
Management of airway complications after lung transplantation: is there an ideal stent?
Despite significant advances in surgical technics, airway complications (ACs) after lung transplantation (LT) occur in 7–8% on average with extremes from 2% to 33% depending on the different studies (1). ACs include anastomotic infection, necrosis or dehiscence within the first month. Others develop later such as excess granulation tissue, bronchomalacia, fistula and stenosis, which is the most frequent. Stenosis is due to the final healing process of the ischemic and necrotic bronchial mucosa. Risk factors associated with ACs are those that increase anastomotic ischemia. A possible mechanism of immunologic rejection has also been hypothesized by some authors (2). The preferred surgical technique for LT at most institutions consisted of an end-to-end anastomosis which is performed closer to the secondary carina. This is the key factor, although others have also been identified such as organ preservation, hypoperfusion, infections, donor and recipient factors and immunosuppression agents. The treatment is mainly based on bronchial balloon dilation, often in combination with endobronchial placement of silicone, self-expanding metallic and biodegradable stents (3,4). The use of cryotherapy, laser, electrocautery, high-dose endobronchial brachytherapy and surgery has been also reported by some authors.
Sinha and colleagues proposed to investigate the results of using covered metallic stents for ACs after LT (5). In a retrospective study at Temple University Hospital (Philadelphia, Pennsylvania) between April 2016 and April 2021, they identified 645 lung transplants. In 50 cases (7.8%), there was a postoperative AC requiring the placement of a covered metallic stent (n=376) under bronchoscopy (n=774). The most common locations were the Right Main Stem Bronchus (n=147, 39.10%), the Left Main Stem Bronchus (n=82, 21.81%) and the Bronchus Intermedius (n=77, 20.48%). Bonastent®, Atrium iCASTTM or AERO® stents were used respectively in 219, 130 and 27 cases. The median duration per stent was 22.5 days (13–29 days). Four (±8.75) stents were used per patient. Stent removal was performed because of minor complications like secretions (n=193), granulation tissue (n=131), migration (n=51) and fracture (n=15). Stent fracture occurred more frequently in the Bonastent® subgroup (P=0.04). In 2 cases, major bleeding was observed after stent removal. The authors provided the largest series of covered metallic stents inserted to treat ACs after LT. This manuscript, however, highlights some limitations to the use of this third generation Self-Expanding Metallic Stents (SEMS), that are for most of them reported in the manuscript. The median duration per stent is very short (22.5 days) compared to what can offer other devices, in particular silicone stents: 266 days in a population of 17 LT patients, with 70% of anastomotic complications being healed after a planned stent removal (6). This short duration is explained by the high rate of complications requiring stent removal, that also contrasts with what was reported with silicone stents: 0.13 complication/patient/month (6). The following results observed by the authors of the present study (193 mucus plugging, 131 granulation tissue reactions, 51 migrations, 15 stent fractures, and 2 severe hemorrhages: 4% of patients, one lethal, the other requiring a pneumonectomy) in a population of 50 patients do not clearly support the conclusions of the authors regarding the safety of these devices. More than 80% of stents were placed in a main bronchus or the bronchus intermedius, so probably the treatment of smaller airways cannot explain the rate of mucus plugging. It is also not clear how the authors treat non-anastomotic stenoses that can involve the secondary carina and often requires a bifurcated device (7). Thirty-nine percent of covered SEMS have been placed in the right main bronchus, which may mean the authors either use very short devices with a high risk of migration; or temporarily cover the right upper lobe. Again, a recent report demonstrated in a limited cohort (n=15) the efficacy and safety of onsite customized silicone bifurcated stents in this setting (7). Also, the need for rigid bronchoscopy is reported as a pitfall of silicone stents, but complications of rigid bronchoscopy are extremely rare, and they represent a safe way to insert and manage potential immediate complications of stents (SEMS or silicone in our experience). Obviously, as long as no comparative study is conducted, it won’t be possible to conclude whether one stent is superior to the other, but in this specific indication, it seems silicone offer more durable outcomes and a lower rate of complications. SEMS fit better to distorted airways, a frequent situation after LT, and 3D printed stents can overcome this limitation (8).
