 | 0.467 | 0.379 | 0.866 | 0.291 | 0.328 | |
| Pesee (cmH2O) | 2.945 | 2.728 | 2.608 | 1.261 | 1.146 | 0.709 | 0.354 | 0.781 | |
| ΔPes (cmH2O) | 7.457 | 8.003 | 8.303 | −0.846 | −0.300 | 0.159 | 0.025 | 0.446 | |
| PLei (cmH2O) | 21.941 | 21.969 | 22.688 | −0.747 | −0.719 | 0.949 | 0.155 | 0.099 | |
| PLee (cmH2O) | 5.536 | 6.187 | 6.161 | −0.625 | 0.026 | 0.195 | 0.071 | 0.954 | |
| DPL (cmH2O) | 16.595 | 15.937 | 16.820 | −0.224 | −0.883 | 0.234 | 0.535 | 0.180 | |
| DP (cmH2O) | 17.010 | 16.960 | 17.477 | −0.467 | −0.517 | 0.886 | 0.225 | 0.233 | |
| EMGdi (μV) | 15.378 | 16.225 | 15.403 | 0.000 | 0.001 | 0.713 | 0.986 | 0.462 | |
| Vt (mL) | 385.506 | 373.525 | 379.065 | 6.441 | −5.540 | 0.175 | 0.358 | 0.334 | |
| RR (breaths/min) | 27.023 | 26.175 | 26.871 | 0.161 | −0.657 | 0.097 | 0.638 | 0.123 | |
| PEEP (cmH2O) | 8.802 | 8.844 | 8.811 | −0.010 | 0.033 | 0.157 | 0.896 | 0.615 | |
| Ppeak (cmH2O) | 25.664 | 25.552 | 26.073 | −0.409 | −0.521 | 0.735 | 0.297 | 0.213 | |
| Cre (L/cmH2O) | 24.181 | 24.575 | 25.114 | −0.933 | −0.539 | 0.725 | 0.611 | 0.725 | |
Data are expressed as the “marginal mean, B” if not otherwise specified, B represents coefficient of different prone positions compared to middle/late-PPV group in GEE model. PFR, PaO2/FiO2 ratio; Pesei, inspiratory esophageal pressure; Pesee, expiratory esophageal pressure; ΔPes, esophageal pressure swings; PLei, inspiratory transpulmonary pressure; PLee, expiratory transpulmonary pressure; DPL, transpulmonary driving pressure; DP, driving pressure; EMGdi, diaphragmatic electromyogram; Vt, tidal volume; RR, respiration rate; PEEP, total positive end expiratory pressure; Ppeak, peak airway pressure; Cre, respiratory compliance; SPV, supine position ventilation; PPV, prone position ventilation; GEE, generalized estimating equations.
A stratified analysis according to patient outcome (death vs. survival)
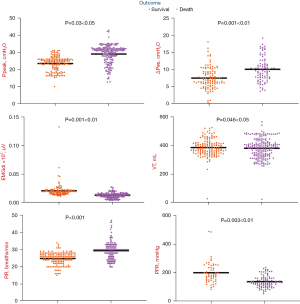
The Ppeak, ΔPes and RR in the death group were significantly higher than the survival group (27.48 vs. 24.23 cmH2O Ppeak; P<0.05), (8.95 vs. 6.97 cmH2O ΔPes; P=0.001) (30.33 vs. 23.34 breaths/min RR; P<0.001). In contrast, the Vt, EMGdi and PFR in the death group were significantly lower than the survival group (359.60 vs. 401.11 mL Vt; P<0.05), (10.79 vs. 21.53 µV EMGdi; P=0.001), (132.68 vs. 211.55 mmHg PFR; P=0.003). It may suggest that patients with a poor prognosis had more difficulty ventilating and poorer oxygenation, and suffered from more severe dysfunction of their pulmonary ventilation. What’s more, these findings may also suggest that even after PPV, the patients who died did not experience a significant improvement in alveolar ventilation, and the effects of pulmonary recruitment were unsatisfactory (Table 3 and Figure 5).
