Serum cystatin C is a potential predictor of short-term mortality and acute kidney injury in acute aortic dissection patients: a retrospective cohort study
Introduction
Acute aortic dissection (AAD) is a life-threatening aortic syndrome with high mortality (1,2). In a large autopsy series, the prevalence of aortic dissection ranged from 0.2–0.8% (3). Population-based study estimated the incidence of AAD to be between 2.5 and 7.2 cases per 100,000 per year (4). It is reported that the mortality rate of AAD ranged from 10.8–74.5% in the last 30 years (4). Despite the gradual standardization of AAD management, the mortality rate remains high. It has been reported that if untreated with swift open surgical repair, acute type A aortic dissection has a mortality rate as high as 90% (5). Due to its poor prognosis, early rapid assessment of prognosis, as well as organ functional damage, and timely and appropriate treatment interventions are crucial.
Currently, the diagnostic gold standard for AAD still relies on imaging, such as computed tomography CT scan, echocardiography or magnetic resonance angiography (6). AAD is featured with the rapid development of an intimal flap that separates the true lumen from the false lumen (7). The formation of the false lumen causes multi-organ dysfunction and even death. Thus, some indicators for early post-diagnosis assessment need to be identified.
In recent decades, some studies have shown that some biomarkers, such as D-dimer, monocytes/high-density lipoprotein ratio, and a triglyceride/high-density lipoprotein cholesterol ratio, have certain value in the prognosis assessment of AAD patients (8-10). However, we still need to explore some simple and readily available indicators for organ function and the prognosis assessment of AAD patients.
Cystatin C is a potent cysteine protease inhibitor that plays a pleiotropic role in human vascular pathophysiology, particularly in regulating cathepsins S and K (11,12). Cystatin C levels increase earlier than urea and creatinine when renal is injured (13). A previous study showed that cystatin C is strongly associated with cardiovascular disease risk in a dose-dependent manner (14). However, to date, the role of cystatin C in the renal function and prognosis assessment of AAD patients has not been closely examined. In the present study, we aim to evaluate the relationship between serum cystatin C levels and the renal function and prognosis of AAD patients. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-937/rc).
Methods
Study design and population
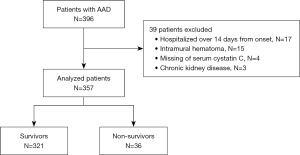
We conducted a retrospective observational study of AAD patients admitted to the Department of Cardiovascular Surgery at The First Affiliated Hospital of Soochow University from November 2019 through January 2022. The diagnostic criteria was based on guidelines proposed by European Society of Cardiology (ESC) 2014 on the treatment and diagnosis of aortic diseases (15). The Stanford classification system was used to categorize AAD type (16). Only patients admitted to hospital within 14 days of AAD onset were included in our study. Patients with intramural hematoma, chronic kidney disease, and missing cystatin C data were excluded from the study. The flow chart of patient inclusion is shown in Figure 1.
This study was reviewed and approved by the Ethics Committee of The First Affiliated Hospital of Soochow University (IRB No. 2022–212). As a retrospective study, the requirement of informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data collection
Data on patients’ demographic information, chronic comorbidities (including hypertension, diabetes, and hyperlipemia), clinic symptoms, Stanford classification type, previous treatments, laboratory tests, complications, the time from symptom onset to admission, and patients’ survival at 60 days were collected. All the data were obtained from the electronic medical records system using data collection forms.
The definition of hypertension was systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DPB) ≥90 mmHg. Accelerated hypertension was defined as SBP ≥180 mmHg and/or DPB ≥110 mmHg (17). Shock was defined as SBP <90 mmHg. Patients were diagnosed with diabetes if they had a fasting plasma glucose level >7.0 mmol/L, a random blood glucose level >11.1 mmol/L, or used any hypoglycemic drugs. Patients were diagnosed with heart failure if they had an ejection fraction <50% according to echocardiography. Patients were diagnosed with liver injury if they had elevated bilirubin and aminotransferase serum levels. Acute kidney injury (AKI) was defined according to the Kidney Disease: Improving Global Outcomes (KIDGO) clinical practice guidelines (18). Patients who met the criteria for AKI during hospitalization were diagnosed. All the data were collected from electronic medical record system and cross-checked independently by 3 physicians. Missing data were excluded from the analysis.
