Mediastinal micro-vessels clipping during lymph node dissection may contribute to reduce postoperative pleural drainage
Introduction
Surgical intervention remains an important and effective treatment option for early stage lung cancer. During the perioperative period, decreasing morbidity and shortening the hospital stay may enhance patient outcomes by accelerating recovery, return to work, and minimizing economic burden on the healthcare system.
Postoperative pleural effusion often occurs after major pulmonary resection and systematic mediastinal lymph node dissection (SMLD), which is associated with prolonged duration of chest tube, prolonged hospital stay and higher hospital costs (1,2). Meanwhile the concomitant sequelae, including postoperative pain, functional limitation, and potential risk of infection, may lower the quality of surgery. Furthermore, some studies (3-5) have investigated the role of immune complexes, lymphocytes and cytokines existing in the pleural fluid, implicating a potential immune impairment after losing significant pleural fluid in a short time after surgery.
The present study aimed to detect potentially predisposing factors related with high-output pleural drainage after curative thoracic surgery in a group of non-small cell lung cancer (NSCLC) patients retrospectively and explore the impact of micro-vessels clipping in the mediastinum on pleural drainage control after lymph node dissection.
Materials and methods
Patients
From February 2012 to November 2013, 339 NSCLC patients admitted to the department of Thoracic Surgery II, Beijing Cancer Hospital and Institute, Peking University School of Oncology and treated with lobectomy or above and SMLD were eligible for the study. In addition, pyothorax after surgery (n=4), and reoperation due to postoperative hemorrhage (n=2) or chylothorax (n=2) were excluded because these specific complications could affect postoperative drainage dramatically. Pneumonectomies (n=9) were excluded for different physiology of the empty pleural space after the removal of the whole lung. The Institutional Review Board at Beijing Cancer Hospital provided ethical approval for the present retrospective study and patient consent was waived. The routine preoperative staging included computed tomography of the chest, brain magnetic resonance imaging (MRI), abdominal ultrasonography, bone scintigraphy or positron emission tomography/computed tomography (PET/CT). Fibrobronchoscopic biopsy was applied to central localized tumor. Pulmonary function testing and cardiac evaluation were required for preoperative assessment.
Surgical technique
Pulmonary resections, including lobectomy, bilobectomy and sleeve-lobectomy were selected on the basis of tumor location. SMLD was performed to each patient using a standard procedure according to the International Association for the Study of Lung Cancer (IASLC) (6).
Electric cautery was used to perform en-bloc dissection during operation and micro-vessels were cauterized and transected. Due to the existence of direct lymphatic communication between the pulmonary parenchyma and the mediastinum (7), some surgeons preferred to clip hilar/mediastinal lymphatic node pedicle and tube-like structures, such as micro-vessels, linked with surrounding anatomic landmarks by using clip applier during en-bloc dissection of lymphatic and fatty tissue (Figure 1). The application of clip applier was at the discretion of the attending surgeons.
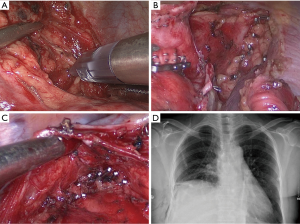
Measurements of pleural fluid were recorded at morning rounds. All of chest tube were always connected with water-sealed drainage bottles and no external suction system was applied. The criteria for chest tube removal were as follows: (I) drainage volume less than 200 mL in 24 hours; (II) absence of air leakage and intrathoracic hemorrhage; (III) absence of signs of purulent pleural effusion and atelectasis.