Table 3
| Indices | Survival group | Death group | B (coefficient) | P value |
|---|---|---|---|---|
| Gas exchange | ||||
| pH | 7.378 | 7.347 | −0.030 | 0.074 |
| PaO2 (mmHg) | 111.126 | 96.612 | −14.514 | 0.391 |
| PaCO2 (mmHg) | 54.048 | 58.251 | 4.203 | 0.288 |
| PFR (mmHg) | 211.550 | 132.680 | −89.685 | 0.003 |
| Respiratory mechanics | ||||
| Pesei (cmH2O) | 2.429 | 4.529 | 2.100 | 0.200 |
| Pesee (cmH2O) | 2.905 | 2.602 | −0.303 | 0.832 |
| ΔPes (cmH2O) | 6.972 | 8.951 | 1.979 | 0.001 |
| PLei (cmH2O) | 21.266 | 23.167 | 1.901 | 0.549 |
| PLee (cmH2O) | 5.364 | 6.576 | 1.212 | 0.454 |
| DPL (cmH2O) | 15.501 | 17.479 | 1.978 | 0.237 |
| DP (cmH2O) | 15.845 | 18.564 | 2.719 | 0.059 |
| EMGdi (μV) | 21.530 | 10.785 | −0.011 | 0.001 |
| Vt (mL) | 401.108 | 359.599 | −41.509 | 0.046 |
| RR (breaths/min) | 23.338 | 30.325 | 6.986 | <0.000 |
| PEEP (cmH2O) | 8.532 | 9.139 | 0.606 | 0.398 |
| Ppeak (cmH2O) | 24.233 | 27.484 | 3.251 | 0.030 |
| Cre (L/cmH2O) | 31.379 | 18.114 | −13.265 | 0.055 |
Data are expressed as the “marginal mean, B” if not otherwise specified. B represents coefficient of death group compared to survival group in GEE model. PFR, PaO2/FiO2 ratio; Pesei, inspiratory esophageal pressure; Pesee, expiratory esophageal pressure; ΔPes, esophageal pressure swings; PLei, inspiratory transpulmonary pressure; PLee, expiratory transpulmonary pressure; DPL, transpulmonary driving pressure; DP, driving pressure; EMGdi, diaphragmatic electromyogram; Vt, tidal volume; RR, respiration rate; PEEP, total positive end expiratory pressure; Ppeak, peak airway pressure; Cre, respiratory compliance; GEE, generalized estimating equations.
Effects of NMBAs on the respiratory mechanics during PPV
With regard to the effects of NMBAs on respiratory mechanics, the Pesei increased significantly when NMBAs were used (5.91 vs. 0.93 cmH2O; P<0.01). The ΔPes, PLei, DPL and DP all decreased significantly when NMBAs were used (7.08 vs. 8.76 cmH2O ΔPes; P<0.01), (19.71 vs. 24.69 cmH2O PLei; P<0.001), (14.82 vs. 18.08 cmH2O DPL; P<0.001), (16.73 vs. 17.56 cmH2O DP; P<0.05). It may suggest that NMBAs may improve man-machine synchronization and reduce muscle work during inspiration, thereby achieving improved positive pressure ventilation. What’s more, the rational use of NMBAs may exert a synergistic effect on lung-protective ventilation (Table 4).
Table 4
| Indices | NMBAs treatment | No NMBAs used | B (coefficient) | P value |
|---|---|---|---|---|
| Gas exchange | ||||
| pH | 7.367 | 7.360 | −0.007 | 0.732 |
| PaO2 (mmHg) | 97.400 | 110.443 | 13.043 | 0.243 |
| PaCO2 (mmHg) | 55.521 | 56.544 | 1.024 | 0.766 |
| PFR (mmHg) | 149.310 | 176.820 | 27.514 | 0.056 |
| Respiratory mechanics | ||||
| Pesei (cmH2O) | 5.908 | 0.929 | −4.979 | 0.007 |
| Pesee (cmH2O) | 3.592 | 1.929 | −1.663 | 0.127 |
| ΔPes (cmH2O) | 7.082 | 8.760 | 1.678 | 0.003 |
| PLei (cmH2O) | 19.705 | 24.693 | 4.988 | <0.000 |
| PLee (cmH2O) | 5.271 | 6.652 | 1.382 | 0.068 |
| DPL (cmH2O) | 14.822 | 18.079 | 3.257 | <0.000 |
| DP (cmH2O) | 16.734 | 17.564 | 0.830 | 0.031 |
| EMGdi (μV) | 16.263 | 15.074 | −0.001 | 0.323 |
| Vt (mL) | 388.174 | 370.556 | −17.618 | 0.088 |
| RR (breaths/min) | 27.068 | 26.311 | −0.757 | 0.501 |
| PEEP (cmH2O) | 9.111 | 8.527 | −0.583 | 0.079 |
| Ppeak (cmH2O) | 25.812 | 25.713 | −0.099 | 0.770 |
| Cre (L/cmH2O) | 25.270 | 23.977 | −1.293 | 0.303 |
Data are expressed as the “marginal mean, B” if not otherwise specified. B represents coefficient of no NMBAs group compared to NMBAs group in GEE model. NMBAs, neuromuscular blocking agents; PFR, PaO2/FiO2 ratio; Pesei, inspiratory esophageal pressure; Pesee, expiratory esophageal pressure; ΔPes, esophageal pressure swings; PLei, inspiratory transpulmonary pressure; PLee, expiratory transpulmonary pressure; DPL, transpulmonary driving pressure; DP, driving pressure; EMGdi, diaphragmatic electromyogram; Vt, tidal volume; RR, respiration rate; PEEP, total positive end expiratory pressure; Ppeak, peak airway pressure; Cre, respiratory compliance; GEE, generalized estimating equations.
