
Donor lung preservation for transplantation—where do we go from here?
Introduction
The first successes with heart-lung transplantation published by the Stanford Group in the N Eng J Med in 1982 (1) and with isolated lung transplantation reported by the Toronto group in the same journal in 1986 (2) date back to nearly four decades ago (3). Meanwhile, methods and technologies for donor lung preservation for transplantation have evolved over time.
Methods for graft cooling
Autoperfusion by normothermic machine preservation (NMP) of heart and lungs during distant procurement was first reported by Hardesty and Griffith in 1987 (4). Donor core cooling on cardio-pulmonary bypass was propagated by the Harefield group for distant procurement of heart and lungs (5). Simple hypothermic immersion of the collapsed pulmonary graft was the original method used by the Toronto Group in its early reported series of single-lung transplantation (2). This simple technique i.e., topical cooling alone was also advocated later by Steen and colleagues as a safe and efficacious method for prolonged lung preservation up to 12 hours in a series of pig lung transplantation (6). In his first clinical case report of successful single-lung transplantation from an uncontrolled donor after circulatory death determination (DCD), donor lungs were cooled for 3 hours inside the donor’s pleural cavity by infusion of cold low-potassium dextran glucose (LPDG) solution via two chest drains after a period of 65 min of warm ischemia (7). This method was described as an ethically acceptable way to preserve the organ in the deceased body.
In the early days of lung transplantation obtaining satisfactory lung preservation for a longer period with topical cooling alone was difficult. In a series of left single-lung transplantation in dogs, Haverich and colleagues in 1986 demonstrated that safe and reliable lung preservation for 24 hours was possible with flush-perfusion with 60 cc/kg Euro-collins solution over 4 minutes with a pulmonary artery pressure around 20 mmHg (8). This technique of pulmonary artery flush followed by simple topical hypothermia was rapidly adopted by most transplant centers for distant procurement of heart and lungs (9,10). Currently, the vast majority of transplant centers have adopted a single pulmonary artery flush technique in combination with topical cooling to preserve the lungs because of its technical simplicity.
Preservation solution
The type of preservation solution to flush-perfuse donor lungs has also changed over time. During the late eighties and nineties modified Euro-collins solution, a crystalloid containing a high potassium level, was the standard lung preservation solution already widely used at that time for abdominal organs (10). Other centers preferred to use University of Wisconsin solution (11) or a colloid solution based on donor blood (Papworth solution) (12). Further research demonstrated that the high potassium content in the intracellular type solutions was responsible for strong pulmonary vasoconstriction (13), but can also directly cause endothelial injury (14) resulting in an increased risk of hydrostatic as well as permeability edema upon reperfusion. This resulted in a switch to extracellular type solutions such as LPDG, commercialized as Perfadex™ (XVIVO AB, Göteborg, Sweden). The presence of both the dextran 40 and the low potassium content in the solution are critical components improving pulmonary microcirculation and preserving the endothelial-epithelial barrier (15). Clinical studies have shown superiority for LPDG (16) that has become the standard lung preservation solution in most lung transplantation centers worldwide for more than three decades. Excellent post-transplant outcome has also been reported with the use of Celsior® (Institut Georges Lopez, Lissieu, France), another extra-cellular type solution containing high amounts of antioxidants such as reduced glutathione, histidine, and lactobionate, which may play an important role in the prevention of free radical injury (17).
Antegrade and retrograde flush
In addition to the antegrade flush via the pulmonary artery, a retrograde flush via the left atrial appendage or the individual pulmonary veins, either inside the donor with both lungs ventilated, or on the back table after explantation, has the potential of flushing both the bronchial and pulmonary vessels. An additional advantage of this technique is the wash out of any possible emboli from the pulmonary vasculature. A retrograde flush was found to improve lung preservation (18). Most transplant teams have now adopted a technique combined of an antegrade flush via the pulmonary artery followed by a retrograde flush through each of the pulmonary veins. The rationale to perform a second retrograde flush on the back table immediately prior to graft implantation is to wash out post-ischemic inflammatory agents that may improve immediate post-transplant graft function (19).
The role of ex-vivo lung perfusion (EVLP) for lung preservation
NMP for preservation and evaluation of lungs, now commonly named EVLP, was first reported by Steen and colleagues in 2001 using LPDG as lung perfusate with human serum albumin added to optimize colloid oncotic pressure, later commercialized as STEEN Solution™ (XVIVO AB, Göteborg, Sweden) (7). The EVLP technique was further pioneered and clinically introduced by the Toronto group (20). Reassessment of questionable donor lungs is currently the most common EVLP indication in lung transplant centers worldwide with run times between 3 to 6 hours. Portable EVLP can also be utilized as a platform for prolonged preservation of healthy donor lungs for logistic reasons to prolong cross-clamp time on indication such as to convert the transplantation into a semi-elective procedure, to allow long distance travel, or to facilitate combined organ transplantation.