Statistical analysis
The pleural drainage volume was recorded each day after surgery until chest tube removal and the sum of each day’s volume was recorded as cumulative drainage volume. We calculated the propensity score by incorporating the variables that can potentially affect pleural drainage, including gender, age, operative side, upper/lower lobectomy, neoadjuvant chemotherapy, video-assisted thoracic surgery (VATS) approach, resection volume, pleural adhesion, duration of operation, mediastinal lymphadenectomy meets National Comprehensive Cancer Network (NCCN) criteria and complications such as chylothorax, cardiac arrhythmia, atelectasis, representing the probability of being assigned to either the group applying clip (group clip) or the group no clip was applied (group control). We matched propensity scores one to two using nearest neighbor methods, no replacement and 0.2 caliper width. Finally, matched 73 patients from group clip and 124 patients from group control were included in the analysis. The characteristics of both group clip and group control were compared before and after propensity score matching. Multivariable logistic regression analyses were performed for binary outcomes to predict the factors significantly associated with pleural drainage. Continuous variables were expressed as the mean ± standard deviation. Frequencies and percentages were expressed as categorical variables. The student’s t-test was used for the analysis of normally distributed data after the assurance of homogeneity by Lavene’s test. The Pearson’s chi-square (χ2) test was used to compare proportions (or the Fisher’s exact test as required). The P values for differences were calculated with a significance level of P<0.05. SPSS software (version 18.0; SPSS, Chicago, IL, USA) was used for all analysis.
Results
Demographic and perioperative data
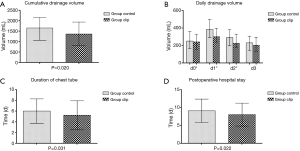
Three hundred and twenty-two cases met the inclusion criteria and entered the final analysis, which was divided into group clip and croup control according to applying clip or not. The demographic and perioperative variables of the patients in two groups were compared before and after propensity score matching in Table 1. In matched cohort, cumulative drainage volume of group clip was significantly less than that of group control (1,465.4±817.3 vs. 1,758.9±865.4 mL, P=0.020, Figure 2A). Group clip showed less daily drainage volume when compared with group control in the day of surgery, the first postoperative day and the second postoperative day (P<0.05). However, the difference was absent after the third operative day (Figure 2B). Meanwhile, group clip presented shorter duration of chest tube (5.2±2.7 vs. 6.0±2.3 d, P=0.031, Figure 2C) and postoperative hospital stay (8.0±3.2 vs. 9.1±3.3 d, P=0.022, Figure 2D) compared with group control.
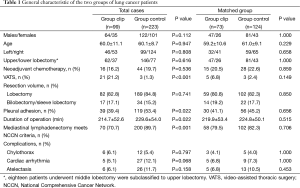
Full table
Multivariable logistic regression analysis for factors associated with high-output pleural drainage
Total postoperative pleural drainage was 1,639.8±840.1 mL in all cases. High-output was defined as pleural drainage exceeded 1,804 mL, which was 10% above the average. Risk factors significantly associated with high-output drainage in multivariable logistic regression analysis were being male (P=0.003), age >60 years (P=0.001), bilobectomy/sleeve lobectomy (P=0.032), pleural adhesion (P=0.009), the application of clip applier (P=0.005), duration of operation ≥220 minutes (P<0.001) and chylothorax (P=0.001) (Table 2).

Full table
The application of clip applier was the only factor that negatively correlated with high-output drainage [odds ratio (OR) 0.410; 95% confidence interval (CI): 0.221–0.763], as showed in Table 2.
Discussion
Pleural fluid filters from the capillaries of the parietal pleura and drains through the lymphatic stomata of the parietal pleura under normal physiological conditions (8). However, there is increasing evidence suggesting that stomata drainage coexists with absorption through the visceral mesothelium and electrolyte-coupled liquid absorption through both visceral and parietal mesothelial (9). An increase in pleural liquid volume after thoracic operations enhances the contractility of lymphatic smooth muscle, and causes an increase in lymphatic flow through the stomas, which could drain more than 20 times greater than that reported under physiological conditions (10). Although several mechanisms promote absorption of increased pleural liquid due to the thoracic surgery, it still seems a necessary procedure to place intercostal tubes postoperatively. Chest tubes could prevent pleural effusion and life-threatening tension pneumothorax, measure the blood loss and air leakage (11). However, chest tubes are associated with postoperative pain, functional limitation (1), risk of infection (12) and hinder pulmonary mechanics (13). The timing of tube removal is limited by several reasons, of which postoperative drainage volume might be the major rate-limiting factor.
In this multivariate analysis, we demonstrated that several clinical factors were notably linked with high-output drainage for this research group, such as male, age >60 years, bilobectomy/sleeve lobectomy, pleural adhesion, duration of operation ≥220 minutes and chylothorax. These factors might induce more pleural effusion after surgery based on their pathophysiological features. However, our purpose is to find any procedure which might help to reduce postoperative pleural effusion and remove the chest tube as soon as possible.