Laboratory tests
A complete blood cell count, a high-sensitivity C-reactive protein (hs-CRP) test, serum biochemistry tests, including tests to determine the level of serum cystatin C, creatinine, and total cholesterol, routine blood coagulation tests, including tests for D-dimer were conducted. All the tests were performed in the Clinical Testing Center at The First Affiliated Hospital of Soochow University.
Statistical analysis
The continuous variables are expressed as the mean ± standard deviation of the mean (normal distribution) or median with interquartile range (Q1, Q3) (skewed distribution), while the categorical variables are expressed as frequencies or percentages. Kruskal-Wallis H (skewed distribution) and One-Way ANOVA analysis of variance tests (normal distribution) or χ2 tests (categorical variables) were performed to compare the different groups.
Univariate and multivariate Cox proportional-hazards regression analyses were conducted to identify the independent risk factors for mortality, and the adjusted hazard ratios (HRs) of mortality are presented in a forest plot. Given the total number of deaths in our study and to avoid overfitting in the model, all variables with P<0.1 in univariate analysis except for creatinine were chosen for the multivariate analysis based on previous findings and the clinical constraints. Kaplan-Meier analyses were conducted to compare the survival rates among the patients with different levels of serum cystatin C using the log-rank test.
The association between cystatin C and AKI was evaluated in different multivariate logistic regression models. In Model I, covariates including sex, age, and BMI were incorporated for adjustment. In Model II, the covariates in Model I and comorbidities were adjusted. In Model III, the covariates in Model II and other covariates (white blood cell counts, platelet counts, hs-CRP, total bilirubin and D-dimer) were adjusted. Area under the receiver operating characteristic curves (AUROCs) were conducted to evaluate the predictive value of cystatin C in terms of the mortality of and the incidence of AKI in AAD patients.
A 2-sided α value of <0.05 was considered statistically significant. All statistics were analyzed using SPSS statistical software program package (SPSS version 26.0 for Windows, IBM), and graphs were collated and created with GraphPad Prism 9.0 software (GraphPad Software).
Results
Study participants and baseline characteristics
A total of 396 patients were diagnosed with AAD and hospitalized in the Department of Cardiovascular Surgery at The First Affiliated Hospital of Soochow University from November 2019 to January 2022. Among them, 17 patients were admitted to hospital >14 days from onset, 15 patients were diagnosed with intramural hematoma, 4 patients had missing serum cystatin C data, and 3 patients with CKD were excluded. Ultimately, 357 patients met the inclusion criteria for the study. The flow chart of patients selection is shown in Figure 1.
All the participants were categorized into four groups according to quartiles of serum cystatin C concentration (Q1 <0.81 mg/L; Q2 =0.81–0.98 mg/L; Q3 =0.98–1.18 mg/L; and Q4 >1.18 mg/L). Base line characteristics are presented in Table 1. Notably, the proportion of males and the frequency of hypertension were higher in the high serum cystatin C concentration group (P<0.001). The higher the concentration of cystatin C, the higher the level of serum creatinine and the higher the incidence of AKI (P<0.001). Mortality was significantly higher in the Q4 group (P=0.003).
