
Middle to long-term outcomes of surgical repair for atrioventricular septal defect: a single-center study
Introduction
Atrioventricular septal defect (AVSD) is related to severe pulmonary hypertension and heart failure in early childhood. In 1966, Rastelli classified three types of AVSD based on anatomical structure: complete (CAVSD), transitional (TAVSD), and partial (PAVSD) (1). Developed surgical techniques and peri-operative management have reduced AVSD-related mortality in children to about 10% over the past few decades (2,3).
However, despite improved survival, left atrioventricular valve (LAVV) regurgitation may develop as a major event after surgery in 8% to 10% of patients, sometimes requiring re-operation (4,5). The surgical repair for AVSD still remains a great challenge in patients younger than three months, less than four kilograms, or with other genetic disorders (e.g., Down syndrome). This study aimed to evaluate the middle to long-term surgical outcomes in patients with three types of AVSD and identify the risk factors associated with mortality and LAVV re-operation. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-790/rc).
Methods
Patients
From January 2013 to December 2021, patients who underwent AVSD repair were recruited from the database of the Cardiovascular Center of the Children’s Hospital of Fudan University. Patients who had transposition of the great arteries, double outlet right ventricle, tetralogy of Fallot, or a single ventricle were excluded from the analysis. In total, 150 patients were included and divided into three groups according the Rastelli classification: Complete defect group (C-group), Transitional defect group (T-group), and Partial defect group (P-group). Age (≤3.0 months) and weight (≤4.0 kg) were defined according to the findings reported by other studies (2). Early mortality was defined as death within 30 days after corrective surgery. Echocardiography was regularly used to assess the severity of LAVV regurgitation (mild, moderate, severe) using the color Doppler jet area before and after surgery. The pulmonary arterial pressure was evaluated based on tricuspid regurgitation pressure gradient using echo and further classified as none (PH <30 mmHg), mild (30≤ PH <50 mmHg), moderate (50≤ PH <70 mmHg), and severe (≥70 mmHg) (6). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Ethics Review Board of the Children’s Hospital of Fudan University [No. (2022)132]. The need for patient consent was waived because of the retrospective nature of the study.
Study endpoints
The primary outcomes were set as time of death, freedom from overall re-operation, and LAVV re-operation. For patients who did not experience these outcomes, times were censored at the last follow-up date (December 31, 2021).
Data collection and follow-up
Demographic and surgical data were obtained from the Center. Follow-up data were collected from clinical examinations and echocardiography during outpatient clinics. The mean follow-up period of the entire cohort was 33.37±27.13 months (range, 1–100 months).
Statistical analysis
Mean ± standard deviation (SD) was used to describe the continuous variables in normal distribution, and median (interquartile range, IQR) to describe data that were not normally distributed. Categorical variables are presented as n (%). Differences among AVSD groups were analyzed using the Chi-squared test and one-way ANOVA analysis. Survival and freedom from LAVV re-operation were assessed using Kaplan-Meier survival analysis. The univariable Cox regression analysis was used to evaluate risk factors for mortality and LAVV re-operation: age ≤3.0 months, weight ≤4.0 kg, gender, Down syndrome, left atrium diameter (LAD) and left ventricular diastolic diameter (LVDD) before surgery, severe pulmonary hypertension before surgery, ≥ moderate LAVV regurgitation before surgery, cardiothoracic ratio (CTR) ≥0.6 before surgery and within 24 hours after surgery, re-operation after AVSD repair, LAVV re-operation, severe LAVV regurgitation within 24 hours after the first surgery, ≥ moderate LAVV regurgitation within 24 hours after the first surgery, and surgery era [2017–2021]. The results of the models are reported as hazard ratios (HR), 95% confidence intervals (CI), and P values. Variables with P<0.1 were then included in the multivariable Cox regression analysis. Statistical significance was set as P<0.05. All statistical analyses were carried out using the IBM SPSS statistics 25.0 on Windows (SPSS, Inc., Chicago, IL, USA). Figures were created using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA).
Surgical techniques
Surgical repair was performed via median sternotomy with full cardiopulmonary bypass (CPB) at moderate hypothermia. Upon cardiac arrest, the structure of the LAVV was re-observed to determine the type of AVSD and surgical approach. Simple cleft closure, as the dominant approach, was performed in 48 (71.6%), 19 (73.1%), and 40 (70.2%) patients, followed by cleft closure with annuloplasty strategy in 17 (25.4%), 5 (19.2%), and 15 (26.3%) patients, and no repair in two (3%), two (7.7%), and two (3.5%) patients in the C-group, T-group, and P-group, respectively.
