
Mediastinal lymph node evaluation, especially at station 4L, in left upper lobe lung cancer
Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide (1). Despite the availability of treatment options, pulmonary resection with lymph node (LN) harvesting remains the best therapeutic tool for the treatment of primary non-small cell lung cancer (NSCLC). This procedure has been universally accepted as the standard surgery for lung cancer for >50 years (2-4). However, to date, reports show inconsistent effects on prognosis, and the scientific basis for LN dissection remains unclear (5-8).
The American College of Surgery Oncology Group Z0030 study showed that there was no difference in median overall survival (OS), 5-year disease-free survival (DFS), and recurrence patterns between mediastinal LN dissection and LN sampling in early-stage NSCLC (9). However, an important criterion for inclusion in this comparative study was that the LN stations, which are the most common sites of metastatic N2 disease, were confirmed to be pathologically benign in frozen sections before LN dissection. Cerfolio et al. (10) stated that the methodology used in the Z0030 study was an uncommon practice for most thoracic surgeons and did not show a survival benefit over mediastinal LN dissection compared to LN sampling. Moreover, they reported that complete mediastinal LN dissection detected more patients with pathological N2 disease among those who were clinically node-negative. They also reported that mediastinal LN dissection not only identified up to 4% of N2 oversights but also caused no complications and had a time difference of only 15 min. The purpose of systematic LN dissection in radical surgery is to remove metastatic LNs, although accurate staging with sufficient LN dissection has provided the opportunity for early intervention in patients with latent micrometastases (11).
Left upper mediastinal LN dissection, especially at station 4L, is more difficult to perform than right lung LN dissection due to anatomical limitations caused by the aortic arch, left recurrent laryngeal nerve, and thoracic duct (12-14). Therefore, some thoracic surgeons do not dissect station 4L. However, recent studies showed that station 4L LN metastasis was an independent risk factor for poor prognosis and that station 4L LN dissection led to a more favorable outcome (15-19).
Recurrent laryngeal nerve palsy is a major complication that can become life-threatening or affect patients’ activities of daily living due to recurrent aspiration pneumonia or difficulty in expectoration (20). Even if there are differences in the degree of tumor progression or anatomical variations in the recurrent laryngeal nerve, it is important to consistently perform surgical procedures above a certain level, considering safety and the need for an accurate diagnosis.
Herein, we evaluated intraoperative videos to elucidate the most important key point during dissection for safely obtaining LN information, especially station 4L LN, in left upper lobe lung cancer. Additionally, we considered the need for mediastinal LN dissection. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-537/rc) (21).
Methods
Ethical statement
The study was conducted following the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shiga University of Medical Science on November 11, 2019 (No. R2019-261). The requirement for individual consent was waived due to the retrospective nature of the study.
Patient selection
Between January 2009 and December 2019, we retrospectively reviewed 151 consecutive patients with left upper lobe primary lung cancer. Patients who underwent radical lung resection (left upper lobectomy without pneumonectomy) with LN dissection were included. Patients with other concurrent active malignant tumors and those who received neoadjuvant therapy were excluded. The reason for excluding patients who received neoadjuvant therapy was to ensure the accuracy of postoperative pathological assessment of the presence of LN metastasis. Finally, 139 patients were enrolled in this study, including 69 who underwent simultaneous mediastinoscopy and 91 who had video recordings of sufficient quality for evaluation.
Clinical information
Data on the following clinical variables were collected from electronic medical records: basic background characteristics; Brickman Index; global initiative for chronic obstructive lung disease grade; respiratory history and comorbidities; clinical stage and clinical N descriptors; abnormal nodal uptake on fluorodeoxyglucose-positron emission tomography (FDG-PET); mediastinoscopy; surgical procedure and intraoperative information; tumor histology; pathological nodal status at stations 4L, 5, and 6; pathological N descriptors; adjuvant therapy; postoperative respiratory and circulatory complications; recurrent laryngeal nerve palsy; prognosis; and recurrence. All the patients included in this study were reassessed according to the eighth edition of cancer staging by the American Joint Committee on Cancer (22,23). Preoperatively, LN metastasis was suspected if the short axis was >1 cm in the contrast-enhanced computed tomography (CT) scan and the focally increased FDG uptake was higher than the normal background and not symmetrical on FDG-PET. Resected lung cancer samples and LNs were evaluated histopathologically by at least two experienced pathologists. LN stations were classified according to the LN map proposed by the International Association for the Study of Lung Cancer (24).
Mediastinoscopy
At our institution, mediastinoscopy was performed in almost all patients before 2014. Since 2015, mediastinoscopy has been performed in principle in patients, in whom a definitive diagnosis of primary lung cancer was obtained preoperatively, including those without any imaging findings. In particular, endobronchial ultrasound-transbronchial needle aspiration was performed preoperatively in patients with clinically suspected N2/N3 disease. Preoperative treatment was introduced in node-positive cases; even in node-negative cases, mediastinoscopy was performed at the time of surgery. Mediastinoscopy was performed before lobectomy on the day of surgery. Specimens routinely biopsied from stations 2R, 2L, 4R, 4L, and 7 were rapidly confirmed intraoperatively for the presence of metastases. In the presence of metastases, lobectomy was discontinued, and neoadjuvant therapy was initiated.
