
The effect of aggressive management of intraoperative body temperature on postoperative APACHE II score and prognosis in high-risk patients undergoing thoracoscopic surgery
Introduction
Intraoperative hypothermia refers to a core body temperature <36.0 ℃, which is a common perioperative complication (1). Yi et al. (2) reported that the incidence of intraoperative hypothermia was as high as 44.3%, and the rate of active warming remains low. Intraoperative hypothermia is associated with numerous adverse outcomes, including postoperative drug metabolism disorders (3,4), surgical site infections (5-7), perioperative bleeding and the need for transfusion (8,9), postoperative shivering, cardiovascular events (10,11), and even mortality (12). Intraoperative hypothermia also prolongs post-anesthesia care unit or intensive care unit stays (7,13,14), reduces patient satisfaction, and increases medical costs (5,15). Similar results were shown in thoracoscopic surgery (16,17).
Intraoperative normothermia is usually defined as a core body temperature ≥36 ℃. However, even at the lowest point of circadian rhythm, which usually occurs at around 3.00 am, the core body temperature does not fall below 36.5 ℃ (18). Conversely, at around 3 pm the core body temperature is usually around 37.5 ℃ (18). Generally, the normal body temperature of humans is about 37 ℃ rather than the widely recognized 36 ℃ (11,19).
Knaus et al. (20) first established the Acute Physiology and Chronic Health Evaluation II (APACHE II), and after long-term application and extensive verification, it has become the most commonly used evaluation system for assessing the severity and clinical prognosis of patients, and its ability to predict mortality risk and the fatality rate closely reflect reality (21-23). The higher the APACHE II score, the worse the prognosis.
To date, study has been conducted to confirm the effect of intraoperative temperature on APACHE II scores and prognosis in patients undergoing elective video-assisted thoracoscopic surgery (VATS). Thus, this study sought to explore whether active intraoperative warming prevented increases in APACHE II scores and improved patient prognosis after thoracoscopy. We present the following article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-873/rc).
Methods
The study used the data of a previous multicenter randomized controlled trial (PROTECT) (24), which is registered at clinicaltrials.gov (NCT03111875). This multicenter trial explored the relationship between aggressive intraoperative warming during non-cardiac surgery and incidence of a 30-day composite of major cardiovascular outcomes. The Ethics Committee of the China-Japan Union Hospital, Jilin University approved the original randomized controlled trial (No. 2018120503), and all the patients agreed to and signed informed consent forms to participate in that study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
To be eligible for inclusion in that study, patients undergoing thoracoscopic surgery had to meet the following inclusion criteria: (I) be aged ≥45 years; (II) be undergoing surgery with an expected duration of 2–6 hours; and (III) have at least 1 of the following risk factors: (I) an age ≥65 years; (II) have coronary atherosclerotic heart disease; (III) have a history of stroke or transient ischemic attack; (IV) have diabetes requiring medication; (V) have hypertension requiring medication; or (VI) have a smoking history and not quitting smoking. Patients with a body mass index (BMI) ≥30 kg/m2 were excluded from the study.
Patients were randomly assigned (1:1) to the routine management group (Group R) or the aggressive warming group (Group A). The patients in Group R did not receive any prewarming and were only warmed during blood transfusion, the temperature in the operating room was maintained at about 20 ℃, a forced-air cover was placed in the appropriate non-operative site, and heating only started when a patient’s core temperature dropped to 35.5 ℃. The patients in Group A were preheated with a forced-air cover for 30 minutes before anesthesia induction, all the intravenous infusion pathways were warmed, and a heating blanket was used to actively raise the core temperature to ≥37 ℃ during the operation. It was not possible to blind the patients to the prewarming or the clinicians to the intraoperative warming. However, this trial was assessor blinded.
We conducted a secondary analysis of data from a prospective study. As our sample size was based on the primary study, we did not perform a sample size estimate for this analysis. We retrieved electronic medical information that had been automatically recorded in an anesthesia record system (Docare V5.0, Medical) in 5-min intervals. We selected patients with at least 1 record of a core temperature >37.0 ℃ or <36.0 ℃, and on this basis, we allocated the patients to the aggressive warming group (Group A) and the routine management group (Group R), respectively.