Two randomized trials (Inspire trial, Vienna trial) using standard-criteria donor lungs have reported the safety and feasibility of normothermic lung preservation, but lacked convincing evidence regarding the superiority of EVLP versus standard cold storage in terms of patient in-hospital outcome and early survival (21,22).
Temperature during cold static preservation
The optimal temperature for cold storage has been debated since the early start of lung transplantation. Traditionally, after cold flush-perfusion donor lungs are preserved around 4 ℃ in a cooler on ice. Experimental work during the nineties demonstrated that graft function may be better preserved if lungs could be stored around 10 ℃. For practical reasons, however, the icebox has been used over the years. A recent study by the Toronto group demonstrated that static lung storage at 10 ℃ maintains mitochondrial health and preserves donor organ function. Levels of metabolites protecting mitochondrial health including itaconate, glutamine, and N-acetylglutamine were higher in lungs stored at 10 ℃ compared to 4 ℃. Functional assessments performed during EVLP demonstrated that porcine lungs stored for 36 hours at 10 ℃ had lower airway pressures, higher lung compliances, and better oxygenation capabilities, indicative of preserved pulmonary physiology, as compared to lungs stored conventionally at 4 ℃ (23). A clinical feasibility proof-of-concept study (Clinical Trials.gov NCT04616365) conducted in Toronto and Vienna storing donor lungs overnight at 10 ℃ showed promising results (data presented at the 103rd Annual Meeting of the American Association for Thoracic Surgery, Boston, MA, USA, May 14–17, 2022). The authors concluded that preservation at 10 ℃ could become the standard of care for prolonged pulmonary preservation, providing benefits to both patients and health care teams. A multicenter randomized clinical trial comparing standard donor lung storage in the icebox with storage in an incubator at 10 ℃ is currently being set up in North-America and Europe.
Following the initial promising experience with donor hearts (24) preserved in the Sherpapak™ (Paragonix, Cambridge, MA), a 72% reduction (P=0.005) in severe primary graft dysfunction and a 39% reduction (P=0.03) in need for mechanical circulatory support in addition to improvement in 1-year survival was recently reported in abstract form at the 2022 annual meeting of the international Society for Heart and Lung Transplantation, Boston, MA, USA, April 25–30, 2022. Using the same cooling technology validated to maintain temperatures between 4–8 ℃ for over 40+ hours, the same company has now commercialized an FDA-cleared and CE-marked medical device (LUNGguard™) for lung preservation. The GUARDIAN-LUNG is a clinical non-randomized post-market registry study currently ongoing in the USA and Europe (Clinical Trials.gov NCT04930289) comparing the outcome after lung transplantation using this new transportation and preservation device with the standard icebox. Data collection is ongoing and results are being awaited.
In-situ donor lung recruitment and assessment for transplant suitability
Partial atelectasis of donor lungs is not uncommon in patients ventilated for some days in the intensive care unit prior to becoming an organ donor. Atelectatic areas in donor lungs should be reexpanded with the aid of bronchoscopic airway suctioning and increased ventilatory pressures and volumes prior to pulmoplegia. In addition, by reversing the atelectatic zones, lung perfusion will be more homogenous and lungs can be fully inflated with 50% oxygen prior to extraction and packing. In this way, immediate post-transplant graft function is expected to be better with preserved surfactant function and prolonged cold ischemic tolerance as a result of the alveolar oxygen reserve during cold storage.
Important to understand is that the timing for assessment of lung compliance and elasticity after full lung recruitment and final acceptance for transplantation differs between donor types. In donation after brain death (DBD), all organs including lungs can be inspected and assessed while the donor’s heart is still beating prior to cardioplegia and circulatory arrest. A blood sample for gas analysis taken from left and right pulmonary veins will more accurately assess oxygenation capacity of each lung individually. In contrast, in DCD’s, lungs cannot be inspected and assessed until after opening the chest upon declaration of death. Rapid organ procurement is then needed to limit the total warm ischemic period. The length of the asystolic phase required to respect the dead donor rule differs across national legislations (5–20 min). Pulmoplegia in DCD is therefore usually started prior to full macroscopic inspection and assessment of donor lungs.
