Mediastinal lymphadenectomy fulfilling NCCN criteria may improve the outcome of clinical N0–1 and pathological N2 non-small cell lung cancer
Introduction
Lymphadenectomy is an integral component of surgical treatment for clinical early-stage lung cancer (1). The extent of lymph node removal (dissection versus sampling) and its impact on survival has been addressed by several randomized trials (2,3). The biggest randomized trial Z0030 demonstrated that mediastinal lymph node dissection did not improve survival in patients with early stage non-small cell lung cancer after systematic sampling of mediastinal and hilar lymph nodes (3).
Based on the current literature, individual academic societies and research groups have published different recommendations about nodal definitions and requirement of nodal assessment. For example, the National Comprehensive Cancer Network (NCCN) guidelines suggest investigating at least three mediastinal lymph node stations for accurate pathological staging (1). The International Association for the Study of Lung Cancer (IASLC) suggested that adequate N staging is generally considered to include sampling or dissection of lymph nodes from stations 2R, 4R, 7, 10R and 11R for right-sided tumors, and stations 5, 6, 7, 10L and 11L for left-sided tumors (4). The American College of Surgeons Oncology Group (ACOSOG) (5) and European Society of Thoracic Surgery (ESTS) guideline (6) also have their featured recommendations on lymph node dissection.
Thoracic surgeons may choose to follow a specific guideline for their routine practice. The general consensus is that radical mediastinal lymphadenectomy will provide accurate information for pathological staging and guiding adjuvant therapy (7-9). However, it is not clearly established whether mediastinal lymphadenectomy compliant with international criteria will improve the oncological outcomes of clinical early-stage lung cancer.
This retrospective study was aimed to compare the long-term survival between the cases treated with lymphadenectomy fulfilling the NCCN criteria and other cases not met the criteria in a group of patients with clinical early-stage lung cancer.
Materials and methods
From September, 2003 to January 2010, 726 consecutive patients admitted to Peking University Cancer Hospital were diagnosed with clinical early stage NSCLC (cN0–1) and treated with pulmonary resection and lymphadenectomy. None of the patients received neoadjuvant therapy. The Institutional Review Board at Beijing Cancer Hospital approved this retrospective study. The requirement of patient consent was waived. Informed consent, including written and oral, has also been obtained from the participants.
Preoperative staging
The routine preoperative staging included contrast computed tomography (CT) of the chest, brain magnetic resonance imaging (MRI), abdominal ultrasonography, bone scintigraphy or positron emission tomography/CT (PET/CT, about 30% of patients receiving the exam in this cohort). Fibrobronchoscopic biopsy was applied to central localized tumor.
The clinical N0–1 (cN0–1) stage was defined as follows: any mediastinal lymph node with longest diameter less than 10 mm on chest CT and with a standardized uptake value maximum lower than 2.0 on a PET/CT scan if it was done. All other patients were referred to invasive mediastinal node evaluation (such as endobronchial ultrasound-guided transbronchial needle aspiration, EBUS-TBNA) and multidisciplinary team discussion and thus excluded from further analysis.
Surgical resection
Pulmonary resections, including wedge resection, lobectomy, bilobectomy, sleeve-lobectomy and pneumonectomy were selected on the basis of tumor location. The status of the residual tumor after surgical treatment was defined as three categories: R0 resection (no residual tumor present), R1 resection (microscopically residual tumor) and R2 resection (macroscopically residual tumor) (10). R1 and R2 resection were excluded from the study. Lymph-node stations were defined according to the IASLC (11). Lymphadenectomy was routinely performed after pulmonary resection. Tissues and lymph nodes were sent for routine pathologic analysis with paraffin blocks. Lymph nodes were bivalved along their longitudinal axis and totally submitted for microscopic evaluation. Small nodes (0.4 cm or less) were submitted without bivalving. A single hematoxylin-eosin (H&E)-stained slide was produced from each block.