Table 1
| Characteristics | Cystatin C, mg/L | P | |||
|---|---|---|---|---|---|
| Q1 (n=91) | Q2 (n=87) | Q3 (n=91) | Q4 (n=88) | ||
| Age, years | 50.09±12.64 | 53.01±13.75 | 55.43±13.84 | 53.10±13.56 | 0.068 |
| Male, n (%) | 64 (70.3%) | 74 (85.1%) | 87 (95.6%) | 78 (88.6%) | <0.001 |
| BMI, kg/m2 | 24.50 (21.48, 26.57) | 26.06 (23.66, 29.06) | 25.65 (23.51, 28.73) | 25.868 (23.55, 29.06) | 0.001 |
| AAD type A, n (%) | 53 (58.2%) | 46 (52.9%) | 47 (51.6%) | 54 (61.4%) | 0.521 |
| Hypertension, n (%) | 48 (52.7%) | 58 (66.7%) | 73 (80.2%) | 71 (80.7%) | <0.001 |
| DM, n (%) | 4 (4.4%) | 5 (5.7%) | 4 (4.4%) | 3 (3.4%) | 0.904 |
| Hyperlipemia, n (%) | 22 (24.2%) | 17 (19.5%) | 17 (18.7%) | 21 (23.9%) | 0.729 |
| Smoker, n (%) | 20 (22%) | 16 (18.6%) | 29 (31.9%) | 27 (30.7%) | 0.119 |
| Heart rate, bpm | 77 (73, 89) | 80 (70, 92) | 80 (72, 92) | 80 (72, 94) | 0.766 |
| SBP, mmHg | 140 (126, 153) | 140 (127, 157) | 147 (126, 162) | 145 (120, 165) | 0.546 |
| DBP, mmHg | 75 (65, 87) | 75 (61, 88) | 76 (63, 93) | 76 (64, 88) | 0.854 |
| WBC, ×109/L | 10.93 (8.42, 14.01) | 11.51 (8.46, 13.92) | 11.22 (8.90, 13.63) | 12.50 (9.93, 14.86) | 0.099 |
| PLT, ×109/L | 162.00 (137.00, 204.00) | 169.50 (132.25, 217.50) | 172.00 (128.00, 210.00) | 159.50 (127.25, 203.75) | 0.835 |
| TBIL, μmol/L | 16.40 (12.90, 22.10) | 17.75 (12.13, 27.98) | 18.20 (13.20, 25.40) | 17.55 (11.90, 23.175) | 0.608 |
| TCHOL, mmol/L | 4.27 (3.70, 4.87) | 4.34 (3.47, 4.99) | 4.03 (3.59, 4.62) | 3.79 (3.21, 4.58) | 0.013 |
| sCr, μmol/L | 59.00 (50.40, 68.10) | 72.20 (63.80, 79.48) | 88.40 (74.90, 101.00) | 131.05 (105.15, 186.52) | <0.001 |
| hs-CRP, mg/L | 7.14 (2.45, 27.07) | 11.20 (2.70, 37.76) | 7.83 (3.69, 25.71) | 8.59 (2.71, 42.55) | 0.557 |
| D-Dimer, μg/mL | 4.07 (1.61, 12.16) | 5.43 (1.81, 16.76) | 4.76 (1.93, 11.83) | 9.88 (2.51, 20.00) | 0.016 |
| IVST, mm | 10.00 (10.00, 11.00) | 11.00 (10.00, 12.00) | 11.00 (10.00, 13.00) | 12.00 (10.00, 13.00) | <0.001 |
| AKI, n (%) | 8 (8.8%) | 21 (24.1%) | 25 (27.5%) | 70 (79.5%) | <0.001 |
| Death, n (%) | 6 (6.6%) | 6 (6.9%) | 6 (6.6%) | 18 (20.5%) | 0.003 |
The data are shown as mean ± standard or median (Q1, Q3). Q1: <0.81 mg/L; Q2: 0.81–0.98 mg/L; Q3: 0.98–1.18 mg/L; Q4: >1.18 mg/L. BMI, body mass index; AAD, acute aortic dissection; DM, diabetes mellitus; SBP, systolic pressure; DBP, diastolic pressure; WBC, white blood cell count; PLT, platelet counts; TBIL, total bilirubin; TCHOL, total cholesterol; sCr, serum creatinine; CRP, C-reactive protein; IVST, interventricular septum thickness; AKI, acute kidney injury.