It is a novel finding that using micro-vessel clipping might reduce postoperative pleural drainage after lobectomy and lymph node dissection. As non-penetrating titanium clips provide a close apposition of the tissues without creating a suture tract and the use of titanium clips is easier and faster (14,15), it gradually changes to be a routine instrument at surgery. At the beginning of our practice, we clipped some fine vascular vessels to avoid potential bleeding after dissecting. With the accumulation of experience, we found there were also plenty of lymphatic networks in the mediastinum besides micro-blood vessels. En-bloc dissecting fatty-lymphatic tissue could produce a traumatic surface with severe exudation (16). Therefore, we clipped these tube-like structures as many as possible for effusion control after surgery. Sometimes, lymphatic node pedicle retracted when it was transected. Therefore, the lymphatic node pedicle was often clipped before transection in order to block micro-blood and lymphatic vessels exactly. Our results suggested that the application of clip applier was the negative factor which could reduce the odds of high-output drainage by 59%. Mediastinal micro-vessels clipping could also lead to a shorter chest tube duration and postoperative hospital stay in this study. These findings may be attributed to extensive lymphatic and vascular network existing in the mediastinum (17). After dissection, this network was transected and exudation from surgical surface could be obvious, especially in a drainage system with negative intrathoracic pressure. Blocking these micro-blood and lymphatic vessels with clip could occlude the network, thus then avoid exudation of lymph fluid or interstitial fluid. There was other research focusing on identifying patients at higher risk of developing a large pleural effusion (18). We suggest using clip-appliers in these patients who may develop postoperative high-output drainage under researchers’ speculation.
Titanium clips are not expected to dislodge in a magnetic field (19,20) and thus patients’ safety can be secure during MRI examination. In very rare cases, a tear in the great vessel due to friction of a titanium clip would cause major bleeding after surgery (21). Considering possible tissue excursions triggered by the heartbeat, all clips must be rechecked before closing incision to avoid abrasion to the vascular wall. If necessary, the option of local tamponade over clips using hemostatic sponge should be adopted to make vascular wall free from friction. In this group, no such cases were recorded after clip application.
Several methods could theoretically reduce pleural fluid, either by clipping or energy sealing the micro-vessels structure or by increasing intrathoracic pressure, such as intermittent chest tube clamping. There were some reports investigating the role of vessel sealing instruments, such as ultrasonic dissection device, bipolar sealing device and LigaSure on pleural fluid control. Kamiyoshihara et al. (22) found the drainage volume after using bipolar sealing device in right superior mediastinal lymph node dissection was same as the combination of clip applier and monopolar electrocautery. Further data is needed to elucidate the benefits and side-effects of these different methods on pleural effusion control.
Nevertheless, retrospective analysis and non-randomization are the major limitations of this study. Therefore, we used a propensity score-matched analysis to minimize selection bias. Although our investigation shows the tendency of superiority in using clip applier, we need prospective study to confirm these results. A randomized controlled trial will be suitable to compare the clip applier with vessel sealing instruments in the efficacy of reducing postoperative pleural drainage for further study.
Conclusions
In conclusion, NSCLC patients with postoperative high-output drainage present with certain clinical characteristics. The application of clip-applier could reduce cumulative drainage volume, and we suggest using clip-appliers in patients possessing risk factors of postoperative high-output drainage.
