
Intraoperative fluorescence imaging in esophagectomy and its application to the robotic platform: a narrative review
Introduction
The use of robotic-assisted esophagectomy, a type of minimally invasive esophagectomy (MIE), is increasing and a growing body of literature demonstrates its advantages over other operative techniques (1). MIE, in general, has been associated with shorter length of stay, decreased complication rates, and improved quality of life, without comprising surgical outcomes or survival (2-4). In addition, robotic-assisted esophagectomy provides the surgeon with improved visualization with 10× magnification and 3D high-definition modalities, increased mobility due to a wider range of motion of the wristed instruments and user-friendly ergonomics. Recent studies have indicated that even compared to MIE, robotic assistance has resulted in superior outcomes, including lower rates of conversion to open, shorter length of stay, higher lymph node yield, less operative blood loss, and higher rates of R0 resection (5-7).
Esophagectomy, a complex procedure with high morbidity and mortality, represents an operation with great potential for improvement with robotic techniques (8). In this procedure, the resected esophagus is replaced with a tubularized conduit, most commonly gastric, that is dependent on blood flow from the right gastroepiploic artery. Therefore, visualization of this vessel to ensure preservation during dissection, as well as assessing perfusion at the conduit tip to determine the site of the gastroesophageal anastomosis, are critical. The superior visualization by 3D technology enhances the identification of these critical structures, and the wristed instruments allow improved management of tight operative spaces, facilitating key steps including complete 3-field lymph node dissection. In addition, the use of adjunct visualization tools is optimized in the robotic setting, where 3D optics and built-in near-infrared (NIR) imaging are standard components of the robotic platform. Intraoperative fluorescence imaging has demonstrated promising preliminary results in reducing complications following esophagectomy (9). Although there are multiple fluorescence modalities available, NIR fluorescence with the use of indocyanine green (ICG) is the only modality approved by the Federal Drug Administration, and as such, is widely used for esophagectomy, along with other gastrointestinal cancers, in both open and minimally invasive settings (10).
As robotic-assisted esophagectomy becomes more routine, the reliance on adjunct visualization tools will increase. These tools allow for both standardization and quality control across surgeons and hospital centers by facilitating clear visualization of conduit perfusion, mapping of lymphatic channels, and identification of critical anatomy. The aim of this narrative review is to evaluate the specific uses of intraoperative fluorescence imaging as an adjunct tool while performing robotic-assisted esophagectomy. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-456/rc).
Methods
A literature search was conducted via PubMed in February 2022 to look specifically at the use of intraoperative fluorescence imaging in esophagectomy. The following keywords and their combinations were used: esophagectomy, esophageal cancer, infrared, NIR and fluorescence. The inclusion criteria were peer-reviewed academic journal articles published in English between 2000 and 2021. Bibliographies of relevant studies were reviewed and appropriate citations were included. Editorials, commentaries, abstracts and articles without full text were excluded (Table 1).
Table 1
| Items | Specifications |
|---|---|
| Date of search | February 20, 2022 |
| Databases and other sources searched | PubMed |
| Search terms | Esophagectomy, esophageal cancer, infrared, near-infrared, fluorescence |
| Timeframe | 2000–2021 |
| Inclusion and exclusion criteria | Peer-reviewed academic journals published in English were included. Editorials, commentaries, abstracts and articles without full text were excluded |
| Selection process | A single author (MVP) reviewed the results of the initial search, along with relevant bibliographies and excluded those unrelated to the topic. All authors reviewed the final list of studies included in the review |
Results
Assessment of perfusion
Anastomotic leak rate following esophagectomy has been reported to be anywhere from 6–41% and is associated with significant morbidity and mortality (11). Anastomotic leaks are a result of mechanical tension and poor perfusion, which has motivated the development of adjunct tools to assess intraoperative blood flow at the anastomotic site (12). Prior to the development of these techniques, the risk of anastomotic leak was predicted by clinical judgement, which is not reliably correlated with clinical results (13,14). Studies have evaluated the role for intraoperative fluorescence imaging as related to feasibility, selection of anastomotic site, and defining qualitative and quantitative measures of perfusion. As we will discuss below, we would argue that these metrics are best leveraged in the robotic platform, with several groups, including our own, advancing the role of these quantitative metrics in evaluating quality and outcomes for esophagectomy.
