A retrospective comparative cohort study on the efficacy and safety of bi-port robotic-assisted lobectomy and multi-port robotic-assisted lobectomy
Introduction
Surgical resection is still the optimal treatment for patients with early-stage lung cancer (1,2). In the past 20 years, the advent of the da Vinci robotic surgical system has ushered in a new era of minimally invasive surgery. The high-definition imaging technology and the 3-dimensional (3D) surgical field with scaled movement and tremor reduction ensure the accuracy of the operation and provide convenience and safety (3). Many studies have confirmed that robotic-assisted thoracic surgery (RATS) is an effective alternative to video-assisted thoracic surgery (VATS), and it has the advantages of less intraoperative blood loss, a faster postoperative recovery time, fewer complications, and a less steep learning curve (4-6). Robotic-assisted surgery is becoming increasingly popular; however, the surgical method is relatively fixed to the multi-port pattern.
The pursuit of less surgical incisions has led to a multitude of clinical innovations and research. Studies from home and abroad have compared the advantages and disadvantages of multi- and single-port VATS. In addition to reducing the number of incisions, single-port surgery has also been shown to reduce the hospitalization time of patients, relieve postoperative pain, and greatly improve the postoperative experience of patients (7,8). The reduction of the size of the robotic arm in the new generation da Vinci robotic XI surgical system enabled us to apply our surgical experiences of uniport VATS to RATS. A case report had demonstrated the feasibility of uniportal RATS (9). However, only 1 patient was involved in the report and the complex surgical techniques of uniportal surgery narrowed its application. Thus, between February 2021 and May 2021, we preliminarily explored the feasibility and the surgical approach of robotic-assisted lobectomy with a bi-port and completed 20 cases. This study collected and analyzed the perioperative data of these 20 cases and compared it to 40 cases of simultaneous multi-port RATS. The advantages and disadvantages of this surgical method are discussed. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1003/rc).
Methods
Patients
Between February 2021 and May 2021, there were a total of 73 cases of RATS. we performed 20 cases of bi-port RATS and the rest 53 cases were multi-port RATS. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have a tumor with a maximum diameter <5 cm as shown on the chest CT scan; (II) no mediastinal lymph node with a maximum diameter >1 cm or no Standardized uptake value (SUV) uptake as shown in the positron emission tomography-computed tomography (PET-CT) scan; and (III) have no abnormality detected in the preoperative examinations.
Patients who underwent wedge resection or segmentectomy or who lacked complete clinical data were excluded; Patients were also excluded from the study if the surgery was stopped because the patient appeared to have a clinical tumor, node, metastasis (TNM) stage IIIB tumor. Eventually 20 cases of bi-port RATS and 40 cases of multi-port RATS were enrolled. These cases were selected and examined to compare the safety and efficacy of the new surgery strategy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Shanghai Chest Hospital (No. KS1735) and informed consent was taken from all the patients.
The patients were strictly required to quit smoking for 2 weeks, and the preoperative examinations were finished and evaluated before surgery, including pulmonary function testing, coronary computed tomography (CT) angiography, ultrasound cardiogram, brain magnetic resonance imaging, and abdominal ultrasound. Bone scintigraphy or PET-CT was performed to rule out metastasis. Surgery was arranged after the strict evaluation of the results of the above-mentioned tests.
Surgical procedure
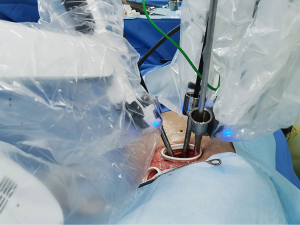
The da Vinci Surgical System (Xi) was used in all the surgeries. All the patients underwent general anesthesia with double-lumen endotracheal intubation. The bi-port RATS requires 2 ports; that is, an operation port and an assistant port. The operation port is set in the 4th or 5th intercostal space along the anterior axillary line and the incision is about 2.5–3 cm. A protective sleeve covers the incision and groups all the surgical instruments, including the camera and 2 arms. The camera is inserted vertically into the sleeve, and 2 interchangeable arms carrying surgical instruments are crossed at the plane of the incision. The assistant port is set in the 4th intercostal space between the mid-axillary line and the anterior axillary line, 1.5 cm from the edge of the incision. During the surgery, the stapler, suction, and clamps are held carried by the bedside assistant through the assistant port (see Figure 1).
