The optimal sequence for bronchial brushing and forceps biopsy in lung cancer diagnosis: a random control study
Introduction
Bronchoscopy, while essential for diagnosing and staging lung cancer, can give variable diagnostic yields ranging from 37–77% (1-5). One reason for this variability is limitations in tissue sampling techniques, which can make it impossible to obtain the most representative area of neoplastic tissue. Numerous techniques have been devised to circumvent these limitations and thereby increase diagnostic yield; they range from the relatively simple and inexpensive, such as biopsy, washing, brushing, and conventional transbronchial needle aspiration (TBNA), to the sophisticated and expensive, including autofluorescence bronchoscopy, bronchoscopic cryotechnology, and endobronchial ultrasound (EBUS) (1,6-9). Concurrent application of different sampling techniques can improve the yield, such as the combination of forceps biopsy and brushing, as well as a combination of histology and cytology. These various techniques, depending on the clinical context, may or may not perform better than biopsy alone, which typically gives a diagnostic yield of 71–91% (1-4,10). For endobronchial visible exophytic tumors, for example, cryobiopsy gave a diagnostic yield of 97.3–100%, compared to only 69.3–89.5% for forceps biopsy alone (11,12). Autofluorescence imaging (AFI) video bronchoscopy has shown good diagnostic sensitivity, albeit poor specificity, for diseases of bronchial mucosa (8). EBUS combined with TBNA and EBUS-guided transbronchial biopsy (TBB) are highly specific and sensitive methods for examining submucosal lesions, mediastinal lymph nodes and peripheral pulmonary lesions (9,13,14). All these approaches can improve the ability of bronchoscopy to diagnose lung cancer, but most require expensive instrumentation out of reach for primary hospitals around the world and for many health care centers in developing countries. Thus, especially for developing countries, improving the diagnostic yield of basic techniques, such as biopsy and brushing, remains an important challenge.
The detail of bronchoscopic procedures may affect the diagnostic yield. For example, the different sequence of TBNA showed difference in sampling quality which may influence the diagnostic yield (15). Bronchial brushing gives a diagnostic yield of 52–77% for endoscopically visible tumors (1-4), which is not significantly better than the yield obtained with biopsy alone. It is possible that the efficacy of brushing may depend on its sequence performed before or after biopsy, but this has not been studied in detail. One study examining the optimal sequence of washing before or after biopsy found no difference in diagnostic yield between the two sequences of steps (16), but we are unaware of analogous studies examining the sequence of bronchial brushing and forceps biopsy. In the present study, the diagnostic yields of bronchial brushing performed before or after forceps biopsy during flexible bronchoscopy was measured prospectively in suspicious lung cancer patients with endoscopically visible tumors or mucosal invasion. Subgroup analysis based on tumor type and bronchoscopic morphology was also performed to determine whether the two sequences of steps gave different diagnostic yields in specific contexts.
Materials and methods
Patients and sampling
Between June 2012 and July 2013, 420 consecutive patients who underwent bronchoscopy examination at the First Affiliated Hospital of China for suspected endobronchial lung carcinoma were prospectively enrolled in this study. Patients were randomly assigned to a pre-biopsy brushing group, who received two brushings before forceps biopsy and two afterwards, or to a post-biopsy brushing group, who received two brushings after forceps biopsy. The randomization was performed by simple randomization with table of random number. Only patients with a definitive cytological or histological diagnosis of lung cancer based on bronchoscopy or other confirmatory techniques were included. Patients were excluded if they had submucosal lesions, extrinsic compressions, pulmonary metastasis of extrapulmonary malignancies or uncommon non-small cell lung carcinoma (NSCLC), such as salivary gland carcinoma.
This study was approved by the Ethics Review Board of The First Hospital of China Medical University. Each patient provided written informed consent.
Bronchoscopic procedures
The same pulmonologist (G Hou) with extensive bronchoscopy experience performed all procedures using a standard video bronchoscope (1T260, Olympus, Tokyo, Japan) under topical anesthesia (2% lidocaine). Endobronchial biopsy was performed using a flexible long biopsy forceps (FB21C-1, Olympus, Tokyo, Japan) and four tissue pieces from each patient were fixed in 10% formalin and processed for histopathological examination.
Bronchoscopic brushing was carried out using straight brushes (BC-202D-2010, Olympus, Tokyo, Japan); different brushes were used for pre- and post-biopsy brushings to avoid contamination. After sampling, brushes were smeared on four clean slides, then fixed in 95% ethyl alcohol for cytological examination.