In this issue of the journal, authors from the University of Okayama in Japan published their findings of an experimental study on the best timing for recruitment of atelectatic donor lungs using a porcine left single-lung transplantation model. Recipient animal outcome during the first 6 hours was compared between two study groups following implantation of a 24-hour atelectatic left donor lung. The parenchyma was fully recruited either before versus after cardioplegic circulatory arrest prior to the start of cold pulmoplegia (25). The authors reported that post-transplant oxygenation capacity expressed as PaO2/FiO2 ratio was significantly better in the group with lungs not recruited until after circulatory arrest (P=0.015). Wet-to-dry weight ratio, histological findings of graft injury and tissue interleukin-8 expression, however, did not differ between the groups. The authors postulated that the mechanism of injury is similar to the phenomenon of rapid reexpansion edema in patients with complete lung collapse receiving a chest tube for a large pneumothorax or pleural effusion. The authors hypothesized that circulating proinflammatory mediators may cause lung injury after lung reperfusion with whole blood upon recruitment of the atelectatic parenchyma. In lungs recruited in the absence of blood circulation, this phenomenon will not occur.
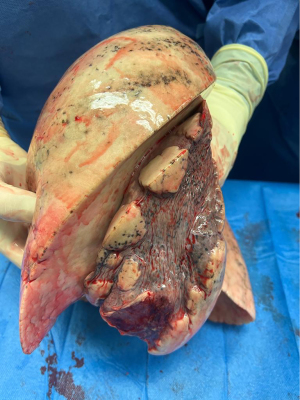
Several points are important to consider before this practice of delayed parenchymal recruitment can be implemented in daily lung transplantation practice. First, due to the time investment using a large animal model, the number of pigs in each group was limited (n=5 in each study group and n=3 in the control group with no atelectatic lungs). Therefore, the power to find any significant differences between groups was limited. Second, no differences in wet-to-dry weight ratio and no differences in histological and biomolecular markers were observed to support their hypothesis of reexpansion edema. Third, no other physiologic parameters reflecting graft quality such as hemodynamics and ventilatory findings were recorded and compared between groups. Fourth, in their model, the authors decided to induce complete atelectasis in the left donor lung by ligating the left main bronchus for 24 hours prior to the transplantation procedure. Atelectasis of the entire lung is rarely observed in lung donors (Figure 1). Parenchymal collapse in ventilated donors is often observed on chest computed tomography scan in the dorsal region of the lower lobes.
By delaying parenchymal recruitment until after cardiac arrest, conventional functional assessment prior to lung acceptance and transplantation is no longer possible. It is well known that donor oxygenation will improve after reversing atelectatic lung zones as witnessed when sampling blood from individual pulmonary veins for gas analysis before and after parenchymal recruitment. Moreover, physiologic parameters reflecting physiologic graft performance such as lung compliance, elasticity, and airway pressures should be measured in lungs while fully ventilated. The authors argue that functional assessment of atelectatic grafts nowadays can easily be done with EVLP prior to acceptance and transplantation. Routine EVLP for every donor lung, however, is a demanding procedure in terms of logistics, personnel, and finances.
Niman, Miyoshi, and other co-authors are to be congratulated in performing this research using a very demanding model. Their findings are preliminary and should be repeated by other groups before a clinical trial can be conducted in humans to investigate the potential advantage of delaying parenchymal recruitment until after cardiac arrest in the absence of blood circulating through the donor lungs. Meanwhile, in-situ inspection and assessment after securing full ventilation of both lungs for final acceptance should be continued prior to cardiac arrest and pulmoplegia.
Conclusions
Donor lung preservation by antegrade and retrograde cold flush-perfusion with an extracellular type solution with high oncotic pressure followed by static cold storage in the icebox at 0–4 ℃ remains the current standard practice performed worldwide. Results of the ongoing trial investigating the potential superiority of lung storage at 10 ℃ are awaited.
Recruitment of atelectatic donor lung parenchyma in heart-beating donors preceding cold flush preservation remains the preferred technique for final graft assessment prior to acceptance. Further clinical studies are needed to investigate whether delaying parenchymal recruitment until after cardiac arrest results in improved immediate graft function and hospital outcome.