Feasibility
Multiple studies have demonstrated the use of intraoperative fluorescence to identify the vascular network and associated conduit perfusion (15-21). Among patients undergoing robotic-assisted esophagectomy, Sarkaria et al. utilized ICG in a cohort of 30 patients and were able to successfully identify the termination of the vascular arcade in all patients. In addition, the use of ICG resulted in visualization of small transverse vessels, which were otherwise unidentified, and confirmation of the arcade during mobilization of the greater curve and omentum, illustrating the advantages of the improved optics and visualization capabilities of the robotic system (22).
Creation of anastomosis
The placement of the gastroesophageal anastomosis is a critical decision, as anastomosis to areas with poor perfusion threaten the integrity, increasing the risk for anastomotic breakdown and leak. Egberts et al. describe their fully robotic technique in 75 patients, which includes administration of fluorescing ICG in order to identify a potential deficiency in perfusion of the gastric conduit, allowing for gastric tube length adaptation as needed (23). Similarly, both Pötscher et al. and DeLong et al. illustrate their experience with the robotic system, explaining the use of fluorescence in their identification of vascular anatomy and creation of the gastric conduit and esophagogastric anastomosis, highlighting the ease, feasibility and technical advantages that come with the robotic system (24,25). Lastly, Hodari et al. evaluated 54 patients who underwent robotic-assisted Ivor Lewis esophagectomy, utilizing ICG and the FireFly Fluorescence Imaging system to evaluate real-time perfusion. Ultimately, only 3 patients developed a leak, and the team hypothesized that the use of ICG to evaluate real-time perfusion improved outcomes as they were able to consistently identify a demarcation zone of perfusion on the esophageal remnant and tip of esophageal mucosa, guiding their suture placement (26). The adoption of the robotic platform for MIE further enhances the scope of these findings, with the ease of the built-in Firefly camera and the opportunity to quantify perfusion intensity and time to perfusion, and to standardize these metrics across surgeons and centers.
Qualitative and quantitative measures of perfusion
Given the association of poor perfusion with development of anastomotic leak, assessing perfusion quality provides a unique opportunity to quantify the risk for anastomotic leak and the quality of the anastomosis, allowing a new level of standardization and development of quality of care metrics, which is critically important for a procedure with historically high morbidity. However, essential to improving surgical care in esophagectomy is to quantify what defines a “good” conduit in objective rather than subjective terms. Preliminary studies in open and MIE techniques have shown that longer intraoperative fluorescence visualization time and slower gastric conduit perfusion are associated with anastomotic leak, with timing thresholds ranging from 30–90 seconds (27-33). Slooter et al. prospectively evaluated 84 patients who underwent Ivor Lewis or McKeown esophagectomy, many of which were performed with robotic assistance. This group determined that time between ICG injection and tip enhancement was predictive for anastomotic leakage with a cut-off value of 98 seconds [specificity 98%, sensitivity 17%, positive predictive value (PPV) 50%, negative predictive value (NPV) 91%] (27). In these situations, in which seconds may lead to improved outcomes, the accuracy of timing can be ideally quantified in a robotic system, particularly with the use of the da Vinci Firefly camera, allowing for more precise and standardized measures of perfusion and enhanced monitoring of surgical technique across surgeons and hospital centers.