The relationship between cystatin C and in-hospital mortality
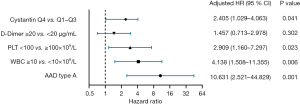
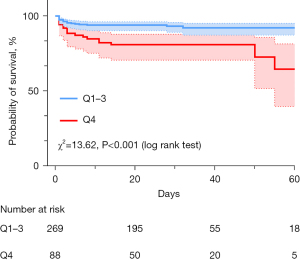
In the univariate Cox regression analysis for in-hospital mortality, patients in the Q4 group had an increased risk of death [HR =3.184, 95% confidence interval (CI), 1.656–6.121, P=0.001]. Subsequently, in the multivariate Cox regression analysis, type A AAD, a white blood cell (WBC) count >10×109/L, a platelet (PLT) count <100×109/L, and a serum cystatin C concentration level >1.18 mg/L (adjusted HR =2.405, 95% CI, 1.029–4.063, P=0.041) were independent risk factors for in-hospital mortality (see Table 2). The adjusted HRs of each independent risk factor for in-hospital mortality are presented in a forest plot (see Figure 2). All the patients were categorized into two groups (Q1–3 and Q4) according to the lower quartile, and the Kaplan-Meier analysis indicated that the Q4 group had a significantly higher cumulative death rate than the Q1–3 group (log-rank test, χ2=13.62, P<0.001; see Figure 3). A ROC curve was used to further investigate the predictive power of serum cystatin C in evaluating in-hospital mortality in AAD patients, and the area under the curve (AUC) was 0.655 (95% CI, 0.551–0.760), which shows that cystatin C can act as a prognostic predictor for AAD patients (see Figure 4).
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age, years | 0.999 (0.976–1.023) | 0.927 | – | – | |
| Male | 0.773 (0.340–1.757) | 0.539 | – | – | |
| BMI, kg/m2 | 1.021 (0.947–1.100) | 0.588 | – | – | |
| AAD | |||||
| Type B | 1 (Ref) | – | 1 (Ref) | – | |
| Type A | 9.174 (2.818–29.870) | <0.001 | 10.631 (2.521–44.829) | 0.001 | |
| Hypertension | 1.212 (0.589–2.496) | 0.602 | – | – | |
| DM | 1.238 (0.298–5.145) | 0.769 | – | – | |
| Hyperlipemia | 0.934 (0.428–1.279) | 0.865 | – | – | |
| Smoke | 0.535 (0.224–1.270) | 0.159 | – | – | |
| Heart rate per 10 bpm | 1.136 (0.931–1.387) | 0.210 | – | – | |
| SBP, mmHg | |||||
| <90 | 2.549 (0.759–8.565) | 0.130 | – | – | |
| ≥90 to ≤140 | 1 (Ref) | – | – | – | |
| >140 | 0.567 (0.288–1.115) | 0.100 | – | – | |
| WBC, ×109/L | |||||
| <10 | 1 (Ref) | – | 1 (Ref) | – | |
| ≥10 | 3.630 (1.409–9.348) | 0.008 | 4.138 (1.508–11.355) | 0.006 | |
| PLT, ×109/L | |||||
| ≥100 | 1 (Ref) | – | 1 (Ref) | – | |
| <100 | 2.608 (1.139–5.971) | 0.023 | 2.909 (1.160–7.297) | 0.023 | |
| TBIL, μmol/L | |||||
| ≤23 | 1 (Ref) | – | – | – | |
| >23 | 0.893 (0.407–1.960) | 0.779 | – | – | |
| TCHOL, mmol/L | |||||
| ≤5.2 | 1 (Ref) | – | – | – | |
| >5.2 | 1.344 (0.520–3.475) | 0.542 | – | – | |
| sCr per 10 μmol/L | 1.078 (1.039–1.119) | <0.001 | – | – | |
| CRP, mg/L | 0.998 (0.990–1.006) | 0.639 | – | – | |
| D-Dimer | |||||
| <20 | 1 (Ref) | – | 1 (Ref) | – | |
| ≥20 | 2.815 (1.441–5.501) | 0.002 | 1.457 (0.713–2.978) | 0.302 | |
| IVST, μg/mL | |||||
| ≤10 | 1 (Ref) | – | – | – | |
| >10 | 1.326 (0.675–2.604) | 0.413 | – | – | |
| Cystatin C, mg/L | |||||
| Q1–Q3 | 1 (Ref) | – | 1 (Ref) | – | |
| Q4 | 3.184 (1.656–6.121) | 0.001 | 2.405 (1.029–4.063) | 0.041 | |
Q1: <0.81 mg/L; Q2: 0.81–0.98 mg/L; Q3: 0.98–1.18 mg/L; Q4: >1.18 mg/L. AAD, acute aortic dissection; HR, hazards ratio; CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; SBP, systolic pressure; WBC, white blood cell counts; PLT, platelet counts; TBIL, total bilirubin; TCHOL, total cholesterol; sCr, serum creatinine; CRP, C-reactive protein; IVST, interventricular septum thickness.