Video imaging assessment
The following points were considered as indicators of sufficient quality for the evaluation of total intrathoracic surgical procedures, particularly the dissection of mediastinal LNs: whether the LN status was sufficiently observed at the station that had not been pathologically examined (the criteria for “sufficient observation” in the video image were defined as confirmation of the presence of the left-side tracheobronchial wall for station 4L LNs, ligamentum Botalli for station 5 LNs, and aortic arch wall for station 6 LNs). Considering the basic procedure of mediastinal LN dissection, as shown in Figure 1, the following surgical procedures were evaluated in the video for preventing recurrent laryngeal nerve palsy: confirmation and dissection of the ligamentum Botalli, the vagal nerve traveling along the central and peripheral sides of the recurrent laryngeal nerve bifurcation, and the shallow and deep branches of the recurrent laryngeal nerve before the start of LN dissection, as well as the use of a conventional electric knife (EK) and vessel sealing system (VSS) near the recurrent laryngeal nerve (distance approximately <3 mm) and pulmonary artery (PA) taping during LN dissection. Intraoperative video images were evaluated independently according to the checklist prepared by two thoracic surgeons in a blinded manner. If the evaluations of the two thoracic surgeons differed, the video image was confirmed and discussed again with a third thoracic surgeon. Cases wherein video evaluation could be performed were mainly those after 2015, and almost all cases were targeted.
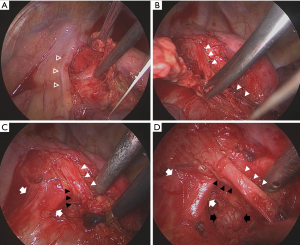
Recurrent laryngeal nerve palsy
After two postoperative days, patients with hoarseness or dysphagia, including those with mild symptoms were suspected of having recurrent laryngeal nerve palsy, and the Otolaryngology Department at our institution was consulted. Recurrent laryngeal nerve palsy was diagnosed on laryngoscopy based on various measurements such as the maximum phonation time. Thereafter, the incidence of complications and changes in symptoms were monitored regularly, and management, such as conservative or surgical treatment, was decided.
Follow-up
Blood tumor marker [carcinoembryonic antigen (CEA), Cyfra21-1, pro-gastrin releasing peptide (proGRP), squamous cell carcinoma (SCC), neuron specific enolase (NSE) and Sialyl Lewis X-i antigen (SLX)] testing and chest/abdominal CT were routinely performed every 6 months, and brain magnetic resonance imaging and bone scintigraphy were performed annually for 5 years postoperatively. After 5 years, patients were still recommended to undergo annual examinations. DFS was calculated as the time interval from the date of surgery until the first event (relapse, metastasis, or death from lung cancer) or last follow-up. OS was defined as the time interval between the date of surgery and death from lung cancer or last follow-up. The follow-up period ended in December 2020 or at the date of death; both DFS and OS were measured in months. The median follow-up period was 44 (range, 0–137) months.
Statistical analyses
To assess the survival effects of the clinical factors of metastatic LN stations, OS and DFS curves were generated using the Kaplan-Meier method and compared using the log-rank test. The univariate prognostic significance of variables was determined using the Cox proportional hazards model. Variables that were significantly associated with survival in the univariate analysis were included in the multivariate analysis using Cox multiple regression analysis. Fisher’s exact test was used to analyze the association between risk factors or surgical procedures and recurrent laryngeal nerve palsy. All statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) (25). Statistical significance was defined as P<0.05.
Results
Clinical characteristics
Table 1 shows the clinicopathological factors of 139 patients who underwent left upper lobectomy. The average age was 69.39 years (range, 47–90 years), and 103 patients were smokers. FDG-PET was performed preoperatively in 123 patients, of whom 23 showed FDG accumulation in the LNs. Preoperative diagnosis was obtained for 61 patients, and mediastinoscopy was performed in 69. There were 93 (66.91%) and 33 (23.74%) cases of clinical T1 and T2 disease, respectively, accounting for more than 90% of the total. One case of clinical N3 disease was identified; however, LN metastasis was negative by LN sampling using mediastinoscopy and by LN dissection from the thoracic cavity side. Five patients with clinical N2 status were diagnosed with pathological N2 disease; among them, two patients, who did not undergo mediastinal examination due to the lack of a definitive preoperative diagnosis, were positive for station 4L LN metastasis. FDG accumulation was observed in one of the two patients. Pathological N0, N1, and N2 cases comprised 108, 13, and 18 patients, respectively, and the concordance rates of clinical N factor were 86.09%, 36.36%, and 50%, respectively.