CAVSD was repaired using the modified single-patch (MSP) technique in 57 patients and the double-patch (DP) technique in 10 patients. The MSP technique was performed with a patch of glutaraldehyde (GA)-fixed autologous pericardium. The DP technique was performed with a GA-fixed autologous pericardium for the repair of atrial septal defect (ASD), and a Dacron patch (n=5) or a GA-fixed autologous pericardium (n=5) for the repair of ventricular septal defect (VSD). For PAVSD, the ASD was mended using a GA-fixed autologous pericardium. For TAVSD, the VSD was closed directly using a 5-0 proline suture with pledget, and the ASD was closed using a GA-fixed autologous pericardium. As soon as the AVSD was corrected, LAVV function was observed with injection of saline. After releasing from CPB, the degree of LAVV regurgitation was re-assessed using transesophageal echocardiography. If the LAVV regurgitation was graded as severe or the intraoperative echocardiography showed left ventricular outflow tract stenosis, re-exploration was then planned.
Results
Baseline characteristics
The baseline characteristics of the total cohort are summarized in Table 1. There were 67 patients with CAVSD, 26 with TAVSD, and 57 with PAVSD. Among the 67 CAVSD patients, 66 patients (98.5%) presented type A (the common superior bridge valve is divided into two halves at the interventricular septum), and one patient (1.5%) presented type C (the superior valve bridge spans the interventricular septum and is not segmented). The median age at surgery was 6 months (IQR, 3–12.5) in the C-group, 20 months (IQR, 9–60) in the T-group, and 33 months (IQR, 11–48) in the P-group. The average weight in these three groups was 6.78±3.82, 12.98±10.04, and 13.88±8.28 kg, respectively. In total, 21 patients under three months old accepted AVSD repair, including 18 in the C-group, one in the T-group, and two in the P-group; 18 patients (10.2%) had Down syndrome (15 in the C-group, two in the T-group, and one in the P-group). The incidence of severe pulmonary hypertension was 34.3% (n=23) in the C-group, 11.5% (n=3) in the T-group, and 7.0% (n=4) in the P-group before surgery. Pre-operative detection showed that LAVV regurgitation was mild in 43, moderate in 45, and severe in 62 patients.
Table 1
| Characteristics | CAVSD | TAVSD | PAVSD | P value |
|---|---|---|---|---|
| Patient number | 67 | 26 | 57 | |
| Male, n (%) | 30 (44.8) | 9 (34.6) | 30 (53.6) | 0.300 |
| Patient age (months), median [IQR] | 6 [3–12.5] | 20 [9–60] | 33 [11–48] | <0.001 |
| Age ≤3.0 months, n (%) | 18 (26.9) | 1 (3.8) | 2 (3.5) | <0.001 |
| Patient weight (kg), mean ± SD | 6.78±3.82 | 12.98±10.04 | 13.88±8.28 | <0.001 |
| Weight ≤4.0 kg, n (%) | 3 (4.5) | 1 (3.7) | 0 (0) | 0.280 |
| Down syndrome, n (%) | 15 (22.4) | 2 (7.7) | 1 (1.8) | 0.002 |
| Rastelli classification type, n (%) | ||||
| A | 66 (98.5) | |||
| B | 0 (0) | |||
| C | 1 (1.5) | |||
| CTR, mean ± SD | 0.650±0.049 | 0.616±0.059 | 0.616±0.074 | 0.004 |
| Previous surgery, n (%) | 1 (1.5) | 0 (0) | 0 (0) | 0.536 |
| Pulmonary hypertension, n (%) | 60 (89.6) | 18 (69.2) | 46 (80.7) | <0.001 |
| Mild | 11 (16.4) | 8 (30.8) | 29 (50.9) | |
| Moderate | 26 (38.8) | 7 (26.9) | 13 (22.8) | |
| Severe | 23 (34.3) | 3 (11.5) | 4 (7.0) | |
| Left AV valve regurgitation, n (%) | ||||
| Mild | 16 (23.9) | 9 (34.6) | 18 (31.6) | |
| Moderate | 22 (32.8) | 6 (23.1) | 17 (29.8) | |
| Severe | 29 (43.3) | 11 (42.3) | 22 (38.6) |
Data are reported as mean ± SD, median [IQR], or n (%). CAVSD, complete atrioventricular septal defect; TAVSD, transitional atrioventricular septal defect; PAVSD, partial atrioventricular septal defect; IQR, interquartile range; SD, standard deviation; CTR, cardiothoracic ratio; AV, atrioventricular.