The association between cystatin C and AKI in AAD patients
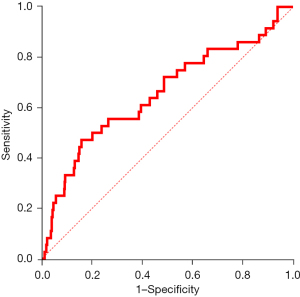
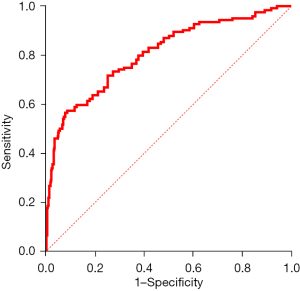
Serum cystatin C concentration was significantly higher in AAD patients with AKI than AAD patients without AKI (1.245, IQR 0.985–1.777 vs. 0.880, IQR 0.770–1.045; P<0.001; see Figure 5). Subsequently, we established 3 logistic regression models to examine the independent effects of cystatin C on the incidence of AKI after adjusting for confounding factors (see Table 3). In the fully adjusted model, the results indicated that serum cystatin C remains an important and independent predictor of AKI. Further, the higher the concentration of serum cystatin C, the higher the incidence of AKI (P for trend <0.001; see Table 3). A ROC curve was used to evaluate the predictive power of cystatin C in determining the incidence of AKI, and the AUC was 0.807 (95% CI, 0.758–0.856; see Figure 6). The results indicated that cystatin C is an appropriate predictor of AKI in AAD patients.
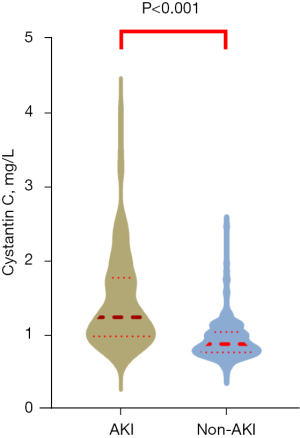
Table 3
| Models | Cystatin C, mg/L | P for trend | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| AKI, n (%) | 8 (8.8%) | 21 (24.1%) | 25 (27.5%) | 70 (79.5%) | – |
| Unadjusted OR (95% CI) | 1 | 3.301 (1.375–7.928) | 3.930 (1.664–9.280) | 40.347 (16.544–98.397) | <0.001 |
| Adjusted OR (95% CI) | |||||
| Model I | 1 | 3.865 (1.518–9.842) | 4.882 (1.889–12.618) | 54.694 (20.499–145.933) | <0.001 |
| Model II | 1 | 4.276 (1.646–11.108) | 5.408 (2.055–14.229) | 60.359 (21.992–165.661) | <0.001 |
| Model III | 1 | 3.707 (1.371–10.021) | 5.351 (1.968–14.522) | 76.489 (25.586–228.660) | <0.001 |
Model I: adjusted for sex, age and BMI; Model II: adjusted for sex, age, BMI, AAD type, hypertension, DM, hyperlipemia and smoke; Model III: adjusted for sex, age, BMI, AAD type, hypertension, DM, hyperlipemia, smoke, WBC, PLT, CRP, TBIL and D-Dimer. Q1: <0.81 mg/L; Q2: 0.81–0.98 mg/L; Q3: 0.98–1.18 mg/L; Q4: >1.18 mg/L. AKI, acute kidney injury; AAD, acute aortic dissection; OR, odds ratio; CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; WBC, white blood cell counts; PLT, platelet counts; CRP, C-reactive protein; TBIL, total bilirubin.