Table 1
| Variables | Values |
|---|---|
| Age (years), mean | 69.39 (range, 47–90) |
| Smoking, n (%) | |
| Non-smoker | 36 (25.90) |
| Smoker | 103 (74.10) |
| Respiratory comorbidities, n (%) | |
| COPD | 49 (35.25) |
| BA | 6 (4.32) |
| IPF | 4 (2.88) |
| Others | 2 (1.44) |
| COPD stage (n=138), n (%) | |
| 0 | 89 (64.49) |
| 1 | 26 (18.84) |
| 2 | 22 (15.94) |
| 3 | 1 (0.72) |
| Respiratory history, n (%) | |
| Tuberculosis | 7 (5.04) |
| Pneumonia | 4 (2.88) |
| Others | 8 (5.76) |
| Clinical T factor, n (%) | |
| 1mi | 5 (3.60) |
| 1a | 19 (13.67) |
| 1b | 39 (28.06) |
| 1c | 30 (21.58) |
| 2a | 25 (17.99) |
| 2b | 8 (5.76) |
| 3 | 10 (7.19) |
| 4 | 3 (2.16) |
| Clinical N factor, n (%) | |
| 0 | 115 (82.73) |
| 1 | 11 (7.91) |
| 2 | 12 (8.63) |
| 3 | 1 (0.72) |
| Clinical stage, n (%) | |
| IA1 | 20 (14.39) |
| IA2 | 36 (25.90) |
| IA3 | 27 (19.42) |
| IB | 18 (12.95) |
| IIA | 6 (4.32) |
| IIB | 13 (9.35) |
| IIIA | 15 (10.79) |
| IIIB | 4 (2.88) |
| FDG-PET (n=123), n (%) | |
| Yes | 23 (18.70) |
| No | 100 (81.30) |
| Preoperative diagnosis, n (%) | |
| Yes | 61 (43.88) |
| No | 78 (56.12) |
| Mediastinoscopy, n (%) | |
| Yes | 69 (49.64) |
| No | 70 (50.36) |
| Bleeding (mL), mean | 209.7 |
| Pathological N factor, n (%) | |
| 0 | 108 (77.70) |
| 1 | 13 (9.35) |
| 2 | 18 (12.95) |
| Histology, n (%) | |
| Ad | 90 (64.75) |
| Sq | 32 (23.02) |
| Others | 17 (12.23) |
| Recurrence, n (%) | |
| Yes | 32 (23.02) |
| No | 107 (76.98) |
| Prognosis, n (%) | |
| Dead | 26 (18.71) |
| Alive | 113 (81.29) |
COPD, chronic obstructive pulmonary disease; BA, bronchial asthma; IPF, idiopathic pulmonary fibrosis; FDG-PET, fluorodeoxyglucose-positron emission tomography; Ad, adenocarcinoma; Sq, squamous cell carcinoma.
Analysis of survival factors in patients with left upper lobe lung cancer
Table 1 shows the clinicopathological characteristics of 139 patients with left upper lobe lung cancer. Pathological N stage ≥2 was a significant independent prognostic factor for DFS (Figure 2). Univariate and multivariate analysis of clinicopathological characteristics affecting OS and DFS revealed that pathological N stage ≥2 was one of the most important independent prognostic factors for DFS (Table 2).
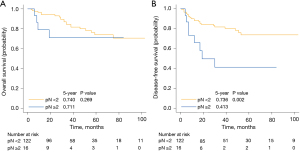
Table 2
| Variables | N | Univariate analysis | Multivariate analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | DFS | OS | DFS | |||||||||||||
| P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | |||||
| Age | ||||||||||||||||
| ≥70 years | 69 | 0.048 | 2.228 | 1.005–4.941 | 0.630 | 1.186 | 0.592–2.378 | 0.048 | 2.384 | 1.008–5.640 | ||||||
| <70 years | 70 | |||||||||||||||
| Sex | ||||||||||||||||
| Male | 96 | 0.114 | 2.361 | 0.813–6.856 | 0.155 | 1.905 | 0.784–4.631 | |||||||||
| Female | 43 | |||||||||||||||
| Brinkman index | ||||||||||||||||
| ≥400 | 94 | 0.015 | 5.973 | 1.41–25.3 | 0.324 | 1.496 | 0.672–3.333 | 0.052 | 4.441 | 0.989–19.950 | ||||||
| <400 | 45 | |||||||||||||||
| Respiratory comorbidities | ||||||||||||||||
| Yes | 56 | 0.048 | 2.192 | 1.006–4.774 | 0.011 | 2.48 | 1.224–5.026 | 0.602 | 1.246 | 0.545–2.848 | 0.058 | 2.257 | 0.972–5.244 | |||
| No | 83 | |||||||||||||||
| COPD stage | ||||||||||||||||
| ≥2 | 23 | 0.073 | 1.469 | 0.965–2.237 | 0.060 | 1.458 | 0.983–2.163 | |||||||||
| <2 | 115 | |||||||||||||||
| Respiratory history | ||||||||||||||||
| Yes | 19 | 0.190 | 1.842 | 0.739–4.596 | 0.163 | 1.816 | 0.785–4.204 | |||||||||
| No | 120 | |||||||||||||||
| Clinical T factor | ||||||||||||||||
| ≥2 | 46 | 0.014 | 2.639 | 1.22–5.71 | 0.001 | 3.188 | 1.584–6.415 | 0.147 | 1.915 | 0.796–4.608 | 0.112 | 1.917 | 0.860–4.271 | |||
| <2 | 93 | |||||||||||||||
| Clinical N factor | ||||||||||||||||
| ≥2 | 13 | 0.131 | 2.277 | 0.783–6.62 | 0.0792 | 2.353 | 0.905–6.116 | |||||||||
| <2 | 126 | |||||||||||||||
| Clinical stage | ||||||||||||||||
| ≥III | 19 | 0.034 | 2.553 | 1.072–6.084 | 0.005 | 3.019 | 1.395–6.531 | 0.839 | 1.110 | 0.406–3.029 | 0.841 | 1.096 | 0.450–2.670 | |||
