Use of a novel digital drainage system after pulmonary resection
Introduction
Management of the postoperative pleural space is central to the care of patients after pulmonary resection. Decisions regarding chest tube drainage after surgery, including the application of negative intrapleural pressure, measurement of fluid output volume, and determination of air leak, all contribute to the patient experience, most directly by impacting chest tube duration which correlates to postoperative pain and hospital length of stay. The utility of digital systems for the management of chest tube drainage post pulmonary resection remains an evolving area of research. Digital drainage systems have a number of advantages over traditional analogue chest drains, such as reporting objective and continuous data, portability, and overall ease of operation. In a number of studies, these advantages have led to improved outcomes for patients, including reduced chest tube duration and decreased length of stay after pulmonary resection (1-5).
The Thoraguard Surgical Drainage System (Centese, Omaha, Nebraska) is a novel, Food and Drug Administration approved [510(k), K181667] system designed to improve the postoperative drainage of fluid and air following cardiothoracic surgery. In this study, we trialed the use of the Thoraguard system in patients undergoing robotic pulmonary resection. Our primary objective was to establish the safety and feasibility of the new drainage system. Secondarily, we sought to compare this cohort against a standard analogue drainage system to assess clinical outcomes. Lastly, we administered a user survey to hospital care providers that utilized the Thoraguard system in order to evaluate its qualitative usability and performance. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-574/rc).
Methods
Study design
This study was conducted in three phases: an initial 50-patient prospective observational safety and feasibility study, followed by a retrospective comparison with 200 prior patients utilizing a traditional analogue chest drain, and lastly, a clinician user-feedback survey. All patients underwent standard preoperative evaluation, which included computed tomography (CT) scan of the chest, integrated positron emission tomography/CT scan (PET/CT), and pulmonary function testing (PFTs). All pulmonary resections were performed on a da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, California, USA) using a completely portal four-arm approach with an assistant port. All patients received the same perioperative care by the same care team. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Study design and data management were approved by the Institutional Review Board at New York University Langone Health (IRB# i18-0135). Informed consent was obtained in the 50 patients who utilized the Thoraguard system. Informed consent was waived for the retrospective arm of this study per IRB approval. This study is registered as NCTS18-01355 as a clinical trial at clinicaltrials.gov.
Initially, we performed a single-center, prospective, open-label, observational study utilizing the Thoraguard Surgical Drainage System in 50 patients undergoing elective robotic pulmonary resection by a single surgeon (MZ). Patients were excluded if they required emergency surgery or had previously undergone ipsilateral thoracic surgery. Safety and feasibility were assessed based on clinical outcomes, air and fluid chest tube drainage, chest tube duration, and associated perioperative complications. Equally, system data were reviewed, including instances of clinical alarms, malfunction, or system-associated failures. Informed consent was obtained in the 50 patients who utilized the Thoraguard system.
Secondarily, we retrospectively compared the 50 patients who utilized the Thoraguard system with 200 consecutive patients who had previously undergone pulmonary resection by the same surgeon (MZ) and utilized an “analogue” Atrium Ocean 2002 wet suction water seal drainage system (Atrium Medical Corp, Merrimack, New Hampshire, USA). These patients were selected using the same inclusion and exclusion criteria as the prospective arm and data was collected retrospectively from the electronic medical record. Clinical outcomes, including detection of air-leak, chest tube duration, and hospital length of stay, were evaluated.
At the completion of the safety and feasibility trial, a post-use qualitative survey was administered anonymously to health care providers involved in the operative and perioperative care of patients with the Thoraguard system. All survey respondents had prior experience with both traditional analogue drainage systems and digital drainage systems.
Thoraguard Digital Drainage System
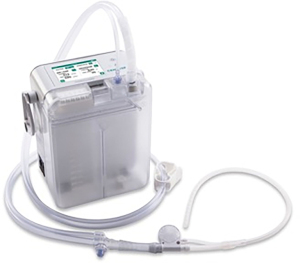
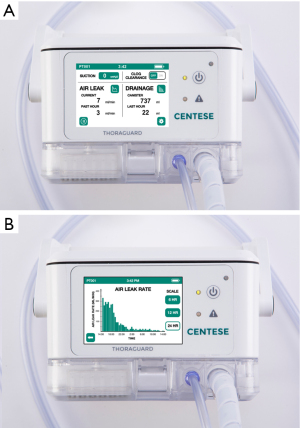
The Thoraguard Surgical Drainage System consists of an electronic control module with digital outputs for continuous monitoring of air and fluid egress, a drainage canister with tubing, and an optional specialized chest tube with clog clearance technology which was not used in this study (Figure 1). To determine air leak rate, the control module uses a tachometer on the pump to count revolutions of the motor in conjunction with pressure measurements and pump speed. The system’s touch screen digital interface reports single digit air and fluid data quantified in milliliters per minute (mL/min) (Figure 2). Data is stored every five minutes and historical data can be accessed and displayed graphically. Additionally, the Thoraguard system has a “pleural assessment” feature that functions as a “digital tidal”, reporting real-time intrapleural pressure. Furthermore, the Thoraguard system has several integrated safety features, including system alarms for excessive air (system disconnection) or fluid output (acute bleeding), system interference or malfunction, and for low battery life.