Discussion
The most typical pathophysiological changes associated with ARDS are caused by the heterogeneity of intrapulmonary air distribution (21), and PPV can homogenize the intrapulmonary air distribution by changing the air distribution in the gravity-dependent and independent regions, thereby relieving VILI to protect the lung.
However, it is not clear if the pathophysiological changes can be maintained at the later stage of PPV (22). Our results showed that during the early stage and middle/late stage of transition from SPV to PPV, the values of PaO2 and PFR were improved while there was no significant change in PaCO2, suggesting that PPV may improve the oxygenation of the patients. Although the ΔPes during PPV was significantly higher, it still fluctuated within a normal range, which indicated that PPV in this stage may affect the patients’ ventilation within an acceptable range, as the prone position is not routine and may cause discomfort to the patients. A highly active anterior thorax is restricted when patients are in the prone position, because it is usually accompanied by discomfort and increased resistance to diaphragmatic movement, leading to a significant increase in the EMGdi (6). Previous studies on the EMGdi in healthy volunteers and stabilized COPD patients showed that the EMGdi in the prone position was significantly higher than the supine position, especially in COPD patients (23,24). However, the above-mentioned change in the EMGdi in the waking state was not observed during the treatment of patients with severe ARDS (9). Thus, it was unclear whether the improvement of tissue oxygenation and the use of deep sedation and paralysis relieved respiratory embarrassment in patients with moderate/severe ARDS. The ΔPes is an index that indirectly reflects the activity of the diaphragm and accessory respiratory muscles (25,26). The increase of ΔPes during PPV and its fluctuation within a safe range in our study suggest that PPV was feasible and probably relatively harmless during the period involved in our study since the patients’ ventilation was only slightly affected. The retention of this ΔPes fluctuation may be beneficial to the retention of dorsal diaphragmatic activity.
The current study showed PPV may promote oxygenation with slight effect on patients, and other studies confirmed that the improvement of oxygenation by PPV does not have an apparent relationship with the patient prognosis (23-25). To further explore this issue, we performed a stratified analysis according to patient outcome (death/survival), showing that the PFR and Vt were significantly higher in survival group patients, while the RR was significantly higher in the death group. The current study also showed significantly higher Ppeak and ΔPes and lower EMGdi in the patients who died than those who survived. Besides, the DPL and DP were higher in the death group, although there was no statistical difference. These findings suggested that, although the oxygenation improved in both groups, the respiratory embarrassment was not improved in the patients who died. Due to the differences in the above respiratory mechanics between the survival group and the death group, further study with more clinical data is necessary to monitor respiratory mechanics and to explore non-responders to prevent futile attempts at repeat PPV.
In addition to deep sedation, patients with moderate/severe ARDS often need to be treated with NMBAs. Although the prognosis of moderate/severe ARDS was not improved by the early application of a NMBA in previous studies (27,28), little is known about the effects of NMBAs on the respiratory mechanics of PPV patients. The results of the present study suggest that the DPL, DP, PLei and ΔPes in the patients treated with NMBAs were all significantly lower than those not treated with a NMBA. When NMBAs were used, the marginal mean of DPL was 14.82 cmH2O, which was close to the safe range recommended in the 2017 SSC Expert Consensus (29), indicating that NMBAs may exert synergistic lung protective effects in such patients. When NMBAs were used, the ΔPes decreased significantly, the Pesei was significantly higher, and there was no significant change in the EMGdi, suggesting that the NMBAs did not simply block the activity of respiratory muscles, but also maintained the proper excitability of the diaphragm or accessory respiratory muscles, reducing the respiratory distress caused by spastic contractions and the “gas pendulum”, making it possible to improve airway and lung protection in the form of man-machine synchronization (30,31).