Patients were assigned to two groups based on the bronchoscopic appearance of their tissue: exophytic tumor, defined as an intraluminal tumor; and mucosal infiltration, characterized by vascular engorgement involving the mucosa and submucosa layers, edema, and irregularity in the mucosa caused by neoplastic cell invasion.
We also evaluated the occurrence of treated bleeding in the two groups. The definition of “treated bleeding” refers to bleeding which needed further intervention with argon plasma coagulation and/or anti-coagulation drugs. In pre-biopsy brushing group, we cannot perform an intra-group comparison of the treated bleeding of the pre-biopsy brushings and post-biopsy brushings due to the impossible differentiation between the bleeding before and after the biopsy absolutely. So the data of occurrence of the treated bleeding in pre-biopsy brushing group were mixed and analyzed together and the comparison was only done by the inter-group comparison.
Sample preparation and analysis
All brushing samples were stained with Papanicolaou stain and examined by two senior pathologists blinded to patient details and group assignment. The two pathologists discussed any discrepancies to reach a consensus diagnosis.
The pathologists determined the histological diagnosis using hematoxylin and eosin-stained sections according to the 2004 World Health Organization (WHO) classification guidelines (17). Only bronchial brush specimens with unequivocal malignant features that allowed tumor typing were considered positive. Specimens where malignancy was suspected were treated as negative in the data analysis. Malignant cells were typed at high magnification (×400) and classified as squamous cell carcinoma-type, adenocarcinomatous, or small cell lung cancer (SCLC)-type. For subgroup analysis, patients were stratified by bronchoscopic morphology (exophytic tumor or mucosal infiltration) and by lung cancer subtype (using the three above-mentioned classifications).
Statistical analysis
The diagnosis of endobronchial disease by bronchoscopy in 30 studies showed the highest sensitivity for endobronchial cytobrushing was 59% (1). In our hospital, the routine procedure is to perform brushing after biopsy, and the sensitivity is approximately 30%. We anticipated a higher diagnostic yield of 50% in our study, and we assumed that we could detect a 20% difference in diagnostic yield between the two sequences of steps. According to the two-tailed test of proportions, a minimal sample size of 55 cases in each group should be sufficient to detect significant differences in diagnostic yield with a power of 0.8 at 95% significance. In order to allow subgroup analysis, we doubled the sample size. The diagnostic sensitivities of the two methods were compared using the chi-square test implemented in SPSS (version 17.0; IBM, USA), with statistical significance set at P<0.05.
Results
Study population and disease characteristics
A total of 420 consecutive patients suspected of lung cancer who underwent bronchoscopy were randomly assigned into the pre-biopsy brushing group and post-biopsy brushing group. After enrollment, 58 patients were excluded because they had benign disease (n=34), metastatic malignancy (n=5), uncommon NSCLC (n=7), or they lacked a definitive diagnosis of lung cancer (n=12).
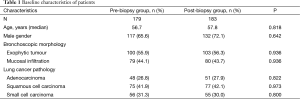
In the end, 362 patients with a definitive diagnosis of lung cancer were analyzed; they were 68.8% male and had a mean age of 57.2 years (range, 26–78 years). The number of patients in the pre-biopsy brushing group was 179, and the number of patients in the post-biopsy brushing group was 183. All patients presented with visible endobronchial abnormalities, which were definitively diagnosed as exophytic tumors in 203 patients (56.1%, 203/362) and as mucosal infiltration in 159 (43.9%, 159/362). Lung cancers were diagnosed based on pathology as squamous cell carcinoma (n=152), adenocarcinoma [99], or SCLC [111]. The two brushing groups did not differ significantly in baseline characteristics of age, gender, endobronchial morphology or tumor type (P>0.05, Table 1).
Full table
Diagnostic yield of bronchial brushing combined with forceps biopsy in two groups
We compared the diagnostic yield of pre-biopsy brushing group (pre-biopsy brushings + forceps biopsy + post-biopsy brushings) with the diagnostic yield of post-biopsy brushing group (forceps biopsy + post-biopsy brushings). The pre-biopsy group had a better diagnostic yield, 89.4% (160/179) vs. 78.8% (144/183) (P=0.006).