Baseline information, including data on patients’ age, gender, American Society of Anesthesiologists (ASA) physical status, BMI, and preoperative complications, was collected. The APACHE II scores were calculated by collecting data on patients’ vital signs (e.g., temperature, heart rate, and blood pressure), and laboratory test outcomes (e.g., complete blood count, blood electrolyte, and liver and kidney function) both preoperatively and on day 1 postoperatively. When the APACHE II scores vary from 0 to 9, postoperative mortality is roughly 1–3%, when they vary from 10–19, postoperative mortality is between 7–12%, when they vary from 20–29, postoperative mortality is about 30–35%, and when they >30, postoperative mortality is about 73–88% (20). Thus, the APACHE II scores were divided into the 4 grades. Increases in APACHE II scores defined as grade up from low level to high level. The Quality of Recovery-15 (QoR-15) scale was administered on postoperative day 3. The QoR-15 is categorized as five quality of recovery dimensions, including physical comfort (5 items), emotional state (4 items), psychological support (2 items), physical independence (2 items), and pain (2 items). Each piece is graded using an 11-point Likert scale. The global QoR-15 score ranges from 0 to150. The length of stay (LoS) in hospital, was defined as time from surgery day to hospital discharge, postoperative complications (Cardiovascular and cerebrovascular complications, such as Chest pain, arrhythmias, postoperative cognitive dysfunction, etc.), infection incidence (Appendix 1), and mortality rate were documented at the 30-day follow-up examination after surgery. Patients who refused postoperative blood gas analysis were excluded from this study, due to they lack primary outcome data.
Statistical analysis
The analyses were performed using R (version 4.1.3, http://www.R-project.org). The categorical data are presented as frequencies and percentages. The differences between the categorical data were analyzed using the Chi-square test or Fisher’s exact test. The continuous data are presented as the mean ± standard deviation (SD) or the median with the interquartile range (IQR). Differences between continuous variable data with normal and skewed distributions were analyzed using the unpaired t-test and Mann-Whitney U test. The confounders were analyzed by a univariate logistic regression. A multivariate logistic regression model using ENTER method was then built with the confounders that had a univariate significance <0.05. Odds ratios (ORs) of the risk factors for the increased postoperative APACHE II score are presented with 95% confidence intervals (CIs). A 2-tailed P value <0.05 was considered statistically significant. Since the patients’ APACHE score grades were all 1 and 2, dichotomous logistic regression was used.
Results
A total of 263 thoracic patients from the PROTECT trial were enrolled in this study. Group R comprised 121 patients and Group A comprised 84 patients. There were significant differences in the smoking status and intraoperative body temperatures between the 2 groups (P<0.05; see Table 1).
Table 1
| Categories | Group R (n=121) | Group A (n=84) | P |
|---|---|---|---|
| Age (years), mean ± SD | 62.08±7.02 | 63.05±8.12 | 0.365 |
| Sex, n (%) | 0.761 | ||
| Male | 68 (56.2) | 49 (58.3) | |
| Female | 53 (43.8) | 35 (41.7) | |
| ASA, n (%) | 0.287 | ||
| II | 89 (73.6) | 56 (66.7) | |
| III | 32 (26.4) | 28 (33.3) | |
| BMI (kg/m2), mean ± SD | 23.78±2.81 | 23.36±3.26 | 0.329 |
| Preoperative complications, n (%) | |||
| Smoking | 52 (44.3) | 22 (30.7) | 0.015 |
| Diabetes | 19 (20.1) | 15 (13.9) | 0.828 |
| Hypertension | 42 (34.7) | 36 (42.9) | 0.301 |
| Coronary atherosclerotic heart disease | 15 (12.4) | 4 (4.8) | 0.108 |
| Stroke | 7 (5.8) | 4 (4.8) | 1.000 |
| Preoperative APACHE II scores, n (%) | 0.307 | ||
| <10 | 120 (99.2) | 81 (96.4) | |
| 10–20 | 1 (0.8) | 3 (3.6) | |
| Intraoperative body temperature (℃), median (IQR) | |||
| Highest body temperature | 36.3 (0.3) | 37.2 (0.1) | <0.001 |
| Minimum body temperature | 35.6 (0.3) | 36.5 (0.2) | <0.001 |
Group R, the routine management group; Group A, the aggressive warming group. SD, standard deviation; ASA, American Society of Anesthesiologists; BMI, body mass index; APACHE II, Acute Physiology and Chronic Health Evaluation II; IQR, interquartile range.