Nodal mapping and dissection
Lymph node dissection during esophagectomy provides improved locoregional control and has been shown to result in improved survival (34). In addition, greater lymph node harvest has been associated with improved staging and prognostic information, influencing post-operative adjuvant therapy decisions (35). Therefore, the ability to identify lymph nodes during esophagectomy is critical for optimal oncological care, especially with the continued improvement in adjuvant therapeutics, including targeted and immunotherapies. The use of intraoperative fluorescence for lymphatic mapping during esophagectomy has been well documented in several studies (36,37). Hachey et al. evaluated the use of endoscopic submucosal injections of ICG in a cohort of 10 patients, four of which were performed robotically, with NIR signals identified in six of the tumor sites (38). Hosogi et al. evaluated 15 patients who underwent robotic-assisted esophagectomy with the use of ICG, identifying 80% of patients with ICG-positive lymph node basins along the right recurrent laryngeal nerve and 73% of patients along the left recurrent laryngeal nerve. All ICG-positive lymph node basins were ultimately found within a common area encompassing the esophagus, trachea, recurrent laryngeal nerves and surrounding lymph nodes (39).
The application of these techniques in robotic esophagectomy remains to be fully established, however ICG under NIR imaging has been found to have better visualization of lymph nodes, particularly within thick fatty tissues, with the use of the robotic system (39,40). This improved visualization, particularly with the use of the da Vinci Firefly camera, allows for en bloc resection of lymph nodes and lymphatics without injury to these structures, thus preventing tumor cell spillage, and safe dissection of lymph node-bearing soft tissue adjacent to critical structures (41).
Identification of anatomy
Chylothorax after esophagectomy occurs in 2–12% of patients and affects not only enteral intake, but also hospital length of stay and overall survival (42). Fluorescence guided dissection, with percutaneous inguinal injection of ICG alone, has been utilized to identify the thoracic duct to avoid injury intraoperatively and in the setting of post-procedural chylothorax (43-45). Jardinet et al. successfully applied these techniques in robotic-assisted esophagectomy by inserting an intra-lymphatic needle in an inguinal node and injecting ICG after mobilization of the inferior pulmonary ligament. This not only identified the thoracic duct, but did so with less time for set-up, more rapid fluorescence, and longer signal duration, as compared to prior studies that did not utilize the robotic platform (46). Similarly, Barbato et al. and Varshney et al. both utilized ICG in 18 and 21 patients, respectively, with identification of the thoracic duct in all patients in the robotic setting (47,48).
Advantages in robotic-assisted esophagectomy
The robotic platform for MIE has the potential to reduce the morbidity and mortality of esophagectomy in several ways, including use of additional built-in diagnostic tools such as intraoperative fluorescence imaging. When considering the use of adjunct tools, the technical advantages of a robotic-assisted platform cannot be understated as improved optics, scaling, and built-in fluorescence camera for rapid angiography allows for a more precise and tailored dissection, and provides quantifiable metrics to define optimal perfusion of the conduit and anastomosis (22,25,38,46) (Figures 1,2). Firefly represents an integrated fluorescent camera which is now standard equipment in all da Vinci Surgical Systems, and includes cameras modified for NIR light, filters and sensors for ICG, and light emitting diode (LED)-based illuminators with a NIR laser to excite ICG (49). In fact, the Firefly technology allows for evaluation of a larger spectrum of wavelengths with various imaging modes (example: white light, unprocessed fluorescence and processed fluorescence) (50).
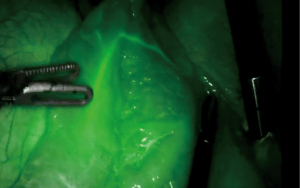
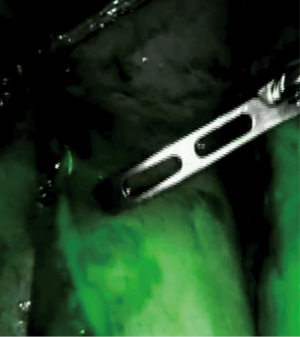
Several limitations to robotic-assisted esophagectomy should be noted, however, including the steep surgeon learning curve, risk of conversion to open, and requisite hospital cost and maintenance, but the benefits in outcomes and potential to advance the field by quantifying optimal surgical technique are certainly worth the investment for surgeons and institutions committed to advancing the field (25,51).