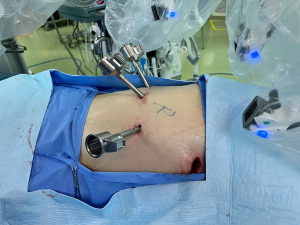
The multi-port RATS requires 4 ports. The camera port is placed in the 7th intercostal space at the mid-axillary line. The other 2 arm ports are placed in the same intercostal space as the camera port and are placed 8 cm away from each other to avoid collision. The assistant port is placed in the 4th intercostal space. The surgical steps are similar to those of the bi-port RATS (see Figure 2).
An intraoperative frozen section diagnosis was made for each patient during the surgery, and the detailed pathology analysis results were available within 3 weeks. The surgery duration and blood loss were immediately documented. The volume and duration of the postoperative chest tube drainage and complications were recorded during the patients’ stay in the hospital.
Statistical analysis
All the statistical analyses were carried out with SPSS 26.0. The normally distributed quantitative variables are expressed as the mean ± standard deviation (), and the Student’s t-test was used to compare the groups. In cases of non-compliance, the continuous variables are expressed as the median [interquartile range (IQR)], and the Wilcoxon rank-sum test was used to compare the groups. The categorical variables were compared using the chi-square test or Fisher’s exact test. The test level between the 2 groups was set at α=0.05 (bilateral), and differences with a P value <0.05 were considered statistically significant. The baseline characteristics of the two group in relation to age, gender, preoperative statues (whether developed diabetes or hypertension), tumor location, and tumor size were comparable (P≥0.05).
Results
A total of 60 surgeries in the 2 groups were enrolled. Among the 20 patients who underwent bi-port RATS, 7 were male and 13 were female. Before surgery, 2 patients had hypertension, and 1 patient had diabetes. None of the surgeries were converted to thoracotomy, and no severe complications occurred during the patients’ stay in the hospital. The baseline characteristics of the 2 groups were listed in Table 1, including their age, gender, and tumor location. The diagnoses of all the lesions, including the dissected lymph nodes, were confirmed by pathology. The maximum diameter of the tumors, pathological TNM staging, and histology were also set out in Table 1. In terms of demographic and clinical characteristics, there was no significant difference between the two groups (P≥0.05).
Table 1
| Baseline characteristics | Bi-port RATS (n=20) | Multi-port RATS (n=40) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 58.5±11.1 | 60.4±8.6 | t=–0.399 | 0.480 |
| Gender | χ2=0.848 | 0.357 | ||
| Male | 7 (35.0) | 19 (47.5) | ||
| Female | 13 (65.0) | 21 (52.5) | ||
| Hypertension | 0.238 | |||
| Positive | 2 (10.0) | 9 (22.5) | ||
| Negative | 18 (90.0) | 31 (77.5) | ||
| Diabetes | ||||
| Positive | 1 (5.0) | 4 (10.0) | 0.656 | |
| Negative | 19 (95.0) | 36 (90.0) | ||
| Tumor location | 0.056 | |||
| RUL | 5 (25.0) | 22 (55.0) | ||
| RML | 2 (10.0) | 5 (12.5) | ||
| RLL | 6 (30.0) | 3 (7.5) | ||
| LUL | 3 (15.0) | 1 (2.5) | ||
| LLL | 3 (15.0) | 8 (20.0) | ||
| RUL and RML | 1 (5.0) | 1 (2.5) | ||
| Max tumor diameter (cm) | 2.0±1.0 | 1.9±0.9 | t=0.464 | 0.711 |
| pTNM stage | 0.808 | |||
| IA | 14 (70.0) | 31 (77.5) | ||
| IB | 3 (15.0) | 3 (7.5) | ||
| IIA | 0 (0) | 0 (0) | ||
| IIB | 0 (0) | 1 (2.5) | ||
| IIIA | 0 (0) | 1 (2.5) | ||
| Other | 3 (15.0) | 4 (10.0) | ||
| Histology | 0.282 | |||
| Atypic Hyperplasia | 1 (5.0) | 0 (0) | ||
| AIS | 0 (0) | 1 (2.5) | ||
| MIA | 3 (15.0) | 4 (10.0) | ||
| IAC | 14 (70.0) | 28 (70.0) | ||
| Other | 2 (10.0) | 2 (5.0) | ||
| Squamous cell carcinoma | 0 (0) | 3 (7.5) | ||
| Granuloma | 0 (0) | 2 (5.0) |
Age and maximum tumor diameter are presented as mean ± standard deviation; Gender, hypertension, diabetes, tumor location, pTNM stage, histology are presented as number (percentage). RATS, robotic-assisted thoracic surgery; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; AIS, adenocarcinoma in situs; MIA, micro invasive adenocarcinoma; IAC, invasive adenocarcinoma.