Peri-operative data
The peri-operative data are summarized in Table 2. In the entire cohort, the mean CPB time was 95.41±60.54 minutes, the mean cross-clamp time was 56.89±27.36 minutes, and the mean postoperative length of stay (LOS) was 15.61±10.78 days. CPB temperature was controlled at an average of 32.44±1.92 ℃. After surgery, the median mechanical ventilation time was 27.5 (61.63) hours and the median of LOS in the intensive care unit (ICU) was 3.5 [2–7] days. CPB time (111.7±63.3 vs. 84.9±22.7, 81.0±64.9 minutes; P=0.011), cross-clamp time (69.5±31.7 vs. 52.8±16.4 and 43.7±18.0 minutes; P<0.001), postoperative LOS (20.6±12.6 vs. 13.0±7.6 and 10.9±6.4; P<0.001), postoperative mechanical ventilation time {71 [42.75–130] vs. 24.5 [19–47.25] and 11.0 [6–24] hours; P<0.001}, and postoperative LOS in the ICU {6 [5–11] vs. 3 [2–4] and 2 [1–3] days; P<0.001} were significantly longer in the C-group compared to both the T-group and P-group. Furthermore, the incidence of complications was 28.4% (19/67) in the C-group, which was significantly higher than 7.7% (2/26) in the T-group and 8.8% (5/57) in the P-group (P=0.005). Among the complications, pulmonary hypertensive crisis occurred most frequently, with seven in the C-group, one in the T-group, and two in the P-group.
Table 2
| Variables | CAVSD | TAVSD | PAVSD | P value |
|---|---|---|---|---|
| Cleft closure, n (%) | ||||
| Simple cleft closure | 48 (71.6) | 19 (73.1) | 40 (70.2) | |
| Cleft closure + annuloplasty | 17 (25.4) | 5 (19.2) | 15 (26.3) | |
| Not to repair, n (%) | 2 (3.0) | 2 (7.7) | 2 (3.5) | |
| CPB time (min), mean ± SD | 111.7±63.3 | 84.9±22.7 | 81.0±64.9 | 0.011 |
| Cross-clamp time (min), mean ± SD | 69.5±31.7 | 52.8±16.4 | 43.7±18.0 | <0.001 |
| CPB temperature (℃), mean ± SD | 31.2±1.9 | 32.7±1.3 | 33.7±1.2 | <0.001 |
| Postoperative mechanical ventilation time, median (h), [IQR] | 71 [42.75–130] | 24.5 [19–47.25] | 11.0 [6–24] | <0.001 |
| ICU stay after surgery (days), median [IQR] | 6 [5–11] | 3 [2–4] | 2 [1–3] | <0.001 |
| Postoperative hospital stay (days), mean ± SD | 20.6±12.6 | 13.0±7.6 | 10.9±6.4 | <0.001 |
| Complications, n (%) | 19 (28.4) | 2 (7.7) | 5 (8.8) | 0.005 |
| Delayed chest closure | 3 | 0 | 2 | |
| Respiratory distress | 5 | 0 | 1 | |
| AV block need pacemaker implantation | 3 | 0 | 1 | |
| Pulmonary hypertensive crisis | 7 | 1 | 2 | |
| Pneumothorax | 1 | 0 | 0 | |
| Peritoneal dialysis/hemodialysis | 0 | 0 | 1 | |
| Total in-hospital mortality, n (%) | 7 (10.9) | 0 (0) | 1 (1.8) | 0.034 |
| LAVV reoperation, n (%) | 7 (10.4) | 3 (11.5) | 2 (3.5) | 0.284 |
Data are reported as mean ± SD, median [IQR], or n (%). CAVSD, complete atrioventricular septal defect; TAVSD, transitional atrioventricular septal defect; PAVSD, partial atrioventricular septal defect; CPB, cardiopulmonary bypass; ICU, intensive care unit; SD, standard deviation; IQR, interquartile range; AV, atrioventricular; LAVV, left atrioventricular valve.