Limitations and future directions
Further scholarship in the robotic platform is required for each of the uses of ICG presented above. In addition, the studies presented highlight the role for intraoperative fluorescence imaging as a tool to facilitate dissection and decrease complications after esophagectomy, but are predominantly case-control studies, with no standardization between groups. More rigorous scientific inquiry is warranted, including published and validated protocols for intraoperative ICG usage, as well as randomized control trials, when possible. In conjunction, new techniques are being developed to improve imaging through more advanced camera technology and more specific tracers. This includes protein-bound ICG formulations to prolong the half-life of intraoperative fluorescence dyes, NIR spectroscopy, thermal imaging, and incorporation of mathematical modeling and software designed specifically for the robotic platform (52-58). In addition, the use of NIR dyes as both cancer imaging and therapeutic modalities is rapidly expanding (59).
Conclusions
Over the last decade, intraoperative fluorescence imaging has demonstrated great potential to facilitate dissection and improve postoperative outcomes following esophagectomy. This technique provides the opportunity to assess perfusion and identify anatomy for more precise and patient-specific dissection and reconstruction. Robotic-assisted esophagectomy is optimally suited to utilize fluorescence imaging to enhance surgical technique, and greater adoption of the robotic approach will enable development of standard metrics to benchmark surgical outcomes of esophagectomy in order to decrease risk and improve patient outcomes of this procedure across surgeons and hospital centers, both nationally and internationally.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-456/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-456/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-456/coif). LYS serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2022 to March 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abdelfatah E, Jordan S, Dexter EU, Nwogu C. Robotic thoracic and esophageal surgery: a critical review of comparative outcomes. Ann Laparosc Endosc Surg 2021;6:10. [Crossref]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Darwish MB, Nagatomo K, Jackson T, et al. Minimally Invasive Esophagectomy for Achieving R0. JSLS 2020;24:e2020. [Crossref] [PubMed]
- Ali AM, Bachman KC, Worrell SG, et al. Robotic minimally invasive esophagectomy provides superior surgical resection. Surg Endosc 2021;35:6329-34. [Crossref] [PubMed]
- Tagkalos E, Goense L, Hoppe-Lotichius M, et al. Robot-assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: a propensity-matched analysis. Dis Esophagus 2020;33:doz060. [Crossref] [PubMed]
- Angeramo CA, Bras Harriott C, Casas MA, et al. Minimally invasive Ivor Lewis esophagectomy: Robot-assisted versus laparoscopic-thoracoscopic technique. Systematic review and meta-analysis. Surgery 2021;170:1692-701. [Crossref] [PubMed]
- Schieman C, Wigle DA, Deschamps C, et al. Patterns of operative mortality following esophagectomy. Dis Esophagus 2012;25:645-51. [Crossref] [PubMed]
- Turner SR, Molena DR. The Role of Intraoperative Fluorescence Imaging During Esophagectomy. Thorac Surg Clin 2018;28:567-71. [Crossref] [PubMed]