| <III | 120 | |||||||||||||||
| FDG-PET accumulation | ||||||||||||||||
| Yes | 23 | 0.215 | 1.77 | 0.717–4.369 | 0.136 | 1.871 | 0.822–4.261 | |||||||||
| No | 100 | |||||||||||||||
| Mediastinoscopy | ||||||||||||||||
| Yes | 69 | 0.241 | 1.63 | 0.720–3.69 | 0.907 | 0.959 | 0.477–1.931 | |||||||||
| No | 70 | |||||||||||||||
| Approach | ||||||||||||||||
| Open thoracotomy | 48 | 0.006 | 3.025 | 1.369–6.687 | <0.001 | 3.765 | 1.836–7.723 | 0.281 | 1.707 | 0.646–4.510 | 0.519 | 1.374 | 0.523–3.609 | |||
| VATS | 91 | |||||||||||||||
| Bleeding | ||||||||||||||||
| ≥200 mL | 35 | 0.002 | 3.308 | 1.526–7.169 | 0.018 | 2.347 | 1.155–4.769 | 0.278 | 1.696 | 0.653–4.406 | 0.167 | 1.854 | 0.772–4.450 | |||
| <200 mL | 104 | |||||||||||||||
| Operation duration | ||||||||||||||||
| ≥300 min | 82 | 0.155 | 1.877 | 0.788–4.467 | 0.292 | 0.689 | 0.345–1.378 | |||||||||
| <300 min | 57 | |||||||||||||||
| Pathological N factor | ||||||||||||||||
| ≥2 | 16 | 0.278 | 1.806 | 0.621–5.253 | 0.003 | 3.379 | 1.511–7.552 | 0.010 | 3.883 | 1.379–10.930 | ||||||
| <2 | 123 | |||||||||||||||
| Histology | ||||||||||||||||
| Ad | 87 | 0.054 | 1.28 | 0.995–1.645 | 0.015 | 1.319 | 1.054–1.651 | 0.890 | 0.937 | 0.367–2.395 | 0.466 | 1.107 | 0.842–1.455 | |||
| Non-Ad | 52 | |||||||||||||||
| Adjuvant chemotherapy | ||||||||||||||||
| Yes | 45 | 0.954 | 1.024 | 0.456–2.30 | 0.015 | 2.363 | 1.181–4.73 | 0.639 | 1.231 | 0.517–2.933 | ||||||
| No | 93 | |||||||||||||||
| Adjuvant radiotherapy | ||||||||||||||||
| Yes | 3 | 0.998 | <0.001 | 0/inf | 0.996 | <0.001 | 0/inf | |||||||||
| No | 135 | |||||||||||||||
| Respiratory complications | ||||||||||||||||
| Yes | 31 | 0.402 | 1.450 | 0.609–3.456 | 0.814 | 1.106 | 0.478–2.56 | |||||||||
| No | 108 | |||||||||||||||
| Circulatory complications | ||||||||||||||||
| Yes | 20 | 0.064 | 2.394 | 0.950–6.03 | 0.004 | 3.105 | 1.43–6.743 | 0.019 | 2.721 | 1.177–6.290 | ||||||
| No | 119 | |||||||||||||||
| RLNP | ||||||||||||||||
| Yes | 20 | 0.312 | 1.604 | 0.642–4.007 | 0.219 | 1.695 | 0.731–3.927 | |||||||||
| No | 119 | |||||||||||||||
OS, overall survival; HR, hazard ratio; CI, confidence interval; DFS, disease-free survival; COPD, chronic obstructive pulmonary disease; FDG-PET, fluorodeoxyglucose-positron emission tomography; VATS, video-assisted thoracic surgery; Ad, adenocarcinoma; inf, infinity; RLNP, recurrent laryngeal nerve palsy.
LN assessment and its effect on metastatic prognosis
When LN dissection was performed at the same time as left upper lobectomy, pathological LN metastasis was observed at stations 4L, 5, and 6 in nine (6.47%), and 11 (7.91%), and nine (6.47%) patients, respectively (Table 3). Among the nine node-positive patients at station 4L, six (66.7%) did not undergo mediastinoscopy, while the remaining three (33.3%) were node-negative on mediastinoscopy. Regarding the effect of nodal metastasis at each station on prognosis, node-positivity at stations 5 and 6 was a significant prognostic factor for DFS (P<0.001 and 0.005, respectively); however, node-positivity at station 4L was not a prognostic factor (P=0.35). Therefore, we evaluated the intraoperative videos of 91 patients to determine whether LN status was observed at each station (Table 4). Compared with other stations, station 4L LNs were not pathologically examined in 33 patients. Of these, only 12 had an observed LN status and were unresected; 21 were not evaluated at all. The effect on prognosis was reexamined by combining the patients in whom LN status was observed with those in whom the pathological examination was performed. The effect of node-positivity at stations 5 and 6 on DFS was similar to those previously reported (15), whereas the P value and 5-year DFS rate at the station 4L decreased from 0.35 to 0.10 and from 57.1% to 48.6%, respectively. Pathological N1 with nodal metastasis at stations 10 and/or 11 was highly correlated with nodal metastasis at stations 4, 5, and 6 (P=0.002).