Acknowledgments
Funding: DVR is supported by the Broere Charitable Foundation. LJC is supported by a KU Leuven University Chair funded by Medtronic and a post-doctoral grant from the University Hospitals Leuven (KOOR-UZ Leuven).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-949/coif). DVR reports that he was a principal investigator for the Inspire Trial and the Expand Trial; both trials were sponsored by Transmedics® (Andover, MA, USA). DVR is supported by the Broere Charitable Foundation. This financial support is not commercial, but an open grant to support research. LJC is supported by a KU Leuven University Chair funded by Medtronic and a post-doctoral grant from the University Hospitals Leuven (KOOR-UZ Leuven). LJC reports unrestricted grant at University of Leuven for emphysema research by MEDTRONIC, and payment or honoraria for clinical immersion and lecture webinar from MEDTRONIC. JVS has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 1982;306:557-64. [Crossref] [PubMed]
- Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Venuta F, Van Raemdonck D. History of lung transplantation. J Thorac Dis 2017;9:5458-71. [Crossref] [PubMed]
- Hardesty RL, Griffith BP. Autoperfusion of the heart and lungs for preservation during distant procurement. J Thorac Cardiovasc Surg 1987;93:11-8. [Crossref] [PubMed]
- Yacoub MH, Khaghani A, Banner N, et al. Distant organ procurement for heart and lung transplantation. Transplant Proc 1989;21:2548-50. [PubMed]
- Steen S, Sjöberg T, Ingemansson R, et al. Efficacy of topical cooling in lung preservation: is a reappraisal due? Ann Thorac Surg 1994;58:1657-63. [Crossref] [PubMed]
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [Crossref] [PubMed]
- Haverich A, Aziz S, Scott WC, et al. Improved lung preservation using Euro-Collins solution for flush-perfusion. Thorac Cardiovasc Surg 1986;34:368-76. [Crossref] [PubMed]
- Baldwin JC, Frist WH, Starkey TD, et al. Distant graft procurement for combined heart and lung transplantation using pulmonary artery flush and simple topical hypothermia for graft preservation. Ann Thorac Surg 1987;43:670-3. [Crossref] [PubMed]
- Colquhoun IW, Kirk AJ, Au J, et al. Single-flush perfusion with modified Euro-Collins solution: experience in clinical lung preservation. J Heart Lung Transplant 1992;11:S209-14. [PubMed]
- Hardesty RL, Aeba R, Armitage JM, et al. A clinical trial of University of Wisconsin solution for pulmonary preservation. J Thorac Cardiovasc Surg 1993;105:660-6. [Crossref] [PubMed]
- Parry A, Higgins R, Wheeldon D, et al. The contribution of donor management and modified cold blood lung perfusate to post-transplant lung function. J Heart Lung Transplant 1999;18:121-6. [Crossref] [PubMed]
- Kimblad PO, Sjöberg T, Massa G, et al. High potassium contents in organ preservation solutions cause strong pulmonary vasocontraction. Ann Thorac Surg 1991;52:523-8. [Crossref] [PubMed]
- Chan BB, Kron IL, Flanagan TL, et al. Impairment of vascular endothelial function by high-potassium storage solutions. Ann Thorac Surg 1993;55:940-5. [Crossref] [PubMed]
- Keshavjee SH, Yamazaki F, Yokomise H, et al. The role of dextran 40 and potassium in extended hypothermic lung preservation for transplantation. J Thorac Cardiovasc Surg 1992;103:314-25. [Crossref] [PubMed]
- Ingemansson R, Massa G, Pandita RK, et al. Perfadex is superior to Euro-Collins solution regarding 24-hour preservation of vascular function. Ann Thorac Surg 1995;60:1210-4. [Crossref] [PubMed]
- Xiong L, Legagneux J, Wassef M, et al. Protective effects of Celsior in lung transplantation. J Heart Lung Transplant 1999;18:320-7. [Crossref] [PubMed]
- Varela A, Cordoba M, Serrano-Fiz S, et al. Early lung allograft function after retrograde and antegrade preservation. J Thorac Cardiovasc Surg 1997;114:1119-20. [Crossref] [PubMed]
- Venuta F, Rendina EA, Bufi M, et al. Preimplantation retrograde pneumoplegia in clinical lung transplantation. J Thorac Cardiovasc Surg 1999;118:107-14. [Crossref] [PubMed]
- Van Raemdonck D, Ceulemans LJ, Van Beersel D, et al. Current achievements and future applications of ex vivo lung perfusion; where do we go from here? J Thorac Cardiovasc Surg 2022; [Crossref] [PubMed]
- Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018;6:357-67. Erratum in: Lancet Respir Med 2018;6:e27. [Crossref] [PubMed]
- Slama A, Schillab L, Barta M, et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial. J Heart Lung Transplant 2017;36:744-53. [Crossref] [PubMed]
- Ali A, Wang A, Ribeiro RVP, et al. Static lung storage at 10°C maintains mitochondrial health and preserves donor organ function. Sci Transl Med 2021;13:eabf7601. [Crossref] [PubMed]
- Naito N, Funamoto M, Pierson RN, et al. First clinical use of a novel hypothermic storage system for a long-distance donor heart procurement. J Thorac Cardiovasc Surg 2020;159:e121-3. [Crossref] [PubMed]
- Niman E, Miyoshi K, Shiotani T, et al. Lung recruitment after cardiac arrest during procurement of atelectatic donor lungs is a protective measure in lung transplantation. J Thorac Dis 2022;14:2802-11. [Crossref] [PubMed]