Chest tube management
Our practice for all patients in this study was to place a single postoperative chest tube (28 French). In all patients, the chest tube was maintained to “suction” at −20 cmH2O immediately after surgery with placement to a “water seal” setting of 0 cmH2O (disconnected from suction), the morning of postoperative day one (POD1). Patients were assessed for air leak in the post-anesthesia care unit (PACU), on the morning of POD1, and (if required) twice daily thereafter. Patients were either positioned sitting in a chair or in an upright in bed. The respective drainage devices were placed to a water seal setting and the patients were instructed to cough as a provocative test. In the analogue group, cessation of air leak was confirmed when air bubbles could not be observed in the analogue chest drain bubble chamber; when no bubbles were observed, the chest tube was removed. In the Thoraguard group, cessation of air leak was defined as a flow rate of 0 mL/min and the chest tube removed. However, in the Thoraguard group, when an air leak flow rate was below 20 mL/min for at least 6 hours, we considered chest tube removal per the discretion of the operating surgeon. These criteria were adapted from prior trials utilizing digital drainage systems (6,7). In both groups, there was no fluid volume output criteria for chest tube removal. Chest tubes were pulled regardless of the number of instances of mobilization or ambulation. All chest tubes were pulled at the end of expiration. Chest tube duration was defined as the number of midnights with a chest tube.
Statistical analysis
Data was gathered and stored in Excel (Microsoft Corp, Redmond, WA, USA). Statistical analyses were performed with IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, New York, USA). Descriptive statistics were used to summarize the data. Continuous variables are reported as median [interquartile range (IQR)] and categorical variables are reported as frequency and percentage. Continuous variables were compared using the Mann-Whitney U test and categorical variables were compared using Fischer’s exact test. A two-sided P value less than 0.05 was predetermined to be statistically significant.
Results
From September 2019 to July 2021, 50 patients who met study inclusion criteria underwent robotic pulmonary resection by a single surgeon (MZ) and utilized the Thoraguard Surgical Drainage System for postoperative drainage. Median patient age was 69 years (IQR 61–78) and 68% were current or former smokers. Patients were relatively healthy with a median Charlson comorbidity score of 4 (IQR 3–5.4), median forced expiratory volume in one second (FEV1) and diffusing capacity for carbon monoxide (DLCO) percent predicted of 84% (IQR 70–96%) and 88% (IQR 78–97%) respectively. The majority of patients (88%) underwent pulmonary resection for a malignant pathology. There were no major differences in preoperative data between the 50 Thoraguard patients and the 200 analogue patients. Preoperative patient data are reported in Table 1.
Table 1
| Variable | Thoraguard (N=50) | Analogue (N=200) | P value |
|---|---|---|---|
| Age, years, median [IQR] | 69 [61–78] | 66 [56–72] | 0.184 |
| Sex, n [%] | 0.432 | ||
| Male | 19 [38] | 92 [46] | |
| Female | 31 [62] | 108 [54] | |
| BMI, mg/kg2, median [IQR] | 25 [23–32] | 26 [23–30] | 0.852 |
| Smoking status, n [%] | 0.281 | ||
| Never | 16 [32] | 76 [38] | |
| Former | 24 [48] | 109 [55] | |
| Current | 10 [20] | 15 [8] | |
| Charlson comorbidity score, median [IQR] | 4 [3–5.4] | 4 [2–5] | 0.930 |
| FEV1% predicted, %, median [IQR] | 84 [70–96] | 90 [75–104] | 0.647 |
| DLCO% predicted, %, median [IQR] | 88 [78–97] | 81 [67–94] | 0.312 |
| Pathology, n [%] | 0.582 | ||
| Malignancy | 44 [88] | 163 [82] | |
| Benign disease | 5 [10] | 34 [17] | |
| Infectious | 1 [2.0] | 3 [1.5] |
IQR, interquartile range; BMI, body mass index; FEV1%, forced expiratory volume in one second, percent predicted; DLCO%, diffusing capacity of lung for carbon monoxide.