Grouping method
NCCN criteria recommended a minimum of three N2 stations sampled or complete lymph node dissection after anatomic pulmonary resection (1). In this study, group A was defined as mediastinal lymphadenectomy fulfilling NCCN criteria, and others not met the criteria were referred as group B.
Statistical analysis
The length of overall survival (OS) was defined as the interval between the date of operation and death due to any cause or the last follow-up visit. The length of the recurrence-free period was calculated from the date of surgical intervention to the initial recurrence or the last follow-up visit. The 5-year OS and 5-year disease-free survival (DFS) between group A and group B were analyzed using the log-rank test, and Kaplan-Meier curves were generated for 5-year OS and 5-year DFS. Multivariate Cox regression analyses were used to determine the factors significantly associated with survival, and hazard ratio (HR) was calculated for 95% confidence interval (CI). Values were expressed as the mean ± standard deviation (or median, ranges). The student’s t-test or Mann-Whitney U-test was used for the analysis of normally or non-normally distributed data, respectively. The Pearson’s chi-square (×2) test was used to compare proportions (or the Fisher’s exact test as required). The P values for differences were calculated with a significance level of P<0.05. SPSS software (version 18.0; SPSS, Chicago, IL, USA) was used for all analysis.
Results
During September 2003 to January 2010, 726 consecutive cases of lung cancer staged as clinical N0–1 were investigated. The final follow-up ended on January, 2014. Two cases were excluded for non-R0 status after surgery and three patients were excluded due to non-cancerous death within the postoperative multi-modality treatment period. Follow-up information was failed to obtain in nine cases during the investigation period and thus excluded from the study. In total, 712 patients were enrolled into data analysis, which was confirmed as 450 cases (63.2%) of pN0, 110 (15.4%) of pN1 and 152 (21.3%) of pN2 after surgery. The base-line features of the whole study group were clinical N0–1, R0 resection. After a median follow-up of 49.5 months (range, 3.1–119.6 months), 196 patients (27.5%) had died. The 5-year OS rates of patients of pN0, pN1 and pN2 status were 80%±2%, 60%±5% and 45%±4% respectively. Of all 712 patients, 702 patients underwent lobectomy or above and 439 patients (61.7%) had at least 3 stations of hilar and bronchopulmonary lymph nodes’ clearance. The most common patterns of mediastinal lymphadenectomy for left lung cancer were station 5+6+7 (52.7%) and station 2R + 4R +7 (56.1%) for right lung cancer.
Five hundred and fifty eight cases were enlisted in group A and 154 cases in group B. Demographics of two groups are summarized in Table 1. There was no significance between the two groups in terms of baseline characteristics: gender, age, pulmonary function, pathological T and N stage, histology and the ratio of lymphovascular invasion. However, group A revealed a larger tumor size compared to group B (3.28±0.069 vs. 2.93±0.141 cm, P=0.021). Group A collected more mediastinal lymph nodes and investigated more N2 stations compared to group B.
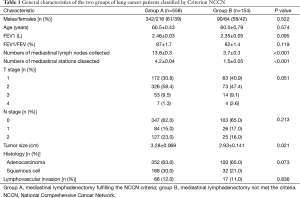
Full table
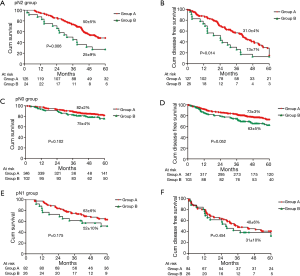
OS and DFS were significantly different between group A and group B in the whole cohort (the 5-year OS rates, 72%±2% vs. 63%±4%, P=0.014; the 5-year DFS rates, 58.0%±2% vs. 49%±4%, P=0.038; Figure 1). Then the whole cohort was stratified into three subgroups by pN status. OS and DFS were significantly different between group A and group B in cN0/1-pN2 status (the 5-year OS rates, 50%±5% vs. 25%±9%, P=0.006; Figure 2A; the 5-year DFS rates, 31.0%±4% vs. 13%±7%, P=0.014; Figure 2B), but not in pN0 (the 5-year OS rates, 82%±2% vs. 75%±4%, P=0.102; Figure 2C; the 5-year DFS rates, 73%±3% vs. 63%±5%, P=0.052; Figure 2D), or pN1 status (the 5-year OS rates, 63%±6% vs. 52%±10%, P=0.175; Figure 2E; the 5-year DFS rates, 40%±6% vs. 31%±10%, P=0.454; Figure 2F).