More patients had a postoperative APACHE II score of 10–20 in Group R (n=18, 14.9%) than Group A (n=5, 6.0%; P=0.046; see Table 2). Compared to Group A (n=17, 14.0%), a higher proportion of patients in Group R increased from an APACHE II score of <10 to an APACHE II score of 10–20 after surgery (n=2, 2.4%) (P=0.005; see Table 3). There were no significant differences in the QoR-15 scores on postoperative day 3, LoS, or the incidence of postoperative complications between the 2 groups (P>0.05). The incidence of postoperative non-surgical related complications in Group R was 9.1% (there was 1 case of delirium, and 10 cases of cardiovascular complications), while that in Group A was 6.0% (there were 5 cases of cardiovascular complications). There were no cases of postoperative infection or death in either group (see Table 4).
Table 2
| APACHE II scores, n (%) | Group R (n=121) | Group A (n=84) | χ2 | P |
|---|---|---|---|---|
| <10 | 103 (85.1) | 79 (94.0) | 3.964 | 0.046 |
| 10–20 | 18 (14.9) | 5 (6.0) |
Group R, the routine management group; Group A, the aggressive warming group. APACHE II, Acute Physiology and Chronic Health Evaluation II.
Table 3
| APACHE II score changes, n (%) | Group R (n=121) | Group A (n=84) | χ2 | P |
|---|---|---|---|---|
| No change | 104 (86.0) | 82 (97.6) | 8.028 | 0.005 |
| Increase in grade | 17 (14.0) | 2 (2.4) |
Group R, the routine management group; Group A, the aggressive warming group. APACHE II, Acute Physiology and Chronic Health Evaluation II.
Table 4
| Categories | Group R (n=121) | Group A (n=84) | χ2/Z | P |
|---|---|---|---|---|
| QoR-15, median (IQR) | 117 (15.0) | 119 (14.0) | –0.611 | 0.541 |
| LoS (day), median (IQR) | 6 (3.0) | 7 (5.0) | –0.338 | 0.736 |
| Complications, n (%) | 11 (9.1) | 5 (6.0) | 0.679 | 0.410 |
Group R, the routine management group; Group A, the aggressive warming group. QoR-15, Quality of Recovery-15; LoS, length of hospital stay; IQR, interquartile range.
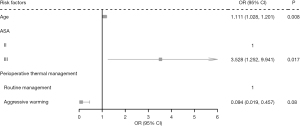
In the regression model for which a postoperative grade increase in the APACHE II score was the outcome, only age, ASA and perioperative thermal management were included in the multivariate logistic regression model. Age and ASA were statistically significant in both the univariate logistic regression and multivariate logistic regression. After controlling for age and ASA, perioperative thermal management was statistically significant. Aggressive warming effectively reduced the risk of postoperative APACHE II grade increasing compared to routine thermal management [odds ratio (OR) =0.097, 95% confidence interval (CI): 0.020 to 0.469, P=0.0789; see Table 5 and Figure 1].
Table 5
| Risk factors | Univariate logistic regression | Multivariate logistic regression | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Age | 1.091 (1.019, 1.168) | 0.013 | 1.111 (1.028, 1.201) | 0.008 | |
| Sex | |||||
| Male | 1 | NA | – | – | |
| Female | 2.481 (0.934, 6.591) | 0.068 | – | – | |
| ASA | |||||
| II | 1 | NA | 1 | NA | |
| III | 3.022 (1.161, 7.870) | 0.024 | 3.528 (1.252, 9.941) | 0.017 | |
| Perioperative thermal management | |||||
| Routine management | 1 | NA | 1 | NA | |
| Aggressive warming | 0.149 (0.034, 0.664) | 0.013 | 0.094 (0.019, 0.457) | 0.003 | |
| BMI | 0.969 (0.828, 1.135) | 0.700 | – | – | |
| Preoperative complications | |||||
| Smoking | 1.012 (0.380, 2.694) | 0.981 | – | – | |
| Diabetes | 0.258 (0.033, 1.998) | 0.194 | – | – | |
| Hypertension | 1.205 (0.462, 3.141) | 0.703 | – | – | |
| Coronary atherosclerotic heart disease | 0.519 (0.065, 4.115) | 0.534 | – | – | |
| Stroke | 0 (0) | 0.999 | – | – | |
APACHE II, Acute Physiology and Chronic Health Evaluation II; OR, odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists; BMI, body mass index; NA, not available.