- Kitagawa H, Yokota K, Marui A, et al. Near-infrared fluorescence imaging with indocyanine green to assess the blood supply of the reconstructed gastric conduit to reduce anastomotic leakage after esophagectomy: a literature review. Surg Today 2022; Epub ahead of print. [Crossref] [PubMed]
- van Boxel G, van Hillegersberg R, Ruurda J. Outcomes and complications after robot-assisted minimally invasive esophagectomy. J Vis Surg 2019;5:21. [Crossref]
- Pham TH, Perry KA, Enestvedt CK, et al. Decreased conduit perfusion measured by spectroscopy is associated with anastomotic complications. Ann Thorac Surg 2011;91:380-5. [Crossref] [PubMed]
- Karliczek A, Harlaar NJ, Zeebregts CJ, et al. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 2009;24:569-76. [Crossref] [PubMed]
- Schlottmann F, Patti MG. Evaluation of Gastric Conduit Perfusion During Esophagectomy with Indocyanine Green Fluorescence Imaging. J Laparoendosc Adv Surg Tech A 2017;27:1305-8. [Crossref] [PubMed]
- Shimada Y, Okumura T, Nagata T, et al. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus 2011;8:259-66. [Crossref] [PubMed]
- Nakano T, Sakurai T, Maruyama S, et al. Indocyanine green fluorescence and three-dimensional imaging of right gastroepiploic artery in gastric tube cancer. World J Gastroenterol 2015;21:369-72. [Crossref] [PubMed]
- Kubota K, Yoshida M, Kuroda J, et al. Application of the HyperEye Medical System for esophageal cancer surgery: a preliminary report. Surg Today 2013;43:215-20. [Crossref] [PubMed]
- Kumagai Y, Ishiguro T, Haga N, et al. Hemodynamics of the reconstructed gastric tube during esophagectomy: assessment of outcomes with indocyanine green fluorescence. World J Surg 2014;38:138-43. [Crossref] [PubMed]
- Murawa D, Hünerbein M, Spychała A, et al. Indocyanine green angiography for evaluation of gastric conduit perfusion during esophagectomy--first experience. Acta Chir Belg 2012;112:275-80. [Crossref] [PubMed]
- Pacheco PE, Hill SM, Henriques SM, et al. The novel use of intraoperative laser-induced fluorescence of indocyanine green tissue angiography for evaluation of the gastric conduit in esophageal reconstructive surgery. Am J Surg 2013;205:349-52; discussion 352-3. [Crossref] [PubMed]
- Rino Y, Yukawa N, Sato T, et al. Visualization of blood supply route to the reconstructed stomach by indocyanine green fluorescence imaging during esophagectomy. BMC Med Imaging 2014;14:18. [Crossref] [PubMed]
- Sarkaria IS, Bains MS, Finley DJ, et al. Intraoperative near-infrared fluorescence imaging as an adjunct to robotic-assisted minimally invasive esophagectomy. Innovations (Phila) 2014;9:391-3. [Crossref] [PubMed]
- Egberts JH, Stein H, Aselmann H, et al. Fully robotic da Vinci Ivor-Lewis esophagectomy in four-arm technique-problems and solutions. Dis Esophagus 2017;30:1-9. [Crossref] [PubMed]
- Pötscher A, Bittermann C, Längle F. Robot-assisted esophageal surgery using the da Vinci® Xi system: operative technique and initial experiences. J Robot Surg 2019;13:469-74. [Crossref] [PubMed]
- DeLong JC, Kelly KJ, Jacobsen GR, et al. The benefits and limitations of robotic assisted transhiatal esophagectomy for esophageal cancer. J Vis Surg 2016;2:156. [Crossref] [PubMed]
- Hodari A, Park KU, Lace B, et al. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg 2015;100:947-52. [Crossref] [PubMed]
- Slooter MD, de Bruin DM, Eshuis WJ, et al. Quantitative fluorescence-guided perfusion assessment of the gastric conduit to predict anastomotic complications after esophagectomy. Dis Esophagus 2021;34:doaa100. [Crossref] [PubMed]