Survival analysis
The 1-, 3-, and 5-year survival rates were, respectively, 94.5%±1.9%, 94.5%±1.9%, and 94.5%±1.9% in the entire cohort, 88.9%±4.0%, 88.9%±4.0%, and 88.9%±4.0% in the C-group, 100%, 100%, and 100% in the T-group, and 98.2%±1.7%, 98.2%±1.7%, and 98.2%±1.7% in the P-group. Statistical significance was observed for survival rate among the three groups (P=0.0404). The Kaplan-Meier survival curves for the three AVSD groups are presented in Figure 1.
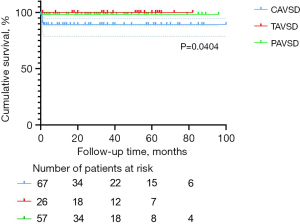
Mortality
The mortality was 5.33% (8/150) in the entire cohort, including seven early deaths (6 in the C-group) and one late death. The 7 (87.5%) deaths included four patients younger than three months in the C-group and 1 (12.5%) in the P-group (Table 2, P=0.034). The two major causes of mortality were cardiopulmonary failure (n=5) and heart failure (n=3). In these patients, 5 (62.5%) were diagnosed with severe pulmonary hypertension, and 7 (87.5%) with ≥ moderate LAVV regurgitation prior to surgery. One patient was treated with mechanical ventilation and one with continuous positive airway pressure (CPAP) therapy due to shortness of breath and poor response during preoperative preparation. Five patients showed recurrent pulmonary infection before surgery, and four showed significant developmental delay. The majority of LAVV in these patients were difficult to repair, causing unsatisfied valve function after surgery. Two patients were diagnosed with severe regurgitation within the first 24 hours after surgery, five with moderate, and only one with mild. Three patients underwent re-operation, including one with moderate LAVV regurgitation and difficulty weaning off the ventilator, one with postoperative mediastinal infection, and one with left ventricular outflow tract stenosis. The details of in-hospital mortality are listed in Table 3.
Table 3
| Variables | Age | Weight (kg) | Preoperative PH | Preoperative left AV valve regurgitation | Postoperative left AV valve regurgitation | Cause of reoperation | LAVV regurgitation after reoperation | Death stage | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| CAVSD | |||||||||
| 1 | 3 mon | 5.5 | Severe | Moderate | Moderate | No | – | 20 days after operation | Cardiopulmonary failure + ventricular fibrillation |
| 2 | 5 mon | 6.6 | Moderate | Severe | Moderate | Moderate LAVV regurgitation + difficulty weaning off the ventilator | Moderate | 17 days after first operation/2 days after reoperation | Cardiopulmonary failure |
| 3 | 3 mon | 4.8 | Severe | Mild | Moderate | No | – | 41 days after operation | Cardiopulmonary failure |
| 4 | 15 mon | 8.66 | Severe | Severe | Moderate | No | – | 28 days after operation | Cardiopulmonary failure |
| 5 | 1 mon | 2.74 | Severe | Severe | Mild | Mediastinal infection | Mild | 22 days after first operation/7 days after reoperation | Cardiopulmonary failure |
| 6 | 3 mon | 4.3 | Moderate | Moderate | Moderate | Left ventricular outflow tract stenosis | Severe | 42 days after first operation/41 days after reoperation | Heart failure + fungal sepsis |
| 7 | 4 mon | 6.1 | Moderate | Severe | Severe | No | – | 10 hours after operation | Heart failure + ventricular fibrillation |
| PAVSD | |||||||||
| 1 | 24 mon | 11 | Severe | Severe | Severe | No | – | Intraoperative death | Heart failure |
PH, pulmonary hypertension; AV, atrioventricular; LAVV, left atrioventricular valve; CAVSD, complete atrioventricular septal defect; mon, month; PAVSD, partial atrioventricular septal defect.
Predictors of mortality
The univariable Cox regression analysis revealed that age ≤3.0 months, severe pulmonary hypertension before surgery, re-operation after AVSD repair, and severe LAVV regurgitation within 24 hours after the first surgery were predictors of mortality (Table 4). In the multivariate Cox regression analysis, severe pulmonary hypertension before surgery (P=0.006) and severe LAVV regurgitation within 24 hours after the first surgery (P=0.023) were independent risk factors for mortality (Table 4), while age ≤3.0 months was not (P=0.222).