There is still a lack of effective monitoring methods for evaluating the efficacy of PPV in patients with severe ARDS after long-term PPV. In this paper, an integrated four-chambered multifunctional gastric tube was used to dynamically monitor the changes in the transpulmonary pressure, DP, Pplat, EMGdi, oxygenation and ventilation before and after prone positioning. The results of this study suggest that it is feasible to monitor transpulmonary pressure in ARDS patients during PPV because the transpulmonary pressure removes the effects associated with the elastic resistance of the chest wall (32). Compared with the compliance and DP, the transpulmonary pressure can better reveal the extent of lung injury caused by mechanical ventilation and more accurately reflect the respiratory mechanical characteristics of ARDS patients’ lungs. We found that the respiratory mechanical indices, including the transpulmonary pressure, were not constant during PPV, but fluctuated with time, conditions, sedation, NMBAs use, etc., and even increased to a critical threshold. This requires us to identify the causes, give symptomatic treatment, and adjust the parameters to avoid causing extra lung injury by the PPV. The patients’ spontaneous breathing is difficult to completely block because of their condition. Even if sedation is deepened at the cost of affecting the circulatory perfusion (33), the depth of sedation cannot be identified, as the RASS score remains consistent in these individuals (≤−4 points) (34). Therefore, maintaining spontaneous breathing with a proper EMGdi in patients with severe ARDS is a requirement that must be accounted for when modifying the sedation, analgesia and optimizing the man-machine synchronization between lung protection and diaphragm protection (35).
There are several limitations of the present study, such as the fact that it was a single-center analysis, covered a large time span, included a small number of patients, and the patients had differences in their basal diseases. For various reasons, PPV was not performed within 36 h for every patient, and not every patient was observed throughout the course of PPV, thus limiting our number of eligible patients. Although we defined SPV, early PPV and middle/late PPV, only a 2-minute record was kept for each stage, and this does not fully represent the changes in respiratory mechanics that occur throughout the process of PPV. Moreover, PPV was not performed every day in each patient. Although the duration of each PPV exceeded 16 h, 16 h was not a fixed duration. Therefore, the comparability of the data may be limited. In the future, dynamic monitoring may be conducted during PPV for patients with severe ARDS to provide more representative data. More patients will be included in future studies, and the monitoring will be prolonged. In addition, the EMGdi will be dynamically monitored based on the transpulmonary pressure, and EIT will also be used to further guide the implementation of LPVS.
Conclusions
In this prospective cohort study involving patients with moderate to severe ARDS, PPV may improve the oxygenation of the patients while ΔPes still fluctuated within a normal range. There are differences in respiratory mechanics between the patients who received PPV with different outcomes. And the rational combination of NMBAs and PPV may exert a synergistic protective effect on the lungs. However, these findings still need more research to confirm.
Acknowledgments
Funding: This work was supported by grants from the Guangzhou Science and Technology Planning Project (No. 202102010353), Scientific and Technological Innovation Strategy Project of Guangdong Province (No. 2020B1111340005); and Guangdong Zhong Nan-Shan Medical Foundation (No. ZNSA-2020001) and the Jack Ma Foundation.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-291/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-291/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-291/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-291/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the First Affiliated Hospital of Guangzhou Medical University (No. 2017-034). Signed informed consent forms (ICF) were obtained from family members of patients with moderate/severe ARDS eligible to receive PPV and an indwelling integrated four-channel multifunctional gastric tube.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Peiris JS, Yuen KY, Osterhaus AD, et al. The severe acute respiratory syndrome. N Engl J Med 2003;349:2431-41. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Guérin C, Beuret P, Constantin JM, et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med 2018;44:22-37. [Crossref] [PubMed]
- Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009;302:1977-84. [Crossref] [PubMed]
- Gattinoni L, Pesenti A, Carlesso E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: impact and clinical fallout through the following 20 years. Intensive Care Med 2013;39:1909-15. [Crossref] [PubMed]
- Dianti J, Matelski J, Tisminetzky M, et al. Comparing the Effects of Tidal Volume, Driving Pressure, and Mechanical Power on Mortality in Trials of Lung-Protective Mechanical Ventilation. Respir Care 2021;66:221-7. [Crossref] [PubMed]
- Mauri T, Lazzeri M, Bellani G, et al. Respiratory mechanics to understand ARDS and guide mechanical ventilation. Physiol Meas 2017;38:R280-H303. [Crossref] [PubMed]
- Sun QW, Li XC, Lin ZM, et al. Assessment of respiratory drive with esophageal diaphragmatic electromyography in patients with acute respiratory distress syndrome treated with prone position ventilation. J Thorac Dis 2019;11:4188-96. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Gattinoni L, Taccone P, Carlesso E, et al. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med 2013;188:1286-93. [Crossref] [PubMed]
- Scholten EL, Beitler JR, Prisk GK, et al. Treatment of ARDS With Prone Positioning. Chest 2017;151:215-24. [Crossref] [PubMed]
- Wang YY, Lin ZM, Chen SB, et al. Assessment of Diaphragm Function with A Newly Designed Multi-function Esophageal Electrode Catheter and Bilateral Anterolateral Magnetic Stimulation of Phrenic Nerves in Patients Underwent Mechanical Ventilation. Chinese Journal of Respiratory and Critical Care Medicine 2014;13:348-52.