To determine whether the diagnostic yield is higher depending on whether the brushing is performed before or after biopsy, both sequences of steps were tested. Forceps biopsy alone gave a similar diagnostic yield in the pre-biopsy brushing group (132/179, 73.7%) and in the post-biopsy brushing group (132/183, 72.1%; P=0.901).
The diagnostic yield when bronchial brushing before or after biopsy was combined with forceps biopsy was significantly higher than when only bronchial biopsy was performed. This result was observed in the pre-biopsy brushing group: pre brushing + biopsy vs. biopsy alone, 87.1% (156/179) vs. 73.7%, χ2=10.23, P=0.001; post brushing + biopsy vs. biopsy alone, 81.9% (149/179) vs. 73.7%, χ2=4.782, P=0.029. A similar result was obtained in the post-biopsy brushing group: post brushing + biopsy vs. biopsy alone, 78.8% (144/183) vs. 72.1%, χ2=4.43, P=0.035. Pre-biopsy brushing combined with forceps biopsy in the pre-biospy group produced higher diagnostic yield than post-biopsy brushing combined with forceps biopsy in the post-biopsy group when compared between two groups, 87.1% vs.78.8%, χ2=4.57, P=0.033.
Diagnostic yield for pre- vs. post-biopsy bronchial brushing
Diagnostic yields of bronchial brushing before and after forceps biopsy were compared in two ways: by intra-group comparison between pre- and post-biopsy brushing in the pre-group, and by inter-group comparison between pre-biopsy brushing in the pre-group and post-biopsy brushing in the post group. Pre-biopsy brushing gave significantly higher diagnostic yield (49.2%, 88/179) than did post-biopsy brushing (31.8%, 57/179) within the pre-group (χ2=11.139, P=0.001, Table 2). This pre-biopsy value of 49.2% was also significantly higher than the 30.6% (56/183) for post-biopsy brushing in the post group (χ2=15.365, P<0.001, Table 3).

Full table

Full table
Comparison of diagnostic yields for pre- and post-biopsy brushings stratified by bronchoscopic morphology
Among patients presenting with exophytic tumors, pre-biopsy brushing gave significantly higher diagnostic yield (59.0%, 59/100) than did post-biopsy brushing (33.0%, 33/100) within the pre-group (χ2=13.607, P<0.001, Table 2). This pre-biopsy value of 59.0% was also significantly higher than the 31.1% (32/103) for post-biopsy brushing in the post group (χ2=16.006, P<0.001, Table 3). Among the patients presenting with mucosal infiltration, no difference in diagnostic yield was found between pre- and post-biopsy bronchial brushing (Tables 2,3).
Comparison of diagnostic yields for pre- and post-biopsy brushing stratified by tumor type
The data were stratified by tumor type into small cell lung carcinoma group, adenocarcinoma group and squamous cell carcinoma group. No matter what type the tumor is, pre-biopsy brushing gave significantly higher diagnostic yield than post-biopsy brushing did except for intra-group comparison in adenocarcinoma group (Tables 2,3).
Comparison of occurrence of bleeding complication for pre- vs. post-biopsy bronchial brushing group
The bleeding complication which need treated was uncommon. No difference in occurrence of treated bleeding for pre- vs. post-biopsy bronchial brushing was found (1.1% vs. 1.7%, P=0.682).
Discussion
Since optimizing basic techniques in diagnostic bronchoscopy is important for improving medical services in developing countries, we sought to determine whether we could increase the diagnostic yield of brushing by performing the brushing and biopsy steps in a particular order. Our results show that performing brushing and biopsy together can significantly improve diagnostic yield, and that pre-biopsy brushing is superior to post-biopsy brushing for diagnosing exophytic tumors. For tumors presented as mucosal infiltration, however, pre- and post-biopsy brushing seems to give similar diagnostic yields.
The overall diagnostic yield of pre- and post-biopsy bronchial brushing was 39.8% in this study, which lies within the range of 37–77% reported in other studies (1-4); this wide range may reflect, in part, differences in what investigators consider to be visible tumors. One reason why our brushing yield may be lower than some other studies is that a greater proportion of our patients presented mucosal infiltration (43.9%). For example, one study reporting a brushing diagnostic yield of 68.4% involved only 22 of 85 (25.9%) patients with mucosal infiltration (4).