Table 4
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age ≤3.0 months | 6.299 | 1.58–25.19 | 0.009 | 2.564 | 0.57–11.62 | 0.222 | |
| Weight ≤4.0 kg | 5.145 | 0.63–41.83 | 0.126 | ||||
| Gender (male =0) | 0.869 | 0.22–3.47 | 0.842 | ||||
| Down syndrome | 1.000 | 0.12–8.13 | 0.999 | ||||
| LAD before surgery | 1.048 | 0.98–1.12 | 0.184 | ||||
| LVDD before surgery | 0.930 | 0.82–1.06 | 0.263 | ||||
| Severe pulmonary hypertension before surgery | 7.032 | 1.68–29.45 | 0.008 | 12.139 | 2.03–72.47 | 0.006 | |
| ≥ Moderate LAVV regurgitation before surgery | 2.886 | 0.36–23.46 | 0.322 | ||||
| CTR ≥0.6 before surgery | 35.97 | 0.07–20,016.56 | 0.267 | ||||
| CTR ≥0.6 within 24 hours after surgery | 1.030 | 0.26–4.12 | 0.966 | ||||
| Reoperation after AVSD repair | 4.686 | 1.12–19.61 | 0.034 | 2.517 | 0.53–12.01 | 0.247 | |
| LAVV reoperation | 2.038 | 0.25–16.57 | 0.506 | ||||
| Severe LAVV regurgitation at first 24 hours after the first surgery | 10.63 | 2.14–52.85 | 0.004 | 12.07 | 1.42–102.90 | 0.023 | |
| Surgical era [2017–2021] | 0.498 | 0.10–2.47 | 0.394 | ||||
HR, hazard ratio; CI, confidence interval; LAD, left atrium diameter; LVDD, left ventricular diastolic diameter; LAVV, left atrioventricular valve; CTR, cardiothoracic ratio; AVSD, atrioventricular septal defect.
Overall re-operation and LAVV re-operation
Freedom from re-operation and LAVV re-operation was associated with good outcomes in the entire cohort. Nineteen patients (12.7%) required re-operation after the initial repair of AVSD, including 12 in the C-group, three in the T-group, and four in the P-group. The main reason for re-operation was severe LAVV regurgitation after surgery (n=11, 57.9%). Among these 11 patients, 10 received LAVV repair and one received LAVV repair with annuloplasty. Other indications for re-operations were AV (atrioventricular) block (n=4, 21.1%), mediastinal infection (n=2, 10.5%), moderate LAVV regurgitation with difficulty weaning off the ventilator (n=1, 5.3%), and left ventricular outflow tract stenosis (n=1, 5.3%). The percentages of patients free from overall re-operation at 1, 3, and 5 years were, respectively, 90.9%±2.4%, 87.8%±2.9%, and 81.2%±4.6% in the entire cohort; 85.4%±4.6%, 82.8%±5.1%, and 71.6%±8.6% in the C-group; 96.2%±3.8%, 90.8%±6.3%, and 84.3%±8.6% in the T-group; 94.7%±3.0%, 92.3%±3.7%, and 92.3%±3.7% in the P-group. These percentages did not differ among AVSD types (P=0.1251; Figure 2), but the C-group had a relatively higher percentage. The percentages of patients free from LAVV re-operation at 1, 3, and 5 years were, respectively, 95.7%±1.7%, 92.5%±2.5%, and 85.9%±4.4% in the entire cohort; 93.1%±3.4%, 90.3%±4.3%, and 79.6%±8.1% in the C-group; 96.2%±3.8%, 90.8%±6.3%, and 84.3%±8.6% in the T-group; 98.2%±1.8%, 95.9%±2.9%, and 95.9%±2.9% in the P-group. These percentages showed no statistical difference related to AVSD type (P=0.2921, Figure 3).
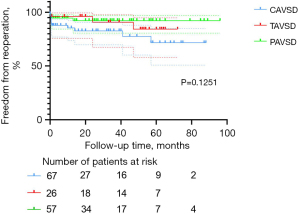
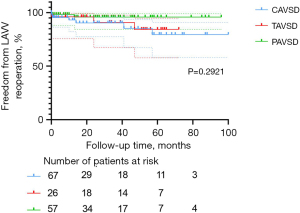
Predictors of LAVV re-operation
On the basis of univariable Cox regression analysis, CTR ≥0.6 within 24 hours after surgery, and ≥ moderate LAVV regurgitation within 24 hours after surgery predicted a higher rate of LAVV re-operation (Table 5). The multivariable Cox regression analysis showed ≥ moderate LAVV regurgitation within 24 hours after surgery (P=0.014, Table 5) was the independent risk factor of LAVV re-operation.