Discussion
This study found that compared to patients whose core temperature was maintained at ≥37.0 ℃, a core temperature <36 ℃ was associated with a significantly increased risk of increased APACHE II scores during elective VATS. The logistic regression analysis confirmed that, unlike age and ASA grade, active warming was the only possible risk factor for preventing an increase in APACHE II scores. The incidence of complications for patients who developed intraoperative hypothermia was increased, but the difference was not statistically significant due to the limited sample size.
The APACHE II score is a widely used scoring system that has a high value in evaluating patients’ prognosis. The system comprises 3 parts: an acute physiological score, an age score; and a chronic health score. The acute physiological score covers 12 physiological measures, including body temperature, mean arterial pressure, heart rate, respiratory rate, Alveolar-artery Oxygen Partial Pressure Gradient (A-aPO2) or Partial Pressure of Oxygen in Arterial Blood (PaO2), Potential of Hydrogen (pH) or HCO3-, serum sodium, serum potassium, blood creatinine, hematocrit, leukocyte count, and Glasgow score, each item receives a single score of 0–4, and the total score ranges from 0–60. The age score is 0–6 and chronic health score is 2–5, respectively, and the total score of the APACHE II varies from 0–71. A higher score indicates a worse prognosis. Naved et al. (25) found that patients who scored between 3 and 10 had a relatively lower mortality rate (10%), while those who scored between 31 and 40 had a relatively higher mortality rate (84.6%). Thus, this score has certain value for classifying patients according to their conditions, and is positively correlated with mortality. Consistent with previous studies (5-15), we found that the provision of active warming to ensure an intraoperative core body temperature ≥37 ℃ effectively reduced increases in postoperative APACHE II score and any rise in the score grade, optimized the recovery of patients, improved the prognosis of patients, and enabled patients to avoid serious adverse events.
We also found that the incidence of postoperative non-surgical procedure–related complications was 9.1% in the routine management group and 6.0% in the aggressive warming group. This trend was consistent with large sample studies that have shown that perioperative thermal management is associated with adverse cardiovascular and cerebrovascular events (3-15). In our study, 1 patient in group R developed delirium. Chen et al. (26) found that hypothermia leads to increased blood viscosity and affects cerebral blood perfusion, which in turn results in an imbalance of cerebral oxygen supply and demand, and ultimately postoperative cognitive dysfunction. No deaths were reported in our study. This may be because the elective surgery patients’ physical state had been improved to a relatively preferable level to undergo surgery, VATS results in minimally invasive operation and has a short operation time, the number of cases of hypothermia was relatively low, and the sample size was limited. There was no significant difference in the incidence of non-surgical related complications between group R and group A. This is consistent with the findings of a systematic review that active body surface warming did not reduce major cardiovascular adverse events and mortality (27). No significant difference was detected in the LoS between the 2 groups in this cohort. Conversely, Li et al. (28) reported that hypothermia prolonged the LoS in hospital after VATS.
QoR-15 is a patient-self-rated outcome used to estimate the quality of recovery after anesthesia and surgery and has good reliability and measurement error (29). There was no significant difference in the QoR-15 scores between the 2 groups in this study, which may be related to the lagging measurement time on the 3rd postoperative day and other confounding factors. Additionally,, previous studies have shown that perioperative hypothermia is associated with an increased risk of infection (7,27,30). Li et al. (31) found that the higher the APACHE II score, the higher the incidence of infection. In this study, while the APACHE II score increased significantly in the hypothermia group, no cases of postoperative infection were observed. Our findings are similar to those of Yi et al. (2), who discovered that the correlation between perioperative hypothermia and infection is unclear in the Chinese population. This may be due to the following related reasons: (I) VATS is a minimally invasive and aseptic surgery with a short surgery time; (II) antibiotics were conventionally administered before and after surgery; and (III) the sample size was relatively limited.