In the patients who utilized the Thoraguard system, the majority underwent an anatomic lung resection (84%): 15 underwent segmentectomy and 27 underwent lobectomy. There were no conversions to open thoracotomy. Median operative time in the Thoraguard group was 122 minutes (IQR 90–148), which was significantly less than the median time of 149 minutes (IQR 110–184) in the analogue group (P=0.003). Detection of a postoperative air leak in the PACU occurred in 36/50 patients (72%) with the Thoraguard system, which was significantly higher than in patients managed with an analogue system (45/200, 23%; P<0.001). Postoperative complications occurred in 3 patients (6%) in the Thoraguard group and 24 patients (12%) in the analogue group—no complications in either group were attributed to the chest drainage systems. There was a decrease in chest tube duration of 1 day (IQR 0–2) versus 2 days (IQR 2–3) (P=0.042) and hospital length of stay of 2 days (IQR 2–3) versus 3 days (IQR 2–4) (P=0.027). No patients in either group developed a pneumothorax after chest tube removal requiring reinsertion. Comparative perioperative outcomes of patients managed with the Thoraguard and analogue chest drainage systems are reported in Table 2.
Table 2
| Variable | Thoraguard (N=50) | Analogue (N=200) | P value |
|---|---|---|---|
| Operation, n [%] | 0.746 | ||
| Wedge resection | 8 [16] | 28 [14] | |
| Segmentectomy | 15 [30] | 66 [33] | |
| Lobectomy | 27 [54] | 106 [53] | |
| Conversion to open thoracotomy, n [%] | 0 [0] | 3 [1.5] | 1.000 |
| Estimated blood loss, mL, median [IQR] | 25 [10–42] | 20 [10–50] | 0.842 |
| Operative time, minutes, median [IQR] | 122 [90–148] | 149 [110–184] | 0.003 |
| Chest tube placement, n [%] | 0.007 | ||
| Single 20 Fr | 2 [4] | 0 [0] | |
| Single 28 Fr | 48 [96] | 200 [100] | |
| Postoperative air leak detected in PACU | 36 [72] | 45 [23] | <0.001 |
| Flow of air-leak detected on removal, mL/min, median [IQR] | 1.5 [0–4; 0–18] | N/A | |
| Flow of air-leak detected 1 hour prior to removal, mL/min, median [IQR] | 2 [0–6; 0–10] | N/A | |
| Maximal air leak flow, mL/min, median [IQR] | 48 [15–285; 5–1,200] | N/A | |
| Chest tube duration, days, median [IQR] | 1 [0–2] | 2 [2–3] | 0.042 |
| Chest tube complications, n [%] | N/A | ||
| Post-pull pneumothorax requiring chest tube reinsertion | 0 [0] | 0 [0] | |
| Postoperative complications, n [%] | 3 [6] | 24 [12] | 1.000 |
| Prolonged air-leak | 0 | 6 | |
| Atrial fibrillation | 1 | 6 | |
| Pneumonia | 1 | 4 | |
| Bleeding requiring reintervention | 1 | 3 | |
| Pulmonary embolism | 0 | 2 | |
| Reoperation | 0 | 2 | |
| Myocardial infarction | 0 | 1 | |
| Stroke | 0 | 1 | |
| Hospital length of stay, median [IQR], days | 2 [2–3] | 3 [2–4] | 0.027 |
| Discharge home with chest tube, median [IQR] | 0 [0] | 2 [1] | 1.000 |
IQR, interquartile range; PACU, post-anesthesia care unit, N/A, not applicable.
Patients managed with the Thoraguard system had a median peak air leak flow of 48 mL/min (IQR 15–285; range, 5–1,200). Patients with a peak air leak flow less than 100 mL/min throughout the chest tube dwell time (32 patients, 64%), had a decreased median chest tube duration of 1 day (IQR 0–1) versus 2.8 days (IQR 1–3) (P=0.004) and a trend towards decreased median hospital length of stay of 2 days (IQR 2–3) versus 3 days (IQR 2.8–6.2) (P=0.072) (Table 3). The median flow of air leak detected by the Thoraguard system was 2 mL/min (IQR 0–6; range, 0–10 mL/min) at one hour prior to removal and a median of 1.5 mL/min (IQR 0–4; range, 0–18 mL/min) at the time of chest tube removal.