Discussion
As is well known, the mortality of AAD patients is high. Further, AAD is often complicated by multi-organ dysfunction, of which AKI is one of the most common organ injuries. As some researches showed that the incidence of AKI following surgery in patients with type A AAD varies from 20% to 67% (19-21). The development of AKI affects the quality of life and prognosis of AAD patients, even those that have partially or completely recovered from the disease (20). Some studies have shown that the occurrence of AKI postoperatively results in prolonged intensive care unit stay and total hospital stay, and the 28-day mortality of patients increases to 53.84% and is as high as 67.5% in those requiring CRRT (21,22). Thus, the early detection of AKI is crucial to the prognosis of AAD patients.
Cystatin C is a non-glycosylated protein produced by nucleated cells at a constant rate. It has been reported that it is a better biomarker than serum creatinine for renal dysfunction (23). Cystatin C is considered an early marker for the diagnosis of AKI, and can predict AKI 1–2 days earlier than serum creatinine (24). In the current study, we also found that the levels of serum cystatin C were associated with AKI in AAD patients. Further, the higher the level of cystatin C, the higher the incidence of AKI. Cystatin C remained an independent risk factor for AKI in AAD patients after adjusting for numerous covariates. In our study, we were surprised to find that when serum cystatin C acting as a predictor of AKI in AAD patients, the AUC was 0.807, which indicates that cystatin C is an appropriate predictor of AKI in AAD patients.
In the present study, cystatin C was also found to be associated with the prognosis of AAD patients. The results of the multivariate Cox regression analysis indicated that cystatin C was an independent risk factor of in-hospital mortality and that a serum concentration >1.18 mg/L was associated with higher mortality. Further, the short-term mortality of AAD patients is well predicted by cystatin C. A previous research indicated that cystatin C is associated with inflammation (25). Recently, it was reported that serum cystatin C concentration is well correlated with hs-CRP levels (26). However, inflammation and oxidative stress were found to play key roles in the pathogenesis and progression of AAD (27,28). This may explain why cystatin C is a good prognostic predictive factor for AAD patients.
We identified a novel predictor of AKI and short-term mortality in AAD patients. However, our research had some limitations. First, it was a single-center retrospective study with a small number of patients. Further, as all the patients included in the study originated from southeast China, the conclusions may not be appropriate for all AAD patients. Second, we only focused on the short-term prognosis of patients and the incidence of AKI and did not consider their long-term prognosis and renal recovery. Thus, a long-term follow-up study needs to be conducted. Third, as a prognostic marker, we only assessed the relationship between static values at admission and prognosis and did not examine dynamic alterations in cystatin C.
Conclusions
In sum, serum cystatin C concentration is a potential predictor of short-term mortality and the incidence of AKI in AAD patients. As a result, it has good clinical applicability and promotion potential.
Acknowledgments
We would like to thank all the hospital staff for their efforts in collecting the information that was used in this study, all the patients included in the analysis, and the medical staff who are on the frontlines in caring for patients.
Funding: This work was supported by the Gusu Health Talents Program (No. GSWS2020006), and the Science and Technology Development Project of Suzhou (No. SKJYD2021074), and the Social Development Project of Jiangsu Province (No. BE2022731).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-937/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-937/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-937/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University (IRB No. 2022–212). As a retrospective study, the requirement of informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Evangelista A, Isselbacher EM, Bossone E, et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018;137:1846-60. [Crossref] [PubMed]