- Mauri T, Yoshida T, Bellani G, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 2016;42:1360-73. [Crossref] [PubMed]
- Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31. [Crossref] [PubMed]
- Brochard L. Measurement of esophageal pressure at bedside: pros and cons. Curr Opin Crit Care 2014;20:39-46. [Crossref] [PubMed]
- Benditt JO. Esophageal and gastric pressure measurements. Respir Care 2005;50:68-75; discussion 75-77. [PubMed]
- Baydur A, Behrakis PK, Zin WA, et al. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 1982;126:788-91. [PubMed]
- Loring SH, O'Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol (1985) 2010;108:515-22. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Cressoni M, Cadringher P, Chiurazzi C, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2014;189:149-58. [Crossref] [PubMed]
- Cornejo RA, Díaz JC, Tobar EA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2013;188:440-8. [Crossref] [PubMed]
- Lee DL, Chiang HT, Lin SL, et al. Prone-position ventilation induces sustained improvement in oxygenation in patients with acute respiratory distress syndrome who have a large shunt. Crit Care Med 2002;30:1446-52. [Crossref] [PubMed]
- Pappert D, Rossaint R, Slama K, et al. Influence of positioning on ventilation-perfusion relationships in severe adult respiratory distress syndrome. Chest 1994;106:1511-6. [Crossref] [PubMed]
- Stocker R, Neff T, Stein S, et al. Prone postioning and low-volume pressure-limited ventilation improve survival in patients with severe ARDS. Chest 1997;111:1008-17. [Crossref] [PubMed]
- Xu YD, Li RF, Luo YM, et al. Impact of pulmonary ventilatory function of patients with stable chronic obstructive pulmonary disease on simultaneous suspended prone position. Chinese Journal of Biomedical Engineering 2011;17:264-8.
- Alhazzani W, Belley-Cote E, Møller MH, et al. Neuromuscular blockade in patients with ARDS: a rapid practice guideline. Intensive Care Med 2020;46:1977-86. [Crossref] [PubMed]
- National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med 2019;380:1997-2008.
- Guervilly C, Bisbal M, Forel JM, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med 2017;43:408-18. [Crossref] [PubMed]
- Hraiech S, Yoshida T, Annane D, et al. Myorelaxants in ARDS patients. Intensive Care Med 2020;46:2357-72. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Gattinoni L, Chiumello D, Carlesso E, et al. Bench-to-bedside review: chest wall elastance in acute lung injury/acute respiratory distress syndrome patients. Crit Care 2004;8:350-5. [Crossref] [PubMed]
- Ebert TJ. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology 2005;103:20-4. [Crossref] [PubMed]
- Dzierba AL, Khalil AM, Derry KL, et al. Discordance Between Respiratory Drive and Sedation Depth in Critically Ill Patients Receiving Mechanical Ventilation. Crit Care Med 2021;49:2090-101. [Crossref] [PubMed]
- Wongtangman K, Grabitz SD, Hammer M, et al. Optimal Sedation in Patients Who Receive Neuromuscular Blocking Agent Infusions for Treatment of Acute Respiratory Distress Syndrome-A Retrospective Cohort Study From a New England Health Care Network. Crit Care Med 2021;49:1137-48. [Crossref] [PubMed]