Table 3
| Variable | Peak air leak <100 mL/min (N=32) | Peak air leak ≥100 mL/min (N=18) | P value |
|---|---|---|---|
| Chest tube duration, median [IQR] | 1 [0–1] | 2.8 [1–3] | 0.004 |
| POD#0 | 12 | 2 | |
| POD#1 | 36 | 5 | |
| POD#2 | 2 | 4 | |
| POD#3 | 0 | 5 | |
| POD#4 or greater | 0 | 2 | |
| Hospital length of stay, median [IQR] | 2 [2–3] | 3 [2.8–6.2] | 0.072 |
| POD#1 | 4 | 0 | |
| POD#2 | 18 | 3 | |
| POD#3 | 6 | 6 | |
| POD#4 or greater | 4 | 9 |
IQR, interquartile range; POD, postoperative day.
Twenty three health professionals (5 surgeons, 18 nurses) completed the post-study user experience survey. Compared to an analogue system, the Thoraguard system was reported to have a better (17/23, 74%) or the same (6/23, 26%) ability to detect air-leaks, better (14/23, 61%) or the same (8/23, 35%) ease of patient ambulation, and better (22/23, 96%) or the same (1/23, 4%) display of clinically relevant information. Compared to alternative digital drainage systems, the Thoraguard system was reported to have a better (19/23, 83%) or the same (3/23, 13%) ability to detect air-leaks, better (21/23, 91%) or the same (2/23, 9%) display of clinically relevant information, and better (16/23, 70%) or the same (6/23, 26%) overall ease of use. User experience survey data is reported in Table 4.
Table 4
| Survey question | Surgeon (N=5) | Nurse (N=18) |
|---|---|---|
| Compared to traditional analog drainage system(s) | ||
| 1. How do you rate Thoraguard’s ability to measure air leak? | Better—4 | Better—13 |
| Same—1 | Same—5 | |
| Worse—0 | Worse—0 | |
| 2. How do you rate patients’ ability to ambulate and/or mobilize with active suction using Thoraguard? |
Better—5 | Better—9 |
| Same—0 | Same—8 | |
| Worse—0 | Worse—0 | |
| N/A—1 | ||
| 3. How does the clinical information displayed on Thoraguard compare? | Better—5 | Better—17 |
| Same—0 | Same—1 | |
| Worse—0 | Worse—0 | |
| Compared to other digital drainage system(s) | ||
| 1. How do you rate Thoraguard’s ability to measure air leak? | Better—4 | Better—15 |
| Same—1 | Same—2 | |
| Worse—0 | Worse—0 | |
| N/A—1 | ||
| 2. How does the amount of clinically-useful information displayed on Thoraguard compare? | Better—4 | Better—17 |
| Same—1 | Same—1 | |
| Worse—0 | Worse—0 | |
| 3. How do you rate the ease of use of Thoraguard’s user interface? | Better—4 | Better—12 |
| Same—1 | Same—5 | |
| Worse—0 | Worse—0 | |
| N/A—1 | ||
N/A, not applicable.
Discussion
The use of digital chest tube drainage systems has advanced the postoperative management of patients undergoing pulmonary resection with the advantages of objective assessment, continuous data gathering, precision adjustments, portability, and improvement in overall usability. The objective nature of these systems reduces inter-observer variability, allowing all members of the team to accurately assess an air leak (8). Digital systems also collect data continuously, allowing interpretation of data trends over time and the ability to capture intermittent leaks. With these evolutions in mind, we trialed the use of a novel digital chest drainage system—the Thoraguard Digital Drainage System—in 50 patients undergoing robotic pulmonary resection and subsequently compared these outcomes with 200 previous patients who were managed with an analogue chest drainage system. We found that the use of the Thoraguard system was safe, feasible, and offered a number of potential clinical advantages over an analogue system.