Among 152 cases of pN2 disease, 127 cases were enlisted in group A and 25 in group B. No differences were showed between two groups in terms of gender, age, pathological T staging, histology, pleural invasion, extent of resection and the rate of adjuvant chemotherapy. However patients in Group A have higher rate of multiple N2 than group B (39% vs. 8%, P=0.003). The patients with pN2 disease (108/152) received 4 cycles platinum-based double regimen chemotherapy after surgery. Thirty patients had early recurrence (<1 year) during follow-up.
In group A, four or more stations of mediastinal lymph nodes dissection did not achieve superior outcome compared to 3 stations of lymph node dissection in cN0/1-pN2 group (the 5-year OS rates, 46%±6% vs. 59%±9%, P=0.152; the 5-year DFS rates, 30%±5% vs. 34%±8%, P=0.598; Figure 3). Meanwhile no differences in the ratio of unexpected N2 were found between the patients with ≥4 stations collected and the patients with 3 stations of N2 node collection (P=0.232).

The spreading pattern of mediastinal nodes among pN2 cases was featured by tumor location. The most frequent involved station for right upper lobe-located lung cancer was 4R (83.7%), followed by 7 (37.2%) and 2R (14.0%). The top 3 involved stations for other cancer locations were 7 (75%), 4R (25%) and 2R (6.3%) for right middle lobe; 7 (81.6%), 4R (34.2%) and 2R (10.5%) for right lower lobe; 5+6 (90.9%), 4L (22.7%) and 7 (4.5%) for left upper lobe; 7 (66.7%), 5+6 (42.4%) and 8 (9.1%) for left lower lobe.
All variates, including age, gender, T staging, histology, lymphovascular invasion, performing a lymphadenectomy fulfilling NCCN criteria and adjuvant chemotherapy were examined in multivariate Cox models among the whole cohort and 152 patients of pN2 status. In the whole cohort, performing a lymphadenectomy fulfilling NCCN criteria (P=0.003), histology type (P=0.009), T staging (P<0.001) and N staging (P<0.001) were found to have significant impact on OS. Meanwhile, performing a lymphadenectomy fulfilling NCCN criteria (P=0.003), pleural invasion (P=0.009), T staging (P<0.001) and N staging (P<0.001) were found to have significant impact on disease free survival. Among the pN2 subgroup, only 2 were found to have significant impact on OS: T staging had a HR of 0.383 (T1 vs. T3/4, 95% CI, 0.171–0.859, P=0.020); performing a lymphadenectomy fulfilling NCCN criteria had a HR of 0.559 (95% CI, 0.323–0.968, P=0.038). Meanwhile performing a lymphadenectomy fulfilling NCCN criteria was also confirmed as an independent prognostic factor for DFS (HR =0.585, 95% CI, 0.353–0.971, P=0.038).
Discussion
Although clinical significance of nodal assessment has been reiterated by different guidelines and recommendations, current practice involving lymphadenectomy is still challenging given the lack of consistent guidelines. According to a recent analysis from Surveillance, Epidemiology, and End Results database (12), 62% of patients with pathologic N0 or N1 NSCLC had no mediastinal lymph node examined, which might be associated with a significant increase of lung cancer-specific mortality. A survey conducted by American College of Surgeons about patterns of surgical care reported that during operation, only 57.8% of patients had lymph nodes either sampled or removed from the mediastinum (13). Another study from the Society of Thoracic Surgeons database revealed that mediastinal lymph nodes were evaluated in 65% of 9,033 cases of pulmonary resection for primary lung cancer (14).