Table 2 sets out the perioperative outcomes. The mean blood loss volumes were 60 and 65 mL for patients who underwent bi-port RATS and multi-port RATS, respectively. The mean operation durations were 95.6 and 101.4 min for patients who underwent bi-port RATS and multi-port RATS, respectively. No significant difference was found between the 2 groups in relation to either of these measurements. The postoperative evaluation also revealed no significant differences between the 2 groups in relation to chest drainage tube removal and Numerical Rating Scales (NRS) pain score. No severe complications were observed in either group. However, 6 and 5 patients suffered from fever in the bi-port RATS group and the multi-port RATS group, respectively. Subcutaneous emphysemas were observed in 2 patients in the bi-port RATS group and 4 patients in the multi-port RATS group. Atelectasis was observed in the postoperative X-ray scan in 4 patients in the multi-port RATS group, but was not observed in any patients in the bi-port RATS group.
Table 2
| Perioperative data | Bi-port RATS | Multi-port RATS | t/χ2 | P |
|---|---|---|---|---|
| Chest tube drainage (mL) | 459.5±262.8 | 673.4±508.0 | t=–1.943 | 0.084 |
| NRS pain score | 2.9±1.0 | 2.6±1.0 | t=1.030 | 0.525 |
| Chest drain removal (d) | 4.3±1.0 | 5.05±1.3 | t=–2.126 | 0.071 |
| Complication | ||||
| Fever | 6 (30.0) | 5 (12.5) | χ2=1.055 | 0.304 |
| Subcutaneous emphysema | 2 (10.0) | 4 (10.0) | 1.000 | |
| Recurrent laryngeal nerve injury | 0 | 0 | ||
| Atelectasis | 0 | 4 (10.0) | ||
| Chylothorax | 0 | 0 | ||
| Operation duration (min) | 95.6±21.4 | 101.4±25.0 | t=–0.881 | 0.328 |
| Blood loss (mL) | 60.0±20.5 | 65.0±30.4 | t=–0.340 | 0.442 |
Chest tube drainage, NRS pain score, chest drain removal are presented as mean ± standard deviation; complications are presented as numbers (percentages). RATS, robotic-assisted thoracic surgery; NRS, numerical rating scale.
A thorough lymph node dissection was performed in the patients diagnosed with invasive adenocarcinoma (IAC) via an intraoperative frozen section. On average, 6 stations of lymph nodes were dissected in the bi-port RATS group and 6.1 were dissected in the multi-port RATS group. The average diameter of the maximum lymph node dissected was 1.2 cm, and the difference between the 2 groups was not statistically significant (see Table 3).
Table 3
| Variables | Bi-port RATS | Multi-port RATS | t | P |
|---|---|---|---|---|
| Lymph node dissected (station) | 6.0±1.4 | 6.1±1.6 | 0.049 | 0.981 |
| Max lymph node diameter (cm) | 1.2±0.4 | 1.2±0.5 | 0.155 | 0.977 |
Dissected lymph node stations and max lymph node diameter are presented as mean ± standard. RATS, robotic-assisted thoracic surgery.
Discussion
The da Vinci robotic surgical system is a developmental milestone that has propelled the advancement of minimally invasive surgery. With the advantages of its 3D view and flexible mechanic arms, robotic-assisted surgery is safe, thorough, and accurate (10,11). A number of studies have validated and proven the advantages of robotic-assisted surgery. Huang et al. discovered that for patients with cN2 non-small cell lung cancer (NSCLC) RATS has similar long time survival to thoracotomy (12). Compared to VATS, RATS is able to dissect lymph nodes more finely and has a relatively shorter operation time (13).
Due to the ever-growing aesthetic demands (14), the single-port technique is becoming highly popular. In recent decades, a tremendous series of changes have occurred in terms of both the technology and the preferred surgical approach. The interchangeable arms in the da Vinci robotic surgical system (XI) have improved the flexibility of the surgery, and the reduction in the size of the robotic arms have made it possible to perform lobectomy and lymphadenectomy with only 1 operation port.
This study compared 20 cases of bi-port RATS and 40 cases of multi-port RATS. No statistically significant differences were found in terms of the intraoperative blood loss, operation duration, and pathology outcomes, including the maximum tumor diameter, pathological TNM staging, and histology, between the 2 groups. The bi-port robotic-assisted lobectomy took 95 min on average, while the multi-port robotic-assisted lobectomy took 101 min. The fastest bi-port lobectomy took only 46 min.