- Golledge J, Eagle KA. Acute aortic dissection. Lancet 2008;372:55-66. [Crossref] [PubMed]
- Pacini D, Di Marco L, Fortuna D, et al. Acute aortic dissection: epidemiology and outcomes. Int J Cardiol 2013;167:2806-12. [Crossref] [PubMed]
- Aboyans V, Boukhris M. Dissecting the epidemiology of aortic dissection. Eur Heart J Acute Cardiovasc Care 2021;10:710-1. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Baliga RR, Nienaber CA, Bossone E, et al. The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging 2014;7:406-24. [Crossref] [PubMed]
- Nienaber CA, Clough RE, Sakalihasan N, et al. Aortic dissection. Nat Rev Dis Primers 2016;2:16053. [Crossref] [PubMed]
- Du X, Zhang S, Xu J, et al. Diagnostic value of monocyte to high-density lipoprotein ratio in acute aortic dissection in a Chinese han population. Expert Rev Mol Diagn 2020;20:1243-52. [Crossref] [PubMed]
- Tian L, Fan X, Zhu J, et al. Plasma D-dimer and in-hospital mortality in patients with Stanford type A acute aortic dissection. Blood Coagul Fibrinolysis 2014;25:161-6. [Crossref] [PubMed]
- Zhou Y, Yang G, He H, et al. Triglyceride/High-Density Lipoprotein Cholesterol Ratio Is Associated with In-Hospital Mortality in Acute Type B Aortic Dissection. Biomed Res Int 2020;2020:5419846. [Crossref] [PubMed]
- Goddard KA, Olson JM, Payami H, et al. Evidence of linkage and association on chromosome 20 for late-onset Alzheimer disease. Neurogenetics 2004;5:121-8. [Crossref] [PubMed]
- Kaeser SA, Herzig MC, Coomaraswamy J, et al. Cystatin C modulates cerebral beta-amyloidosis. Nat Genet 2007;39:1437-9. [Crossref] [PubMed]
- Shlipak MG, Matsushita K, Ärnlöv J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013;369:932-43. [Crossref] [PubMed]
- van der Laan SW, Fall T, Soumaré A, et al. Cystatin C and Cardiovascular Disease: A Mendelian Randomization Study. J Am Coll Cardiol 2016;68:934-45. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. Corrigendum to: 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J 2015;36:2779. [Crossref] [PubMed]
- Sherk WM, Khaja MS, Williams DM. Anatomy, Pathology, and Classification of Aortic Dissection. Tech Vasc Interv Radiol 2021;24:100746. [Crossref] [PubMed]
- Carey RM, Whelton PK. 2017 ACC/AHA Hypertension Guideline Writing Committee. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann Intern Med 2018;168:351-8. [Crossref] [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [Crossref] [PubMed]
- Yuan SM. Acute Kidney Injury after Cardiac Surgery: Risk Factors and Novel Biomarkers. Braz J Cardiovasc Surg 2019;34:352-60. [Crossref] [PubMed]
- Helgason D, Helgadottir S, Ahlsson A, et al. Acute Kidney Injury After Acute Repair of Type A Aortic Dissection. Ann Thorac Surg 2021;111:1292-8. [Crossref] [PubMed]
- Zhang K, Shang J, Chen Y, et al. The prognosis and risk factors for acute kidney injury in high-risk patients after surgery for type A aortic dissection in the ICU. J Thorac Dis 2021;13:4427-37. [Crossref] [PubMed]
- Wang Z, Ge M, Chen T, et al. Acute kidney injury in patients operated on for type A acute aortic dissection: incidence, risk factors and short-term outcomes. Interact Cardiovasc Thorac Surg 2020;31:697-703. [Crossref] [PubMed]
- Huidobro E JP, Guzmán AM, Tagle R. Use of cystatin C to estimate glomerular filtration rate. Rev Med Chil 2021;149:98-102. [PubMed]
- Sinna MM, Altaf FMN, Mosa OF. Serum and Urinary NGAL and Cystatin C Levels as Diagnostic Tools for Acute Kidney Injury and Chronic Kidney Disease: A Histobiochemical Comparative Study. Curr Pharm Des 2019;25:1122-33. [Crossref] [PubMed]
- Muslimovic A, Tulumovic D, Hasanspahic S, et al. Serum cystatin C - marker of inflammation and cardiovascular morbidity in chronic kidney disease stages 1-4. Mater Sociomed 2015;27:75-8. [Crossref] [PubMed]
- Feng WZ, Zhou JQ, Yu GM, et al. Association of serum cystatin C levels with mortality in patients with acute type A aortic dissection. Oncotarget 2017;8:101103-11. [Crossref] [PubMed]
- Lian R, Zhang G, Yan S, et al. Identification of Molecular Regulatory Features and Markers for Acute Type A Aortic Dissection. Comput Math Methods Med 2021;2021:6697848. [Crossref] [PubMed]
- Skotsimara G, Antonopoulos A, Oikonomou E, et al. Aortic Wall Inflammation in the Pathogenesis, Diagnosis and Treatment of Aortic Aneurysms. Inflammation 2022;45:965-76. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)