Acknowledgements
Funding: Beijing Municipal Science & Technology Commission (No. Z161100000516063). Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding support, Code: ZYLX201509; The Capital R&T Resources Platform (No. Z141100003414015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lima VP, Bonfim D, Risso TT, et al. Influence of pleural drainage on postoperative pain, vital capacity and six-minute walk test after pulmonary resection. J Bras Pneumol 2008;34:1003-7. [Crossref] [PubMed]
- Czerny M, Fleck T, Salat A, et al. Sealing of the mediastinum with a local hemostyptic agent reduces chest tube duration after complete mediastinal lymph node dissection for stage I and II non-small cell lung carcinoma. Ann Thorac Surg 2004;77:1028-32. [Crossref] [PubMed]
- Ng CS, Lee TW, Wan S, et al. Thoracotomy is associated with significantly more profound suppression in lymphocytes and natural killer cells than video-assisted thoracic surgery following major lung resections for cancer. J Invest Surg 2005;18:81-8. [Crossref] [PubMed]
- Merrigan BA, Winter DC, O'Sullivan GC. Chylothorax. Br J Surg 1997;84:15-20. [Crossref] [PubMed]
- Szczesny TJ, Slotwinski R, Stankiewicz A, et al. Interleukin 6 and interleukin 1 receptor antagonist as early markers of complications after lung cancer surgery. Eur J Cardiothorac Surg 2007;31:719-24. [Crossref] [PubMed]
- Goldstraw P, editor. IASLC Staging Manual in Thoracic Oncology. Orange Park: Editorial Rx Press, 2009:66-88.
- Riquet M, Hidden G, Debesse B. Thoracic duct collaterals of lymphatic and pulmonary origin. Anatomy and chylothorax after pulmonary surgery. Ann Chir 1989;43:646-57. [PubMed]
- Miserocchi G, Venturoli D, Negrini D, et al. Model of pleural fluid turnover. J Appl Physiol (1985) 1993;75:1798-806. [PubMed]
- Zocchi L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 2002;20:1545-58. [Crossref] [PubMed]
- Broaddus VC, Wiener-Kronish JP, Berthiaume Y, et al. Removal of pleural liquid and protein by lymphatics in awake sheep. J Appl Physiol (1985) 1988;64:384-90. [PubMed]
- Utter GH. The rate of pleural fluid drainage as a criterion for the timing of chest tube removal: theoretical and practical considerations. Ann Thorac Surg 2013;96:2262-7. [Crossref] [PubMed]
- Eren S, Esme H, Sehitogullari A, et al. The risk factors and management of posttraumatic empyema in trauma patients. Injury 2008;39:44-9. [Crossref] [PubMed]
- Abramov D, Yeshayahu M, Tsodikov V, et al. Timing of chest tube removal after coronary artery bypass surgery. J Card Surg 2005;20:142-6. [Crossref] [PubMed]
- Faulkner ND, Finn MA, Anderson PA. Hydrostatic comparison of nonpenetrating titanium clips versus conventional suture for repair of spinal durotomies. Spine (Phila Pa 1976) 2012;37:E535-9. [Crossref] [PubMed]
- Cope C, Lee K, Stern H, Pennington D. Use of the vascular closure staple clip applier for microvascular anastomosis in free-flap surgery. Plast Reconstr Surg 2000;106:107-10. [Crossref] [PubMed]
- Lagarde SM, Omloo JM, Ubbink DT, et al. Predictive factors associated with prolonged chest drain production after esophagectomy. Dis Esophagus 2007;20:24-8. [Crossref] [PubMed]
- Kosugi S, Kanda T, Yajima K, et al. Risk factors influencing the pleural drainage volume after transthoracic oesophagectomy. Eur J Cardiothorac Surg 2013;43:1116-20. [Crossref] [PubMed]
- Hristova R, Pompili C, Begum S, et al. An aggregate score to predict the risk of large pleural effusion after pulmonary lobectomy†. Eur J Cardiothorac Surg 2015;48:72-6. [Crossref] [PubMed]
- Becker RL, Norfray JF, Teitelbaum GP, et al. MR imaging in patients with intracranial aneurysm clips. AJNR Am J Neuroradiol 1988;9:885-9. [PubMed]
- Lawton MT, Ho JC, Bichard WD, et al. Titanium aneurysm clips: Part I--Mechanical, radiological, and biocompatibility testing. Neurosurgery 1996;38:1158-63; discussion 1164. [PubMed]
- Sîrbu H, Herse B, Busch T, et al. Pulmonary vein injury through repetitive clip friction: an unusual cause of hemothorax. Ann Thorac Surg 1998;65:548-50. [Crossref] [PubMed]
- Kamiyoshihara M, Igai H, Ibe T, et al. Right superior mediastinal lymph node dissection in thoracoscopic surgery using a bipolar sealing device. Innovations (Phila) 2013;8:258-63. [Crossref] [PubMed]