Table 3
| LN station | Mediastinoscopy (+), n=69 (%) | Mediastinoscopy (−), n=70 (%) | Total, n=139 (%) | OS | DFS | |
|---|---|---|---|---|---|---|
| P value | P value | |||||
| Station 4 metastasis | 3 (4.35) | 6 (8.57) | 9 (6.47) | 0.59 | 0.35 | |
| Station 5 metastasis | 3 (4.35) | 8 (11.43) | 11 (7.91) | 0.41 | <0.001 | |
| Station 6 metastasis | 4 (5.80) | 5 (7.14) | 9 (6.47) | 0.32 | 0.005 |
LN, lymph node; OS, overall survival; DFS, disease-free survival.
Table 4
| LN station | Lymphadenectomy (−) | Metastasis (+)/cases | OS | DFS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total, (n=91) (%) | Confirmed | Unconfirmed | P value | Survival | P value | Survival | |||
| Station 4 | 33 (32.3) | 12 | 21 | 8/70 | 0.20 | 0.750 | 0.10 | 0.486 | |
| Station 5 | 12 (13.2) | 1 | 11 | 8/80 | 0.92 | 0.875 | <0.001 | 0.250 | |
| Station 6 | 17 (18.7) | 2 | 15 | 6/76 | 0.35 | 1.000 | 0.01 | 0.400 | |
LN, lymph node; OS, overall survival; DFS, disease-free survival.
Recurrent laryngeal nerve palsy
Twenty patients (14.39%) had recurrent laryngeal nerve palsy. The following six risk factors for recurrent laryngeal nerve palsy were extracted by univariate analysis: age ≥75 years, clinical stage ≥II, FDG-PET accumulation, open thoracotomy approach, station 4L LN dissection, and station 4L and/or 5 nodal metastasis. In multivariate analysis, age ≥75 years and station 4L LN dissection were independent risk factors for recurrent laryngeal nerve palsy (Table 5). Table 6 shows the background characteristics and prognoses of patients with recurrent laryngeal nerve palsy. Fourteen (70%) patients with recurrent laryngeal nerve palsy recovered spontaneously in an average of 5.71 months, whereas three (15%) patients required surgical intervention. Aspiration pneumonia associated with recurrent laryngeal nerve palsy was observed in four patients; however, none of the patients died.
Table 5
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P value | OR | 95% CI | P value | OR | 95% CI | ||
| Age ≥75 years | 0.02 | 0.191 | 0.021–0.860 | 0.02 | 0.075 | 0.009–0.621 | |
| Clinical stage ≥II | 0.006 | 3.925 | 1.328–11.944 | 0.92 | 1.090 | 0.205–5.790 | |
| FDG-PET accumulation | 0.006 | 4.717 | 1.383–15.912 | 0.30 | 2.320 | 0.473–11.400 | |
| Open thoracotomy | 0.02 | 3.424 | 1.172–10.556 | 0.10 | 3.8200 | 0.776–18.800 | |
| Station 4 LN dissection | 0.04 | 3.587 | 0.845–13.586 | 0.03 | 7.340 | 1.260–42.800 | |
| Station 4 and/or 5 nodal metastases | 0.05 | 3.297 | 0.985–14.360 | 0.83 | 0.832 | 0.150–4.610 | |
OR, odds ratio; CI, confidence interval; FDG-PET, fluorodeoxyglucose-positron emission tomography; LN, lymph node.
Table 6
| Characteristics | Values |
|---|---|
| Age (years) | |
| Range | 47–80 |
| Mean ± SD | 65.85±8.04 |
| Sex, n (%) | |
| Male | 14 (70.0) |
| Clinical stage, n (%) | |
| ≥II | 11 (55.0) |
| Approach, n (%) | |
| VATS | 9 (45.0) |
| Mediastinoscopy, n (%) | 11 (55.0) |
| Pathological N factor, n (%) | |
| N2 | 5 (25.0) |
| Prognosis, n (%) | |
| Improvement | 14 (70.0) |
| Time required for improvement (months) | |
| Range | 0–15 |
| Mean ± SD | 5.71±3.69 |
| Surgery for RLNP, n (%) | 3 (15.0) |
| RLNP-related complications, n (%) | |
| Aspiration pneumonia | 4 (20.0) |
| Atelectasis | 2 (10.0) |
| Sputum retention | 1 (5.0) |
SD, standard deviation; VATS, video-assisted thoracic surgery; RLNP, recurrent laryngeal nerve palsy.