- Talavera-Urquijo E, Parise P, Palucci M, et al. Perfusion speed of indocyanine green in the stomach before tubulization is an objective and useful parameter to evaluate gastric microcirculation during Ivor-Lewis esophagectomy. Surg Endosc 2020;34:5649-59. [Crossref] [PubMed]
- Kitagawa H, Namikawa T, Iwabu J, et al. Correlation between indocyanine green visualization time in the gastric tube and postoperative endoscopic assessment of the anastomosis after esophageal surgery. Surg Today 2020;50:1375-82. [Crossref] [PubMed]
- Koyanagi K, Ozawa S, Oguma J, et al. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: New predictive evaluation of anastomotic leakage after esophagectomy. Medicine (Baltimore) 2016;95:e4386. [Crossref] [PubMed]
- Kumagai Y, Hatano S, Sobajima J, et al. Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: efficacy of the 90-second rule. Dis Esophagus 2018; [Crossref] [PubMed]
- Yamaguchi K, Kumagai Y, Saito K, et al. The evaluation of the gastric tube blood flow by indocyanine green fluorescence angiography during esophagectomy: a multicenter prospective study. Gen Thorac Cardiovasc Surg 2021;69:1118-24. [Crossref] [PubMed]
- Noma K, Shirakawa Y, Kanaya N, et al. Visualized Evaluation of Blood Flow to the Gastric Conduit and Complications in Esophageal Reconstruction. J Am Coll Surg 2018;226:241-51. [Crossref] [PubMed]
- Visser E, Markar SR, Ruurda JP, et al. Prognostic Value of Lymph Node Yield on Overall Survival in Esophageal Cancer Patients: A Systematic Review and Meta-analysis. Ann Surg 2019;269:261-8. [Crossref] [PubMed]
- Phillips AW, Griffin SM. Chapter 40 - Extent of Lymphadenectomy for Esophageal Cancer. In: Yeo CJ. editor. Shackelford's Surgery of the Alimentary Tract, 2 Volume Set (Eighth Edition). Philadelphia: Elsevier, 2019:431-7.
- Schlottmann F, Barbetta A, Mungo B, et al. Identification of the Lymphatic Drainage Pattern of Esophageal Cancer with Near-Infrared Fluorescent Imaging. J Laparoendosc Adv Surg Tech A 2017;27:268-71. [Crossref] [PubMed]
- Jimenez-Lillo J, Villegas-Tovar E, Momblan-Garcia D, et al. Performance of Indocyanine-Green Imaging for Sentinel Lymph Node Mapping and Lymph Node Metastasis in Esophageal Cancer: Systematic Review and Meta-Analysis. Ann Surg Oncol 2021;28:4869-77. [Crossref] [PubMed]
- Hachey KJ, Gilmore DM, Armstrong KW, et al. Safety and feasibility of near-infrared image-guided lymphatic mapping of regional lymph nodes in esophageal cancer. J Thorac Cardiovasc Surg 2016;152:546-54. [Crossref] [PubMed]
- Hosogi H, Yagi D, Sakaguchi M, et al. Upper mediastinal lymph node dissection based on mesenteric excision in esophageal cancer surgery: confirmation by near-infrared image-guided lymphatic mapping and the impact on locoregional control. Esophagus 2021;18:219-27. [Crossref] [PubMed]
- Vahrmeijer AL, Hutteman M, van der Vorst JR, et al. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol 2013;10:507-18. [Crossref] [PubMed]
- Kwon IG, Son T, Kim HI, et al. Fluorescent Lymphography-Guided Lymphadenectomy During Robotic Radical Gastrectomy for Gastric Cancer. JAMA Surg 2019;154:150-8. [Crossref] [PubMed]
- Brinkmann S, Schroeder W, Junggeburth K, et al. Incidence and management of chylothorax after Ivor Lewis esophagectomy for cancer of the esophagus. J Thorac Cardiovasc Surg 2016;151:1398-404. [Crossref] [PubMed]