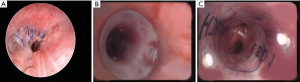
The optimal treatment of ACs after LT remains debated. According to Yserbyt et al., a conservative approach should be discussed as most ACs spontaneously resolve in time (9). Pinillos Robles et al. reported positive results in 2 patients with bronchus intermedius stenosis treated with a Montgomery stent or T-stent (10). Ozgül et al. used Oki stents in 3 post-transplant patients with malacia, stenosis and bronchopleural fistula (11). Guibert et al. reported the successful treatment of post-transplant complex airway stenosis with a three-dimensional, computer-assisted customized airway stent (12). Miyata et al. showed that conservative management may be considered in stable and asymptomatic patients with appropriate pleural drainage (13). Luna et al. reported the closure of post-transplant bronchial dehiscence with endobronchial fibrin sealant (14). Davidson et al. showed that application of Mitomycine C allows reducing the frequency of bronchoscopic balloon dilation in patients with post-lung transplant airway stenosis (15). Walters et al. reported the use of electromagnetic navigational bronchoscopic airway recanalization in 4 patients with vanishing bronchus after LT (16). Mazzetta et al. found that ACs after LT did not influence survival but have a significant effect on lung function and the rate of respiratory infection at long-term follow-up (17). Patoir et al. published a series using widely and safely the third generation of fully covered metallic stents, adapted to situations of tight strictures or tortuous airways (18). Nevertheless, they reminded that silicone stents remain the gold standard especially for malacia and long-term airway supporting. In a retrospective study, Ma et al. concluded that complications related to stent placement were common and that patients treated with airway stents underwent a high volume of repeat procedures (19). Ram et al. reported a hybrid reconstruction of the bronchus using an aortic homograft patch with stenting as a novel technique of management of ischemic airway injury following LT (20). This technique is based on previous works which have demonstrated since 1997 in animals and then in humans the interest of using stented aortic matrices for airway replacement (21,22). Izhakian et al. demonstrated that SEMS insertion was associated with a positive sustained effect on lung function without increasing long-term mortality (23). In addition, they established that bronchial stents were more likely to be colonized with pathologic bacteria and having pneumonia (24). However, stent placement was not associated with increased long-term mortality if stent maintenance is appropriate. In a randomized, controlled trial, Kraft et al. showed that hyperbaric oxygen therapy does not reduce central airway stenosis severity or stenting (25). More recently, Guinde et al. reported that the use of silicone customized bifurcated stent was a safe and efficient procedure to manage ACs after LT (7). Main reported series of ACs after LT from 2015 to present are presented in Table 1. Endoscopic views of a patient treated for ACs (bronchial anastomosis stenosis) after LT by silicone stent insertion are shown in Figure 1.
Table 1
| First author, year of publication | Study period | Number of LT (BA, n) | ACs, n [% of LT] | Type of ACs | Type of stents (n) | Complications of stenting [%] | Stent removal, n [%] |
|---|---|---|---|---|---|---|---|
| Yserbyt, 2016 | 2005–2013 | 490 (924) | 101 [11] | Stenosis | Silicone (n=7); SEMS (n=5) | Fibrotic stenosis [25] | 7 [58] |
| Mazzetta, 2019 | 2009–2014 | 191 (366) | 22 [13.6] | Stenosis; malacia | Silicone; or SEMS (n=9) | NA | NA |
| Patoir, 2020 | 2010–2016 | 121 (203) | 41 [20.2] | Stenosis; dehiscence; malacia | Silicone (n=21); SEMS (n=20) | OG [51.2]; OMP [36.6]; Migration [19.5] | 29 [70.7] |
| Ma, 2020 | 2012–2017 | NA | 47 [NA] | Stenosis; dehiscence; malacia | Silicone (n=29); SEMS (n=6); UCMS (n=12) | OMP [57.5]; OG [36.2] | 28 [59.6] |
| Izhakian, 2021 | 2002–2018 | 582 (813) | 57 [9.8] | Stenosis | SEMS (n=57) | Bleeding [1.8]; Fracture [7.4]; Stenosis [7.4]; Collapse [3.7]; EPU [1.9]; Migration [1.9] | NA |
| Guinde, 2022 | 2010–2020 | 400 (724) | 32 [8] | Stenosis; dehiscence | Silicone; CBS (n=14) | OG [57]; OMP [50]; Stenosis [21] | 11 [78.6] |
AC, airway complication; LT, lung transplantation; BA, bronchial anastomosis; SEMS, self-expandable metal stents; UCMS, uncovered metallic stents; CBS, customized bifurcated stent; OG, obstructive granulomas; OMP, obstruction due to mucus plugging; EPU, endobronchial pressure ulcer; NA, not available.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-858/coif). HD is consultant for Novatech SA, France and received personal fees and royalties. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Crespo MM. Airway complications in lung transplantation. J Thorac Dis 2021;13:6717-24. [Crossref] [PubMed]