Relationship between recurrent laryngeal nerve palsy and surgical procedure
Among the 91 patients in whom the surgical procedure during LN dissection could be confirmed by video imaging, 74 who underwent station 4L LN dissection were included in this study. To prevent recurrent laryngeal nerve palsy, LN dissection should be performed after confirming the location of the recurrent laryngeal nerve; therefore, the surgical procedures considered necessary were set as checkpoints. Univariate analysis was performed to determine the relationship between these checkpoints and recurrent laryngeal nerve palsy (Table 7). EK [odds ratio (OR) =8.278; 95% confidence interval (CI): 0.932–74.895; P=0.03] or VSS (OR =4.224; 95% CI: 0.831–21.757; P=0.04) use near the recurrent laryngeal nerve was a significant risk factor for recurrent laryngeal nerve palsy in univariate analysis. As many of the cases reviewed in the videos were relatively new (since 2014), we investigated the annual transition of the number of cases performed at each checkpoint to confirm whether other factors had a low correlation (Table 7). The comparison was made separately for the first half of 2014–2016 and the second half of 2017–2019. The achievement of checkpoint performance increased significantly, although the incidence of recurrent laryngeal nerve palsy did not decrease.
Table 7
| Surgical procedures† | Annual transition | Univariate analysis | |||||
|---|---|---|---|---|---|---|---|
| 2014–2016, n=27 (%) | 2017–2019, n=47 (%) | Total, n=74 (%) | P value | OR | 95% CI | ||
| Bot-com | 19 (70.4) | 42 (89.4) | 61 (82.4) | 0.68 | 2.060 | 0.241–98.474 | |
| Vce-com | 19 (70.4) | 43 (91.5) | 62 (83.8) | >0.99 | 1.855 | 0.214–89.181 | |
| Vpe-com | 26 (96.3) | 46 (97.9) | 72 (97.3) | >0.99 | Inf | 0.028–inf | |
| Rsu-com | 19 (70.4) | 43 (91.5) | 62 (83.8) | 0.66 | 0.744 | 0.120–8.206 | |
| Rde-com | 16 (59.3) | 39 (83.0) | 55 (74.3) | >0.99 | 1.328 | 0.230–14.118 | |
| EK-use | 0 (0.0) | 6 (12.8) | 6 (0.08) | 0.03 | 8.278 | 0.932–74.895 | |
| VSS-use | 12 (44.4) | 5 (10.6) | 17 (23.0) | 0.04 | 4.224 | 0.831–21.757 | |
| PA-tap | 18 (66.7) | 29 (61.7) | 47 (63.5) | 0.08 | 6.044 | 0.757–279.725 | |
| RLNP | 3 (11.1) | 9 (19.1) | 12 (16.2) | – | – | – | |
†, eight surgical procedures were evaluated: confirmation and dissection of the ligamentum Botalli (Bot-com), the vagal nerve traveling along the central (Vce-com) and peripheral (Vpe-com) sides of the recurrent laryngeal nerve bifurcation, and the shallow (Rsu-com) and deep (Rde-com) branches of the recurrent laryngeal nerve before the start of LN dissection, as well as conventional EK (EK-use) and VSS (VSS-use) use near the recurrent laryngeal nerve (distance approximately <3 mm) and PA taping (PA-tap) during LN dissection. inf, infinity; OR, odds ratio; CI, confidence interval; EK, electric knife; VSS, vessel sealing system; PA, pulmonary artery; RLNP, recurrent laryngeal nerve palsy; LN, lymph node.
Discussion
The prognostic effect of LN dissection remains controversial (5-8). In early-stage NSCLC, effective local tumor control by complete mediastinal LN dissection reduces the local recurrence rate and improves prognosis. However, pathological N2 lung cancer is likely to have micrometastases that cannot be detected preoperatively (26). Therefore, it is important to obtain valuable information regarding patient prognosis from pathological examination of the fully dissected LNs by accurate staging (27). This information helps to determine the indications for adjuvant chemotherapy, which reportedly has therapeutic effects (28). As previously reported, N2 is a prognostic factor for survival in patients with lung cancer who have undergone radical resection (29). The pathological N factor was one of the clinical factors associated with DFS in left upper lobe lung cancer in this study.
Video-assisted mediastinoscopy is a surgical procedure with the high diagnostic ability (30), compensating for the difficulty in performing LN dissection in left lung cancer (31). In this study, although LN metastasis was negative on mediastinoscopy, 4.35% of station 4L LNs were positive for LN dissection using an intrathoracic approach. The reason for this result was the presence of LNs that could not be sampled due to the difficulty in reaching them or the lack of confirmation because mediastinoscopy was limited to LN sampling. As the station 4L LN located in the tracheobronchial site on the dorsal side of the aortic arch has a wide dissection area, the dissection range is slightly different for each approach from the mediastinal or thoracic cavity side, indicating that LN metastasis may not be fully evaluated, even by mediastinoscopy, which is a more direct and important method of LN biopsy. Therefore, even if metastasis is negative in mediastinoscopy, dissection should be performed from the thoracic cavity side.