- Kaburagi T, Takeuchi H, Oyama T, et al. Intraoperative fluorescence lymphography using indocyanine green in a patient with chylothorax after esophagectomy: report of a case. Surg Today 2013;43:206-10. [Crossref] [PubMed]
- Kamiya K, Unno N, Konno H. Intraoperative indocyanine green fluorescence lymphography, a novel imaging technique to detect a chyle fistula after an esophagectomy: report of a case. Surg Today 2009;39:421-4. [Crossref] [PubMed]
- Vecchiato M, Martino A, Sponza M, et al. Thoracic duct identification with indocyanine green fluorescence during minimally invasive esophagectomy with patient in prone position. Dis Esophagus 2020;33:doaa030. [Crossref] [PubMed]
- Jardinet T, Niekel MC, Ruppert M, et al. Fluorescence-Guided Thoracic Duct Dissection in Robotic en Bloc Esophagectomy. Ann Thorac Surg 2022;113:e465-7. [Crossref] [PubMed]
- Barbato G, Cammelli F, Braccini G, et al. Fluorescent lymphography for thoracic duct identification: Initial experience of a simplified and feasible ICG administration. Int J Med Robot 2022;18:e2380. [Crossref] [PubMed]
- Varshney VK, Nayar R, Soni SC, et al. Intra-Nodal Indocyanine Green Injection to Delineate Thoracic Duct During Minimally Invasive Esophagectomy. J Gastrointest Surg 2022;26:1559-65. [Crossref] [PubMed]
- Lee YJ, van den Berg NS, Orosco RK, et al. A narrative review of fluorescence imaging in robotic-assisted surgery. Laparosc Surg 2021;5:31. [Crossref] [PubMed]
- Meershoek P. Multi-wavelength fluorescence imaging with a da Vinci Firefly-a technical look behind the scenes. J Robot Surg 2021;15:751-60. [Crossref] [PubMed]
- Hue JJ, Bachman KC, Worrell SG, et al. Outcomes of robotic esophagectomies for esophageal cancer by hospital volume: an analysis of the national cancer database. Surg Endosc 2021;35:3802-10. [Crossref] [PubMed]
- Yamaguchi K, Nakajima Y, Matsui T, et al. The evaluation of the hemodynamics of a gastric tube in esophagectomy using a new noninvasive blood flow evaluation device utilizing near-infrared spectroscopy. Gen Thorac Cardiovasc Surg 2020;68:841-7. [Crossref] [PubMed]
- Nishikawa K, Fujita T, Yuda M, et al. Quantitative Assessment of Blood Flow in the Gastric Conduit With Thermal Imaging for Esophageal Reconstruction. Ann Surg 2020;271:1087-94. [Crossref] [PubMed]
- Prasetya H, Jansen SM, Marquering HA, et al. Estimation of microvascular perfusion after esophagectomy: a quantitative model of dynamic fluorescence imaging. Med Biol Eng Comput 2019;57:1889-900. [Crossref] [PubMed]
- Ishige F, Nabeya Y, Hoshino I, et al. Quantitative Assessment of the Blood Perfusion of the Gastric Conduit by Indocyanine Green Imaging. J Surg Res 2019;234:303-10. [Crossref] [PubMed]
- Yukaya T, Saeki H, Kasagi Y, et al. Indocyanine Green Fluorescence Angiography for Quantitative Evaluation of Gastric Tube Perfusion in Patients Undergoing Esophagectomy. J Am Coll Surg 2015;221:e37-42. [Crossref] [PubMed]
- Overwater A, Weusten BLAM, Ruurda JP, et al. Feasibility of sentinel node navigated surgery in high-risk T1b esophageal adenocarcinoma patients using a hybrid tracer of technetium-99 m and indocyanine green. Surg Endosc 2022;36:2671-9. [Crossref] [PubMed]
- Kim HK, Quan YH, Oh Y, et al. Macrophage-Targeted Indocyanine Green-Neomannosyl Human Serum Albumin for Intraoperative Sentinel Lymph Node Mapping in Porcine Esophagus. Ann Thorac Surg 2016;102:1149-55. [Crossref] [PubMed]
- Zhu S, Tian R, Antaris AL, et al. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv Mater 2019;31:e1900321. [Crossref] [PubMed]