As the fluency of surgery is not compromised by our new surgical strategy, the indication is also broad. The 20 lobectomies performed using the bi-port technique were all successfully completed, the maximum diameter of the tumors was 3.6 cm and the maximum diameter of the dissected lymph nodes was 1.7 cm. Thus, it is feasible to perform lobectomy via a bi-port and an assistant port if the maximum diameter of the tumor is <3.6 cm and the maximum diameter of the largest lymph node is <1.7 cm. In relation to short-term recovery, the chest drain removal time and chest tube drainage is comparable between the bi-and multi-port RATS groups, and the reduction in operation ports did not prolong patients recovery and did not increase patients suffering.
Lymphadenectomy, as an indispensable part of pulmonary lobectomy, plays a vital role in surgical treatment and has received great attention in all kinds of research (15,16). Wilson et al. drew the conclusion that the rate of nodal upstaging for robotic resection appears to be superior to that of VATS (17). Jin et al. found that robot-assisted lobectomy RAL is associated with a higher number of lymph nodes being dissected (18). The advancement of RATS in lymph node dissection has been confirmed. In this study, the number of lymph node stations dissected and the maximum diameter of the lymph nodes retrieved were documented and compared. No significant differences were found between the 2 groups. However, in complicated situations, such as calcified lymph nodes close to blood vessels or trachea and lymph nodes >2 cm in maximum diameter, multi-port RATS has an advantage over bi-port RATS.
This study had several limitations. First, as a retrospective study, selective bias could not be avoided; second, the size of the sample was relatively small can only reflect the experience of a single center; third, data on long-term survival were not collected in this study. A multicenter randomized clinical trial is warranted to further determine the clinical benefits of bi-port RATS.
Conclusions
Compared to multi-port RATS, Bi-port robotic-assisted lobectomy was safe and showed promising efficacy in patients with early staged operable lung cancer. With continuous endeavor, this technique will be more widely performed in the years to come and bring benefits our patients.
Acknowledgments
Funding: This work was supported by the National Multi-disciplinary Treatment Project for Major Diseases (No. 2020NMDTP) and the National Natural Science Foundation of China (No. 81972176).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1003/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1003/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1003/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics board of Shanghai Chest Hospital (No. KS1735) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Kamigaichi A, Tsutani Y, Mimae T, et al. Prognosis of segmentectomy and lobectomy for radiologically aggressive small-sized lung cancer. Eur J Cardiothorac Surg 2020;58:1245-53. [Crossref] [PubMed]
- Schwartz G, Sancheti M, Blasberg J. Robotic Thoracic Surgery. Surg Clin North Am 2020;100:237-48. [Crossref] [PubMed]
- Gómez-Hernández MT, Fuentes MG, Novoa NM, et al. The robotic surgery learning curve of a surgeon experienced in video-assisted thoracoscopic surgery compared with his own video-assisted thoracoscopic surgery learning curve for anatomical lung resections. Eur J Cardiothorac Surg 2022;61:289-96. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Ng C. Advances in Uniportal Video-Assisted Thoracoscopic Surgery: Pushing the Envelope. Thorac Surg Clin 2016;26:187-201. [Crossref] [PubMed]
- Perna V, Carvajal AF, Torrecilla JA, et al. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: a randomized study. Eur J Cardiothorac Surg 2016;50:411-5. [Crossref] [PubMed]
- Yang Y, Song L, Huang J, et al. A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) Surgical System in the treatment of early-stage lung cancer. Transl Lung Cancer Res 2021;10:1571-5. [Crossref] [PubMed]
- Mahieu J, Rinieri P, Bubenheim M, et al. Robot-Assisted Thoracoscopic Surgery versus Video-Assisted Thoracoscopic Surgery for Lung Lobectomy: Can a Robotic Approach Improve Short-Term Outcomes and Operative Safety? Thorac Cardiovasc Surg 2016;64:354-62. [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Huang J, Tian Y, Li C, et al. Robotic-assisted thoracic surgery reduces perioperative complications and achieves a similar long-term survival profile as posterolateral thoracotomy in clinical N2 stage non-small cell lung cancer patients: a multicenter, randomized, controlled trial. Transl Lung Cancer Res 2021;10:4281-92. [Crossref] [PubMed]
- Huang J, Tian Y, Zhou QJ, et al. Comparison of perioperative outcomes of robotic-assisted versus video-assisted thoracoscopic right upper lobectomy in non-small cell lung cancer. Transl Lung Cancer Res 2021;10:4549-57. [Crossref] [PubMed]
- Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412-41. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Gooseman MR, Brunelli A. Intraoperative Lymph Node Management During Non-small Cell Lung Cancer Surgery. Ann Surg Oncol 2021;28:6925-6. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7. [Crossref] [PubMed]
- Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann Surg 2022;275:295-302. [Crossref] [PubMed]