The presence of metastases was causally associated with DFS in stations 5 and 6 LNs, with a low proportion of undissected cases, whereas station 4L LNs, which had a higher proportion of undissected cases (36.26%), showed no causal relationship. One reason may be the exclusion of node-positive patients at station 4L on mediastinoscopy. Another reason may be that only node-positive patients were pathologically evaluated, and LN status without resection was not considered for pathological examination. LN dissection was not performed in the following cases: (I) after mediastinoscopy or to shorten the operative time and (II) absence of LN enlargement. Intraoperative videos were used to confirm whether LNs were evaluated at the undissected station; however, no significant difference was observed in DFS, thus suggesting that no method was superior for dissection to evaluate metastatic LNs. Recent reports suggesting that station 4L LN dissection has a significant impact on prognosis are consistent with our study (15-19). However, the mediastinal LN dissection sites were selected in these studies; therefore, there may have been selection bias. Another study suggested that there was a significant difference after propensity score matching (18). However, future prospective, multicenter studies are needed to compare the prognosis with station 4L LN dissection as a routine method instead of selective dissection. A high correlation was observed between pathological N1 (station 10 and/or 11) and LN metastasis at stations 4L, 5, and/or 6 (P<0.01). Additionally, station 10 metastasis has been reported to be an independent risk factor for station 4L metastasis in left lung cancer (15,16). Therefore, if pathological N1 is proven intraoperatively, active station 4L LN dissection should be considered regardless of the difficulty in performing the dissection procedure.
Recurrent laryngeal nerve palsy is a major complication of aggressive left mediastinal LN dissection due to not only aspiration pneumonia but also hoarseness or difficulty in bronchial drainage, making perioperative management difficult. In this study, recurrent laryngeal nerve palsy was an independent risk factor for station 4L LN dissection in univariate and multivariate analyses. The incidence of recurrent laryngeal nerve injury varies in the literature and is reported to range from a negligibly low rate to as high as 40% in high-risk procedures, including left-sided lung resection (32). In this study, recurrent laryngeal nerve palsy was observed in 14.39% of patients postoperatively, although 70% of patients recovered spontaneously in an average of 5.71±3.69 months. Aspiration pneumonia associated with recurrent laryngeal nerve palsy was observed in four patients, although no deaths occurred.
In this study, univariate analysis revealed that the use of energy devices near the nerve during LN dissection was an independent risk factor for recurrent laryngeal nerve palsy. Because LN dissection is performed in a small space surrounded by important structures, an EK is unsuitable in terms of thermal injury to adjacent tissues and delayed wound healing due to a significant increase in operating temperature (33). Therefore, the VSS has been primarily used in recent years. This technique eliminates concern about thermal damage to organs due to tip cavitation or frictional heat in ultrasonically activated devices and has the advantage of tip temperature control, regardless of the gripping range (34). Although this device is easy to use, it should be noted that the actual thermal spread to the surrounding tissues is affected by multiple variables, such as tissue type, tissue thickness, cutting time, and surgeons’ use. However, thorough confirmation of the location of the vagus nerve and recurrent laryngeal nerve and identification of the recurrent laryngeal nerve bifurcation site is important, as is ensuring a safe distance from the nerve, even during aggressive dissection (sufficiently peeling and confirming the key structures). Improving the accuracy of LN dissection is essential for oncological evaluation; therefore, it is crucial to establish a method that considers the safety of the procedure and diagnostic certainty.
A more accurate and minimally invasive assessment of the medial LN is important to determine the indications for further oncological treatment induction. The high diagnostic ability of the endoscopic mediastinal LN staging techniques using endobronchial ultrasonography and endoscopic ultrasound has been reported (35); however, at present, it is spread is insufficient. Video-assisted mediastinoscopic lymphadenectomy (36) not only allows bilateral mediastinal LN evaluation but also demonstrates superior sensitivity and negative predictive value relative to the currently prevalent endobronchial ultrasonography. If the procedure is devised to improve the high complication rate, there is a possibility that it is also more effective than the combined use of mediastinal LN dissection from the thoracic cavity side and conventional mediastinoscopy.
Our study limitations include its small sample size and single-center retrospective design. Patients who received preoperative induction therapy for clinically suspected N2 disease on imaging were excluded due to difficulty in evaluating the presence of true LN metastases on postoperative pathological examination. Additionally, patients who received preoperative induction therapy based on positive mediastinoscopy were excluded because the treatment method differed from that of pathological N2 diagnosed on postoperative pathological examination. As the number of patients with pathologically evaluated station 4L LN metastasis was small, we consider that the effect on the prognosis of station 4L LN metastasis may not have been sufficiently evaluated. Furthermore, it is possible that the video evaluation was biased because there were many recent cases wherein efforts were made to improve LN dissection.
Conclusions
In left upper lobe lung cancer, pathological N2 is an important predictor of recurrence. Regardless of the preceding mediastinoscopy, node-positive cases of up-staging by station 4L LN dissection from the chest cavity side were present. We propose to standardize dissection procedures and device usage at each institution for a more accurate assessment of station 4L LN while minimizing complications, including recurrent laryngeal nerve palsy.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-537/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-537/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-537/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-537/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted following the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shiga University of Medical Science (No. R2019-261). The requirement for individual consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Cahan WG, Watson WL, Pool JL. Radical pneumonectomy. J Thorac Surg 1951;22:449-73. [Crossref] [PubMed]
- Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: systematic review and meta-analysis of andomizedd controlled trials. Thorax 2006;61:597-603. [Crossref] [PubMed]
- Huang X, Wang J, Chen Q, et al. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: a systematic review and meta-analysis. pLoS One 2014;9:e109979. [Crossref] [PubMed]
- Meng D, Zhou Z, Wang Y, et al. Lymphadenectomy for clinical early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:597-604. [Crossref] [PubMed]
- Mokhles S, Macbeth F, Treasure T, et al. Systematic lymphadenectomy versus sampling of ipsilateral mediastinal lymph-nodes during lobectomy for non-small-cell lung cancer: a systematic review of randomized trials and a meta-analysis. Eur J Cardiothorac Surg 2017;51:1149-56. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Complete thoracic mediastinal lymphadenectomy leads to a higher rate of pathologically proven N2 disease in patients with non-small cell lung cancer. Ann Thorac Surg 2012;94:902-6. [Crossref] [PubMed]
- Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg 2007;84:1059-65. [Crossref] [PubMed]
- Witte B, Hürtgen M. Video-assisted mediastinoscopic lymphadenectomy. Multimed Man Cardiothorac Surg 2007;2007:mmcts.2006.002576.