Since the previous study suggested that no examination on N2 node might increase the cancer-related mortality, there remains the question of how many stations of N2 nodes is adequate during lymphadenectomy. Although International Association for the Study of Lung Cancer Nodal Chart defines 9 stations of N2 node, most guidelines recommend evaluating a minimal of three N2 stations. The primary aim of this definition is for accurate staging. However, it is still unknown whether a survival benefit could be achieved by performing lymphadenectomy fully compliant with these guidelines.
In this study, we addressed the question by comparison of long-term survival between cases treated with lymphadenectomy fulfilling the NCCN criteria and other cases not met the criteria in a group of patients with clinical early-stage lung cancer.
For the whole cohort, we found a significant difference between these two groups both in OS and in DFS in cN0–1 non-small cell lung cancer patients. Since cN0–1 group might be separated into difference pathological status after surgery, we then ask question which subgroup might contribute to this difference. After stratification by pathological N status, our data demonstrated no significant difference in long-term survival between two groups with pN0 or pN1 disease, which means lymphadenectomy compliant with NCCN criteria may not have impact on survival for pathologically-documented early stage lung cancer.
Interestingly, our results suggested that for cN0/1-pN2 subgroup (unexpected N2), lymphadenectomy fulfilling NCCN criteria might yield a better survival compared to those procedures not met the criteria. Multivariate analysis also suggested that lymphadenectomy compliant with NCCN criteria and T stage were independent prognostic factors for the whole cohort and for pN2 subgroup. Our results predominantly focused on the numbers of N2 station surgeons have taken during operation and its effects on the oncological outcomes, which is slightly different from the design of Z30. The latter trial compared lymph node dissection versus sampling in patients with early stage lung cancer.
Preoperative staging procedures still underestimate pathological stage in 10–20% of patients with early stage disease (15,16) and the proper surgical approach for this unexpected N2 group is still under investigation. According to this study, potential prognostic effect of NCCN lymphadenectomy criteria on cN0/1-pN2 group suggested that lymphadenectomy fulfilling NCCN criteria may be a standard requirement for lung cancer surgery due to its influence on the outcome and staging. However, among patients treated with lymphadenectomy fulfilling NCCN criteria, more extended lymphadenectomy (≥4 stations) did not reveal an extra survival benefit compared with 3 stations dissection. The main reason might be low incidence of node involvement in some N2 stations, such as No. 9 and No. 3. Although lobe-specific lymph node dissection has been promisingly proposed, more data are needed to investigate the best combination mode of N2 node dissection for achieving optimal outcome and minimizing morbidity.
The cN0/1-pN2 subgroup may represent a special population with an optimistic oncological outcome compared with clinical N2 disease. Obiols et al. (17) reported 40% of 5 years OS in unexpected pN2 group and they suggested resection of properly staged unsuspectedpN2 NSCLC is reasonable and surgery should not be avoided if complete resection could be achieved. Cerfolio et al. (18) reported a 35% of 5-year OS in 148 patients with unsuspected N2 disease. A recent study in Korean patients revealed 56.1% of 5-year OS in occult N2 disease (19). In our study, all analyzed cases were treated with R0 resection and 66.5% of all cases were single N2 station involvement, which might contribute to 45% of 5-year OS observed in this group.