In a number of studies, including a limited number of prospective trials, the use of digital drainage systems has reduced the duration of chest tubes after pulmonary resection and decreased hospital length of stay when compared to traditional analog systems (1-4). A meta-analysis assessing 3,399 patients reported a significantly reduced chest tube duration of 0.68 days and reduced length of stay of 1.4 days with the use of a digital drainage system (9). Equally, earlier removal of chest tubes is associated with improved pulmonary function, reduced postoperative pain, and fewer overall complications (1,2,10). Alternatively, while other studies have failed to show a reduction in length of stay, they have found that digital systems reduced chest tube-associated interventions such as clamp trials or obtaining additional chest radiograph studies (11,12). In this study, we found that the Thoraguard system identified a greater number of air leaks in the PACU than the analogue system, 72% versus 23% respectively (P<0.001). Notably, our median chest tube duration of 1 day (IQR 0–2) and hospital length of stay of 2 days (IQR 2–3) in the Thoraguard group are both lower than in previous digital drainage studies (1,2,11). Theoretically, the benefit of a digital chest drain would be greatest in patients with a longer postoperative hospitalization for chest tube drainage.
Changes in pleural pressure and air flow dynamics have been analyzed to help inform timing of chest tube removal and prognostication regarding duration of postoperative air leak (6,13,14). In a cohort of patients undergoing pulmonary resection with a 5.8% rate of prolonged air leak (PAL, defined as an air leak greater than 5 days after surgery), Takamochi et al reported that a peak air leak greater than 100 mL/min was a positive predictor of PAL (6). Furthermore, they described two patterns of air leak over the initial 72 hours after surgery that were associated with PAL: repeated exacerbation and remission of air leak, and an air leak without a progressive trend towards improvement. In this study, we found that patients with a peak air leak flow greater than 100 mL/min did not predict an air leak greater than 5 days, but that these patients had a significantly longer chest tube duration of 2.8 days (IQR 1–3) versus 1 day (IQR 0–1) (P=0.004) and a trend towards longer hospital length of stay of 3 days (IQR 2.8–6.2) versus 2 days (IQR 2–3) (P=0.072) versus than patients with peak air leak less than 100 mL/min (Table 3). Capturing single-digit continuous measurements on the Thoraguard system, we have initiated a follow-up study assessing the predictive potential of data trends regarding pleural pressure and air flow data to potentially predict air leak cessation and timing of chest tube removal.
The clinically appropriate threshold flow of air leak flow for chest tube removal differs substantially across trials, but reported levels generally range from 0 to 50 mL/min over a period of 1–12 hours prior to removal (8,15). In this study, we utilized 20 mL/min as prompting consideration for chest tube removal. Despite this threshold, however, patients had a significantly lower median air leak flow of 1.5 mL/min (IQR 0–4) prior to removal—the largest measured air leak flow immediately prior to removal was 18 mL/min. No patients in the Thoraguard group developed a post-pull pneumothorax requiring chest tube reinsertion. Further research from digital data may help determine an acceptable rate of air leak after pulmonary resection which is safe for chest tube removal. From our early experience, we predict this threshold to be an air leak of approximately 15 mL/min or less. The single digit display of the Thoraguard system aids in this determination. Generally, when compared to an analogue system, the majority of users (22/23, 96%) reported better display of clinically relevant information, including the rate of air leak (Table 4).
An advantage of digital drainage systems is their ability to provide portable suction. Users of the Thoraguard system reported better (14/23, 61%) or the same (8/23, 35%) ease for patient ambulation. While no patients in this study were discharged with a Thoraguard system, the use of chest drainage systems in the outpatient setting is predicated on portability and usability. Utilizing a standardized approach to operative conduct and postoperative protocols, Greer et al. discharged 150/390 (38%) patients after pulmonary lobectomy on POD1 without increased morbidity or rate of 30-day readmission (16). With the anticipated potential use in the outpatient setting, the Thoraguard system was reported as having superior ability to measure an air-leak, clinically-useful display of information, and ease of use versus alternative digital drainage systems.
The primary strength of the observational arm of this study is its prospective design with complete data. Secondarily, the retrospective cohort of patients with similar inclusion criteria, allows for relative comparison between chest drainage systems. The comparison arm of this study is limited primarily by its retrospective design, which is subject to selection bias. While the demographics were well matched overall, the analogue group had a longer operative time and a greater frequency of complications, both of which may have influenced chest tube duration and hospital length of stay. Essentially, these groups were not propensity matched, limiting the strength of our conclusions. Equally, the single-surgeon and single-center design limits translation of our results to all patients and centers.