- Sayar A, Çitak N, Büyükkale S, et al. The incidence of hoarseness after mediastinoscopy and outcome of video-assisted versus conventional mediastinoscopy in lung cancer staging. Acta Chir Belg 2016;116:23-9. [Crossref] [PubMed]
- Liang RB, Yang J, Zeng TS, et al. Incidence and Distribution of Lobe-Specific Mediastinal Lymph Node Metastasis in Non-small Cell Lung Cancer: Data from 4511 Resected Cases. Ann Surg Oncol 2018;25:3300-7. [Crossref] [PubMed]
- Fang L, Wang L, Wang Y, et al. Predictors and survival impact of station 4L metastasis in left non-small cell lung cancer. J Cancer Res Clin Oncol 2019;145:1313-9. [Crossref] [PubMed]
- Wang YN, Yao S, Wang CL, et al. Clinical Significance of 4L Lymph Node Dissection in Left Lung Cancer. J Clin Oncol 2018;36:2935-42. [Crossref] [PubMed]
- Zhao K, Wei S, Mei J, et al. Survival Benefit of Left Lower Paratracheal (4L) Lymph Node Dissection for Patients with Left-Sided Non-small Cell Lung Cancer: Once Neglected But of Great Importance. Ann Surg Oncol 2019;26:2044-52. [Crossref] [PubMed]
- Yang MZ, Hou X, Li JB, et al. Impact of L4 lymph node dissection on long-term survival in left-side operable non-small-cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2020;57:1181-8. [Crossref] [PubMed]
- Gryszko GM, Cackowski MM, Zbytniewski M, et al. The impact of left lower paratracheal (4L) lymph node dissection on survival in patients with surgically treated left-sided NSCLC. Eur J Cardiothorac Surg 2021;60:1201-9. [Crossref] [PubMed]
- Fourdrain A, De Dominicis F, Iquille J, et al. Usefulness of a routine endoscopic assessment of laryngeal lesions after lung cancer surgery. Respirology 2018;23:107-10. [Crossref] [PubMed]
- STROBE (Strengthening the reporting of observational studies in epidemiology). Available online: https://www.strobe-statement.org
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer–- major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Detterbeck FC, Chansky K, Groome P, et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1433-46. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Kanda Y. Investigation of the freely available easy-to-use software’’EZ’’ for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Yamaguchi M, Sugio K. Current status of induction treatment for N2-Stage III non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2014;62:651-9. [Crossref] [PubMed]
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985;312:1604-8. [Crossref] [PubMed]
- NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Xu Y, Li J, Wang J, et al. Association between clinicopathological factors and postoperative radiotherapy in patients with completely resected pathological N2 non-small cell lung cancer. Oncol Lett 2018;15:2641-50. [PubMed]
- Zakkar M, Tan C, Hunt I. Is video mediastinoscopy a safer and more effective procedure than conventional mediastinoscopy? Interact Cardiovasc Thorac Surg 2012;14:81-4. [Crossref] [PubMed]
- Luke WP, Pearson FG, Todd TR, et al. Prospective evaluation of mediastinoscopy for assessment of carcinoma of the lung. J Thorac Cardiovasc Surg 1986;91:53-6. [Crossref] [PubMed]
- Schneider B, Schickinger-Fischer B, Zumtobel M, et al. Concept for diagnosis and therapy of unilateral recurrent laryngeal nerve paralysis following thoracic surgery. Thorac Cardiovasc Surg 2003;51:327-31. [Crossref] [PubMed]
- Loh SA, Carlson GA, Chang EI, et al. Comparative healing of surgical incisions created by the PEAK PlasmaBlade, conventional electrosurgery, and a scalpel. Plast Reconstr Surg 2009;124:1849-59. [Crossref] [PubMed]
- Hruby GW, Marruffo FC, Durak E, et al. Evaluation of surgical energy devices for vessel sealing and peripheral energy spread in a porcine model. J Urol 2007;178:2689-93. [Crossref] [PubMed]
- Liberman M, Sampalis J, Duranceau A, et al. Endosonographic mediastinal lymph node staging of lung cancer. Chest 2014;146:389-97. [Crossref] [PubMed]
- Lozekoot PWJ, Daemen JHT, van den Broek RR, et al. Surgical mediastinal lymph node staging for non-small-cell lung carcinoma. Transl Lung Cancer Res 2021;10:3645-58. [Crossref] [PubMed]