The proposed algorithm according to European Society of Thoracic Surgeons guidelines of nodal staging showed a 5.5% of unsuspected pN2 disease during practice (17). Therefore, combination of preoperative non-invasive and invasive procedures might be feasible to eliminate the possibility of occult positive N2 node as much as possible. But even for early stage, systematic sampling may still leave positive N2 node undetected. In two self-controlled trials comparing systematic sampling and SMLD, 21.8–88% of involved N2 nodes will be neglected if only performing systematic sampling at surgery (8,20). Thus the second checkpoint relies on intraoperative systematic sampling of mediastinal nodes by mediastinoscopy, video-assisted thoracic surgery (VATS) or thoracotomy as proposed by Z30 protocol, which may further reduce the rate of occult N2 to 4% (3). Therefore, we suggested that extensive sampling of N2 nodes and N1 nodes at surgery, even after rigorous preoperative evaluation. Our results suggested that different lobe location of lung cancer might exhibit featured spreading pattern of N2 node and this pattern would help surgeons to target the priority area when performing lymph node sampling. If any N2 nodes were involved, performing mediastinal lymphadenectomy fully compliant with NCCN criteria might improve the outcome in the group of unexpected N2 patients.
Retrospective analysis and lack of randomization are the major limitations of this study, resulting in potential selection bias. However, randomization in cN0/1-pN2 group may expose a certain patients at risk of incomplete resection. Further data from propensity-matched and randomized studies are required to identify the best practice procedure. The relatively small sample size is another limitation of the study, especially for group not met the criteria, which may results in inadequate statistical power to detect differences in complications and outcomes. The other limitation involved slightly higher ratio of unexpected N2 cases in this cohort, which is partly due to low proportion of PET/CT in this series. We majorly chose those cases with N2 nodes less than 1 cm by contrast CT with or without PET/CT scan for analysis to try to minimize the potential bias. The preoperative mediastinal evaluation should be strengthened to reduce the rate of unexpected pN2 patients, such as PET-CT scan and invasive mediastinal evaluation examinations like EBUS-TBNA or mediastinoscopy. Meanwhile, frozen section on sampled N2 nodes during surgery may also help to further detect unexpected N2 cases for multidisciplinary treatments
In summary, mediastinal lymphadenectomy fulfilling NCCN criteria may only improve the survival of unexpected pN2 subgroup among patients with clinical early-stage lung cancer. More extended dissection of mediastinal lymph node (≥4 stations) may not further improve the outcome in this group.
Acknowledgements
We thank Dr. Alan D. L. Sihoe from The University of Hong Kong for discussion of our manuscript and linguistic assistance.
Funding: Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding support, Code: ZYLX201509; Beijing Municipal Science & Technology Commission (No. Z161100000516063).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ettinger DS, Kris MG. Update: NCCN non-small cell lung cancer clinical practice guidelines. J Natl Compr Canc Netw 2004;2 Suppl 3:S-9-13. [PubMed]
- Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest 2011;139:1124-9. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:234S-242S.
- Wu N, Yan S, Lv C, et al. Comparison of systematic mediastinal lymph node dissection versus systematic sampling for lung cancer staging and completeness of surgery. J Surg Res 2011;171:e169-73. [Crossref] [PubMed]
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358-65; discussion 365-6. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol 2012;7:1798-806. [Crossref] [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [Crossref] [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref] [PubMed]
- Obiols C, Call S, Rami-Porta R, et al. Survival of patients with unsuspected pN2 non-small cell lung cancer after an accurate preoperative mediastinal staging. Ann Thorac Surg 2014;97:957-64. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Survival of patients with unsuspected N2 (stage IIIA) nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:362-6; discussion 366-7. [Crossref] [PubMed]
- Cho HJ, Kim SR, Kim HR, et al. Modern outcome and risk analysis of surgically resected occult N2 non-small cell lung cancer. Ann Thorac Surg 2014;97:1920-5. [Crossref] [PubMed]
- Massard G, Ducrocq X, Kochetkova EA, et al. Sampling or node dissection for intraoperative staging of lung cancer: a multicentric cross-sectional study. Eur J Cardiothorac Surg 2006;30:164-7. [Crossref] [PubMed]