In our study combining brushings with forceps biopsy gave higher diagnostic yield than forceps biopsy alone, regardless of whether the brushing was performed pre- or post-biopsy. These results are consistent with previous studies suggesting that a combination of brushing with forceps biopsy is a better and cost-effective strategy for diagnosing visible endobronchial lung cancer (4,18). This improvement in diagnostic yield presumably reflects the ability of brushing to increase the sampling area, as well as the fact that brushing provides an additional diagnostic test to complement biopsy results. But for the cases of mucosal infiltration or submucosal spread, TBNA may be a good alternative (19).
Our study revealed that pre-biopsy brushing group (pre-biopsy + forceps biopsy + post-biopsy brushing group) yielded better than post-biopsy brushing group (forceps biopsy + post-biopsy brushing) in diagnosis of lung cancer. The difference in diagnostic yield maybe due to the increasing times of brushing which agrees with previous study (4). But they have not investigated whether that value depends on the sequence of brushing. Since brushing in most studies, as well as in our hospital, is performed after biopsy, we investigated whether pre-biopsy brushing might give better results. Our study also revealed that pre-biopsy brushing produced higher diagnostic yield than post-biopsy brushing in both intra- and inter-group comparisons. Pre-biopsy brushing combined with forceps biopsy produced higher diagnostic yield than post-biopsy brushing combined with forceps biopsy in inter-group comparison. Subsequent subgroup analysis showed that this difference was limited to exophytic tumors. While among the patients presenting with mucosal infiltration, no difference in diagnostic yield was found between pre- and post-biopsy bronchial brushing. Subgroup analysis also showed that pre-biopsy brushing gave significantly higher diagnostic yield than post-biopsy brushing did when stratified by tumor type, except for intra-group comparison in adenocarcinoma group. It indicated that pathological types of lung cancer did not influence the advantage of pre-biopsy brushing in diagnostic yield. As far as we know, this is the first report examining the dependence of diagnostic yield of bronchial brushing on the order of brushing and biopsy steps. Previous studies have identified several factors influencing diagnostic yield of bronchial brushings, including distance from the carina, endobronchial visibility, tumor size and location (20). Our results extend that literature (18) to show that, in certain circumstances, performing the brushing pre- or post-biopsy will also influence diagnostic yield.
Our finding that pre-biopsy brushings are superior to post-biopsy ones for diagnosing exophytic tumors contrasts with Chaudhary’s study on the sequencing of bronchial washing and biopsy, which suggested that post-biopsy washing gives higher diagnostic yield (2). Chaudhary et al. (2) explained their results by suggesting that the biopsy procedure liberates tumor cells, allowing them to be collected in larger numbers during washing. In our case, we rationalize our finding of better diagnostic yield using pre-biopsy brushings as follows. Before biopsy, exophytic tumors can be easily visualized and sampled because they constitute a nodule or focal mass; after biopsy, bleeding contaminates the limited field available for brushing, making it more difficult to identify and sample appropriate tissue. Similar reasoning may also explain why pre-biopsy brushings did not give higher diagnostic yield than post-biopsy ones in our patients with mucosal infiltration. In this case, the contact surface for mucosal infiltration is larger and more spread out than for an exophytic tumor, making it easier to sample even when contaminated by post-biopsy bleeding. Our study exhibits that there was no difference in the occurrence of bleeding that would need to be treated in pre- and post-biopsy bronchial brushing groups. However, our study indicates that pre-biopsy brushings had the potential advantage to save procedure time. The mild bleeding of the tumors caused by biopsy or brushing had a different impact on the quality of subsequent brushings or biopsies. To maintain a better quality of brushing, we felt that spending more time on the suction of the local bleeding to obtain a good vision field in post-biopsy brushing group. On the contrary, mild bleeding after brushing slightly influenced the quality of biopsy. In many cases we did not need the careful suction of bleeding. Consequently, we deducted that the time spent on the post-biopsy brushing may extend the procedure by a few seconds to minutes.
Despite its insights, the present study does have some limitations. First, although our sample size was adequate based on our power analysis, this was a single-center study and it performed analysis with several relatively small subgroups. Second, twelve patients lacking a confirmatory diagnosis were excluded from the final analysis, which may have biased our results. Third, we did not record the time of bronchoscopy examination in two groups. The deduction that the pre-biopsy brushing is more timesaving was only made by the feeling throughout the procedures.
Conclusions
In conclusion, our study suggests that bronchial brushing improves the diagnostic yield of simple bronchoscopic biopsy, and that pre-biopsy brushing is superior to post-biopsy brushing for patients with endobronchial exophytic tumors.