The logistic regression analysis showed that age and elevated ASA grade were risk factors for increased postoperative APACHE II scores. Similarly, in several studies based on the Epithor database, age, ASA grade, sex, and comorbidities were all considered to be highly associated with postoperative mortality (32-34). However, sex and comorbidities were not associated with an increased postoperative APACHE II score in our study. These differences may be related to the different populations and our insufficient sample size. Active intraoperative temperature management is a modifiable condition, and its management can prevent increases in postoperative APACHE II scores. Our findings are consistent with those of cohort studies and numerous studies that support perioperative active warming (5-15).
Limitations
This study had several limitations. First, this was a single-center, single-disease retrospective study. Second, other unknown confounders were not analyzed. Third, we were unable to control the level of different surgeons, different intraoperative anesthesia management strategies, and the interference of postoperative management methods, which would have had an effect on the research results. The performance of adequate preoperative preparation before the elective VATS would have offset the effect of intraoperative hypothermia on the rising APACHE II scores to some extent. Due to the low incidence of cardiovascular and cerebrovascular complications, postoperative mortality, the infection rate (clean-contamination surgery), and the limited sample size, very few adverse postoperative events were recorded.
Conclusions
For patients with cardiovascular risk factors who are scheduled for VATS, active intraoperative temperature management that maintains a higher than normal body temperature helps to significantly reduce increases in APACHE II scores, which in turn shortens the LoS, reduces the occurrence of non-surgical related cardiovascular and cerebrovascular complications, and improves the prognosis of patients. Elderly and high ASA grade were independent risk factors for increased postoperative APACHE II scores. Various active warming strategies are recommended to maintain an intraoperative core body temperature ≥37.0 ℃, which improves patient prognosis. However, our findings still need to be proven by prospective clinical studies with larger samples.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-873/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-873/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-873/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee of the China-Japan Union Hospital, Jilin University approved the original randomized controlled trial (No. 2018120503), and all the patients agreed to and signed informed consent forms to participate in that study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sessler DI. Mild perioperative hypothermia. N Engl J Med 1997;336:1730-7. [Crossref] [PubMed]
- Yi J, Lei Y, Xu S, et al. Intraoperative hypothermia and its clinical outcomes in patients undergoing general anesthesia: National study in China. PLoS One 2017;12:e0177221. [Crossref] [PubMed]
- Leslie K, Sessler DI, Bjorksten AR, et al. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg 1995;80:1007-14. [PubMed]
- Eger EI 2nd, Johnson BH. MAC of I-653 in rats, including a test of the effect of body temperature and anesthetic duration. Anesth Analg 1987;66:974-6. [Crossref] [PubMed]
- Hart SR, Bordes B, Hart J, et al. Unintended perioperative hypothermia. Ochsner J 2011;11:259-70. [PubMed]
- Madrid E, Urrútia G, Roqué i Figuls M, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev 2016;4:CD009016. [Crossref] [PubMed]
- Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 1996;334:1209-15. [Crossref] [PubMed]
- Winkler M, Akça O, Birkenberg B, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg 2000;91:978-84. [Crossref] [PubMed]
- Rajagopalan S, Mascha E, Na J, et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008;108:71-7. [Crossref] [PubMed]
- Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997;277:1127-34. [Crossref] [PubMed]