- Fujimura S, Kondo T, Handa M, et al. Histologic assessment of bronchial anastomotic healing in canine lung transplantation. J Thorac Cardiovasc Surg 1987;94:323-30. [Crossref] [PubMed]
- Dutau H, Reynaud-Gaubert M, Thomas PA. Endoscopic management of post-lung transplantation anastomotic stenosis: metallic, silicone or biodegradable stents. Eur J Cardiothorac Surg 2012;41:1216-7; author reply 1217-8. [Crossref] [PubMed]
- Lischke R, Pozniak J, Vondrys D, et al. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg 2011;40:619-24. [Crossref] [PubMed]
- Sinha T, Ho TA, van der Rijst N, et al. Safety of hybrid bronchial stents in transplant airway complications: a single center experience. J Thorac Dis 2022;14:2071-8. [Crossref] [PubMed]
- Dutau H, Cavailles A, Sakr L, et al. A retrospective study of silicone stent placement for management of anastomotic airway complications in lung transplant recipients: short- and long-term outcomes. J Heart Lung Transplant 2010;29:658-64. [Crossref] [PubMed]
- Guinde J, Bismuth J, Laroumagne S, et al. Bifurcated Silicone Stents for the Management of Anastomotic Complications in Lung Transplanted Patients: Ten Years' Experience. Respiration 2022;101:675-82. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of complex airway stenoses using patient-specific 3D-engineered stents: a proof-of-concept study. Thorax 2019;74:810-3. [Crossref] [PubMed]
- Yserbyt J, Dooms C, Vos R, et al. Anastomotic airway complications after lung transplantation: risk factors, treatment modalities and outcome-a single-centre experience. Eur J Cardiothorac Surg 2016;49:e1-8. [Crossref] [PubMed]
- Pinillos Robles J, García Luján R, de Pablo Gafas A, et al. Treatment of bronchus intermedius stenosis in lung transplantation with Montgomery T-tube stent. A novel technique. Arch Bronconeumol 2015;51:e5-7. [PubMed]
- Özgül MA, Çetinkaya E, Çörtük M, et al. Oki stent application in different indications: Six cases. Clin Respir J 2018;12:234-40. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of Post-transplant Complex Airway Stenosis with a Three-Dimensional, Computer-assisted Customized Airway Stent. Am J Respir Crit Care Med 2017;195:e31-3. [Crossref] [PubMed]
- Miyata R, Chen-Yoshikawa TF, Hamaji M, et al. Successful conservative management of an anastomotic airway dehiscence at the left main bronchus following bilateral cadaveric lung transplantation. Gen Thorac Cardiovasc Surg 2018;66:368-71. [Crossref] [PubMed]
- Luna BW, Paoletti L, Denlinger CE, et al. Closure of a Post-Transplant Bronchial Dehiscence With Endobronchial Fibrin Sealant. Ann Thorac Surg 2018;106:e193-5. [Crossref] [PubMed]
- Davidson KR, Elmasri M, Wahidi MM, et al. Management of Lung Transplant Bronchial Stenosis With Mitomycin C. J Bronchology Interv Pulmonol 2019;26:124-8. [Crossref] [PubMed]
- Walters DM, Kuckelman JP, Mulligan MS. Electromagnetic navigational bronchoscopic airway recanalization in patients with vanishing bronchus. J Surg Res 2018;231:154-60. [Crossref] [PubMed]
- Mazzetta A, Porzio M, Riou M, et al. Patients Treated for Central Airway Stenosis After Lung Transplantation Have Persistent Airflow Limitation. Ann Transplant 2019;24:84-92. [Crossref] [PubMed]
- Patoir A, Luchez A, Tiffet O, et al. Airway complications after lung transplantation: benefit of a conservative bronchoscopy strategy. J Thorac Dis 2020;12:2625-34. [Crossref] [PubMed]
- Ma KC, Li M, Haas AR, et al. Efficacy and safety of airway stenting to treat anastomotic complications after lung transplant: a cohort study. J Thorac Dis 2020;12:3539-48. [Crossref] [PubMed]
- Ram D, O, Carroll M, Sibal AK. Hybrid reconstruction of bronchial dehiscence following lung transplant. J Card Surg 2020;35:3133-5. [Crossref] [PubMed]
- Martinod E, Chouahnia K, Radu DM, et al. Feasibility of Bioengineered Tracheal and Bronchial Reconstruction Using Stented Aortic Matrices. JAMA 2018;319:2212-22. [Crossref] [PubMed]
- Martinod E, Radu DM, Onorati I, et al. Airway replacement using stented aortic matrices: Long-term follow-up and results of the TRITON-01 study in 35 adult patients. Am J Transplant 2022; Epub ahead of print. [Crossref] [PubMed]
- Izhakian S, Wasser W, Unterman A, et al. Long-Term Success of Metal Endobronchial Stents in Lung Transplant Recipients. Thorac Cardiovasc Surg 2022;70:520-6. [Crossref] [PubMed]
- Izhakian S, Wasser WG, Vainshelboim B, et al. Long-term outcomes of metallic endobronchial stents in lung transplant recipients are not affected by bacterial colonization. Interact Cardiovasc Thorac Surg 2021;32:47-54. [Crossref] [PubMed]
- Kraft BD, Mahmood K, Harlan NP, et al. Hyperbaric oxygen therapy to prevent central airway stenosis after lung transplantation. J Heart Lung Transplant 2021;40:269-78. [Crossref] [PubMed]