Acknowledgements
Funding: This research was supported by a project grant from the Ministry of Science and Technology of the People’s Republic of China (2012BAI05B01), scientific research fund of department of education of Liaoning Province, China (L2013310), a project grant from Science and Technology Department of Liaoning Province (2014225006) and Youth fund of Health and Family Planning Commission of Liaoning Province (LNCCC-D03-2015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S-128S. [Crossref] [PubMed]
- Chaudhary BA, Yoneda K, Burki NK. Fiberoptic bronchoscopy. Comparison of procedures used in the diagnosis of lung cancer. J Thorac Cardiovasc Surg 1978;76:33-7. [PubMed]
- Goel K, Gupta S, Pai MR, Naik R. Comparison of diagnostic accuracy of cytologic sampling techniques in lung cancer: a study of 541 cases from coastal Karnataka. Acta Cytol 2008;52:638-9. [Crossref] [PubMed]
- Karahalli E, Yilmaz A, Türker H, et al. Usefulness of various diagnostic techniques during fiberoptic bronchoscopy for endoscopically visible lung cancer: should cytologic examinations be performed routinely? Respiration 2001;68:611-4. [Crossref] [PubMed]
- Verma A, Lim AY, Tai DY, et al. Timeliness of Diagnosing Lung Cancer: Number of Procedures and Time Needed to Establish Diagnosis: Being Right the First Time. Medicine (Baltimore) 2015;94:e1216. [Crossref] [PubMed]
- Silvestri GA, Feller-Kopman D, Chen A, et al. Latest advances in advanced diagnostic and therapeutic pulmonary procedures. Chest 2012;142:1636-44. [Crossref] [PubMed]
- Sun S, Ge N. Endoscopic ultrasound: An all in one technique vibrates virtually around the whole internal medical field. Journal of Translational Internal Medicine 2014;2:104-6. [Crossref]
- van der Heijden EH, Hoefsloot W, van Hees HW, et al. High definition bronchoscopy: a randomized exploratory study of diagnostic value compared to standard white light bronchoscopy and autofluorescence bronchoscopy. Respir Res 2015;16:33. [Crossref] [PubMed]
- Nguyen P, Fielding D. Optical differentiation between malignant and benign lymphadenopathy by EBUS using grey scale texture analysis. Respirology 2015;20:847-8. [Crossref] [PubMed]
- Shukla S, Malhotra KP, Husain N, et al. The utility of cytology in the diagnosis of adenocarcinoma lung: A tertiary care center study. J Cytol 2015;32:159-64. [Crossref] [PubMed]
- Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J 2012;39:685-90. [Crossref] [PubMed]
- Chou CL, Wang CW, Lin SM, et al. Role of flexible bronchoscopic cryotechnology in diagnosing endobronchial masses. Ann Thorac Surg 2013;95:982-6. [Crossref] [PubMed]
- Czarnecka K, Yasufuku K. Interventional pulmonology: focus on pulmonary diagnostics. Respirology 2013;18:47-60. [Crossref] [PubMed]
- Edey AJ, Pollentine A, Doody C, et al. Differentiating benign from malignant mediastinal lymph nodes visible at EBUS using grey-scale textural analysis. Respirology 2015;20:453-8. [Crossref] [PubMed]
- Sun J, Yang H, Teng J, et al. Determining factors in diagnosing pulmonary sarcoidosis by endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Surg 2015;99:441-5. [Crossref] [PubMed]
- van der Drift MA, van der Wilt GJ, Thunnissen FB, et al. A prospective study of the timing and cost-effectiveness of bronchial washing during bronchoscopy for pulmonary malignant tumors. Chest 2005;128:394-400. [Crossref] [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. Pathology and genetics: Tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004.
- Matsuda M, Horai T, Nakamura S, et al. Bronchial brushing and bronchial biopsy: comparison of diagnostic accuracy and cell typing reliability in lung cancer. Thorax 1986;41:475-8. [Crossref] [PubMed]
- Shital P, Rujuta A, Sanjay M. Transbronchial needle aspiration cytology (TBNA) in endobronchial lesions: a valuable technique during bronchoscopy in diagnosing lung cancer and it will decrease repeat bronchoscopy. J Cancer Res Clin Oncol 2014;140:809-15. [Crossref] [PubMed]
- Lee HS, Kwon SY, Kim DK, et al. Bronchial washing yield before and after forceps biopsy in patients with endoscopically visible lung cancers. Respirology 2007;12:277-82. [Crossref] [PubMed]