- Scott AV, Stonemetz JL, Wasey JO, et al. Compliance with Surgical Care Improvement Project for Body Temperature Management (SCIP Inf-10) Is Associated with Improved Clinical Outcomes. Anesthesiology 2015;123:116-25. [Crossref] [PubMed]
- Billeter AT, Hohmann SF, Druen D, et al. Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery 2014;156:1245-52. [Crossref] [PubMed]
- Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997;87:1318-23. [Crossref] [PubMed]
- Sun Z, Honar H, Sessler DI, et al. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology 2015;122:276-85. [Crossref] [PubMed]
- Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol 2008;22:645-57. [Crossref] [PubMed]
- Li Y, Liang H, Feng Y. Prevalence and multivariable factors associated with inadvertent intraoperative hypothermia in video-assisted thoracoscopic surgery: a single-center retrospective study. BMC Anesthesiol 2020;20:25. [Crossref] [PubMed]
- Fujita T, Okada N, Kanamori J, et al. Thermogenesis induced by amino acid administration prevents intraoperative hypothermia and reduces postoperative infectious complications after thoracoscopic esophagectomy. Dis Esophagus 2017;30:1-7. [PubMed]
- Sessler DI, Lee KA, McGuire J. Isoflurane anesthesia and circadian temperature cycles in humans. Anesthesiology 1991;75:985-9. [Crossref] [PubMed]
- Castrén M, Silfvast T, Rubertsson S, et al. Scandinavian clinical practice guidelines for therapeutic hypothermia and post-resuscitation care after cardiac arrest. Acta Anaesthesiol Scand 2009;53:280-8. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Tian Y, Yao Y, Zhou J, et al. Dynamic APACHE II Score to Predict the Outcome of Intensive Care Unit Patients. Front Med (Lausanne) 2021;8:744907. [Crossref] [PubMed]
- Hansted AK, Møller MH, Møller AM, et al. APACHE II score validation in emergency abdominal surgery. A post hoc analysis of the InCare trial. Acta Anaesthesiol Scand 2020;64:180-7. [Crossref] [PubMed]
- Kisa NG, Kisa E, Cevik BE. Prediction of Mortality in Patients After Oncologic Gastrointestinal Surgery: Comparison of the ASA, APACHE II, and POSSUM Scoring Systems. Cureus 2021;13:e13684. [Crossref] [PubMed]
- Sessler DI, Pei L, Li K, et al. Aggressive intraoperative warming versus routine thermal management during non-cardiac surgery (PROTECT): a multicentre, parallel group, superiority trial. Lancet 2022;399:1799-808. [Crossref] [PubMed]
- Naved SA, Siddiqui S, Khan FH. APACHE-II score correlation with mortality and length of stay in an intensive care unit. J Coll Physicians Surg Pak 2011;21:4-8. [PubMed]
- Chen Y, Ye X, Yu X, et al. Effect of intraoperative warming on delirium during emergence from general anesthesia in elderly patients. Chinese Journal of Anesthesiology 2019:147-9.
- Balki I, Khan JS, Staibano P, et al. Effect of Perioperative Active Body Surface Warming Systems on Analgesic and Clinical Outcomes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Anesth Analg 2020;131:1430-43. [Crossref] [PubMed]
- Li Y, Liang H, Feng Y. Prevalence and multivariable factors associated with inadvertent intraoperative hypothermia in video-assisted thoracoscopic surgery: a single-center retrospective study. BMC Anesthesiol 2020;20:25. [Crossref] [PubMed]
- Kleif J, Waage J, Christensen KB, et al. Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth 2018;120:28-36. [Crossref] [PubMed]
- Hedrick TL, Heckman JA, Smith RL, et al. Efficacy of protocol implementation on incidence of wound infection in colorectal operations. J Am Coll Surg 2007;205:432-8. [Crossref] [PubMed]
- Li HY, Li SJ, Yang N, et al. Evaluation of nosocomial infection risk using APACHE II scores in the neurological intensive care unit. J Clin Neurosci 2014;21:1409-12. [Crossref] [PubMed]
- Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg 2007;133:325-32. [Crossref] [PubMed]
- Bernard A, Rivera C, Pages PB, et al. Risk model of in-hospital mortality after pulmonary resection for cancer: a national database of the French Society of Thoracic and Cardiovascular Surgery (Epithor). J Thorac Cardiovasc Surg 2011;141:449-58. [Crossref] [PubMed]
- Die Loucou J, Pagès PB, Falcoz PE, et al. Validation and update of the thoracic surgery scoring system (Thoracoscore) risk model. Eur J Cardiothorac Surg 2020;58:350-6. [Crossref] [PubMed]