In conclusion, in a feasibility and safety study of 50 patients undergoing robotic pulmonary resection, the Thoraguard Digital Drainage System provided safe and effective pleural drainage of air and fluid. Compared to 200 prior patients who were managed with an analogue drainage system, the Thoraguard system identified a greater number of air leaks after surgery and was associated with reduced chest tube duration and hospital length of stay. Generally, compared to an analogue system, users of the Thoraguard system reported superior ability to measure an air leak, patient ability for ambulation, and display of clinical information. Further research is ongoing to determine the optimal threshold of air leak for chest tube removal, prognostic indicators of air leak duration, system use the in outpatient setting, financial value, and assessment of patient-centered reporting on system usability.
Acknowledgments
The Thoraguard Digital Drainage Systems used in this study were borrowed from Centese. The authors confirm freedom of investigation and full control of the design of the study, methods used, outcome parameters and results, analysis of data, and production of the written report.
Funding: This study was sponsored by Centese (Omaha, Nebraska), which provided financial support and technical support for this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-574/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-574/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-574/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-574/coif). MZ discloses a relationship with Intuitive Surgical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Study design and data management were approved by the Institutional Review Board at New York University Langone Health (IRB# i18-0135). Informed consent was obtained in the 50 patients who utilized the Thoraguard system. Informed consent was waived for the retrospective arm of this study per IRB approval.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miller DL, Helms GA, Mayfield WR. Digital Drainage System Reduces Hospitalization After Video-Assisted Thoracoscopic Surgery Lung Resection. Ann Thorac Surg 2016;102:955-61. [Crossref] [PubMed]
- Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [Crossref] [PubMed]
- Shoji F, Takamori S, Akamine T, et al. Clinical Evaluation and Outcomes of Digital Chest Drainage after Lung Resection. Ann Thorac Cardiovasc Surg 2016;22:354-8. [Crossref] [PubMed]
- Bertolaccini L, Rizzardi G, Filice MJ, et al. 'Six sigma approach' - an objective strategy in digital assessment of postoperative air leaks: a prospective randomised study. Eur J Cardiothorac Surg 2011;39:e128-32. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Solidoro P, et al. Digital air leak monitoring after lobectomy for primary lung cancer in patients with moderate COPD: can a fast-tracking algorithm reduce postoperative costs and complications? J Cardiovasc Surg (Torino) 2010;51:429-33. [PubMed]
- Takamochi K, Imashimizu K, Fukui M, et al. Utility of Objective Chest Tube Management After Pulmonary Resection Using a Digital Drainage System. Ann Thorac Surg 2017;104:275-83. [PubMed]
- Lijkendijk M, Licht PB, Neckelmann K. Electronic versus traditional chest tube drainage following lobectomy: a randomized trial. Eur J Cardiothorac Surg 2015;48:893-8; discussion 898. [Crossref] [PubMed]
- Varela G, Jiménez MF, Novoa NM, et al. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg 2009;35:28-31. [Crossref] [PubMed]
- Chang PC, Chen KH, Jhou HJ, et al. Promising Effects of Digital Chest Tube Drainage System for Pulmonary Resection: A Systematic Review and Network Meta-Analysis. J Pers Med 2022;12:512. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after elective pulmonary resection: a prospective study. Ann Thorac Surg 2008;86:396-401. [Crossref] [PubMed]
- Plourde M, Jad A, Dorn P, et al. Digital Air Leak Monitoring for Lung Resection Patients: A Randomized Controlled Clinical Trial. Ann Thorac Surg 2018;106:1628-32. [Crossref] [PubMed]
- Gilbert S, McGuire AL, Maghera S, et al. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. J Thorac Cardiovasc Surg 2015;150:1243-9. [Crossref] [PubMed]
- Brunelli A, Cassivi SD, Salati M, et al. Digital measurements of air leak flow and intrapleural pressures in the immediate postoperative period predict risk of prolonged air leak after pulmonary lobectomy. Eur J Cardiothorac Surg 2011;39:584-8. [Crossref] [PubMed]
- Shintani Y, Funaki S, Ose N, et al. Air leak pattern shown by digital chest drainage system predict prolonged air leakage after pulmonary resection for patients with lung cancer. J Thorac Dis 2018;10:3714-21. [Crossref] [PubMed]
- Aldaghlawi F, Kurman JS, Lilly JA, et al. A Systematic Review of Digital vs Analog Drainage for Air Leak After Surgical Resection or Spontaneous Pneumothorax. Chest 2020;157:1346-53. [Crossref] [PubMed]
- Greer S, Miller AD, Smith JS, et al. Safety of Next Day Discharge After Lobectomy: Have We Broken the Speed Limit? Ann Thorac Surg 2018;106:998-1001. [Crossref] [PubMed]