Table 5
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age ≤3.0 months | 1.356 | 0.30–6.20 | 0.695 | ||||
| Weight ≤4.0 kg | 3.379 | 0.43–26.45 | 0.246 | ||||
| Gender (male =0) | 2.062 | 0.62–6.87 | 0.239 | ||||
| Down syndrome | 0.040 | 0.000024–68.17 | 0.397 | ||||
| LAD before surgery | 1.033 | 0.96–1.11 | 0.403 | ||||
| LVDD before surgery | 0.932 | 0.84–1.04 | 0.193 | ||||
| Severe pulmonary hypertension before surgery | 0.413 | 0.05–3.21 | 0.398 | ||||
| CTR ≥0.6 before surgery | 2.515 | 0.55–11.50 | 0.234 | ||||
| CTR ≥0.6 at first 24 hours after surgery | 3.095 | 0.84–11.44 | 0.090 | 2.303 | 0.62–8.63 | 0.216 | |
| ≥ Moderate LAVV regurgitation before surgery | 4.571 | 0.59–35.43 | 0.146 | ||||
| ≥ Moderate LAVV regurgitation within 24 hours after the first surgery | 7.875 | 1.72–35.98 | 0.008 | 6.874 | 1.49–31.78 | 0.014 | |
| Surgical era [2017–2021] | 0.186 | 0.02–1.47 | 0.111 | ||||
AV, atrioventricular; HR, hazard ratio; CI, confidence interval; LAD, left atrium diameter; LVDD, left ventricular diastolic diameter; CTR, cardiothoracic ratio; LAVV, left atrioventricular valve.
Impact of pulmonary hypertension analyzed at 3 months of age intervals
The results of patients who had severe PH before the first surgery are displayed in Table 6. These data reflect measurements attained at 3 months of age intervals. Severe pulmonary hypertension before surgery was found most frequently in patient under 3 months of age (n=8), between 3–6 months of age (n=5), and beyond 12 months of age (n=13) compared with patients between 6–9 months of age (n=3) and 9–12 months of age (n=1). Among these patients with pre-operative severe pulmonary hypertension, postoperative pulmonary hypertensive crisis occurred in one patient under 3 months of age, two between 3–6 months of age, and three beyond 12 months of age. Three patients under 3 months of age and two beyond 12 months of age eventually died.
Table 6
| Age | <3 months (n=21) | 3–6 months (n=31) | 6–9 months (n=15) | 9–12 months (n=11) | >12 months (n=72) |
|---|---|---|---|---|---|
| Severe PH before first surgery, n (%) | 8 (38.1) | 5 (16.1) | 3 (20.0) | 1 (9.1) | 13 (18.1) |
| Postoperative pulmonary hypertensive crisis#, n (%) | 1 (12.5) | 2 (40.0) | 0 (0) | 0 (0) | 3 (23.1) |
| Death#, n (%) | 3 (37.5) | 0 (0) | 0 (0) | 0 (0) | 2 (15.4) |
#, the number of patients with postoperative pulmonary hypertensive crisis and death were derived from those with severe PH before the first surgery. PH, pulmonary hypertension.
Discussion
Our mid to long-term results demonstrated a low mortality (5.44%) and an acceptable overall re-operation rate (12.7%) after AVSD repair, which are consistent with the 2.95–15% mortality (7) and 3.6–22% re-operation rate (8) reported by Hoohenkerk et al. and Ginde et al. Patients in the CAVSD group had a higher mortality of 10.4% (7/67), including six early deaths. It is interesting to note that no significant difference was found in freedom from LAVV re-operation among three AVSD types. We further evaluated the effect of age (≤3 months), severe pulmonary hypertension before surgery, severe LAVV regurgitation within 24 hours after the first surgery, AVSD type on mortality, and ≥ moderate LAVV regurgitation within 24 hours after the first surgery, AVSD types and surgical era [2017–2021] on LAVV re-operation rate. The impact of pulmonary hypertension was also analyzed in patients at 3 months of age intervals.
Mortality
No consensus has been reached about whether AVSD repair can increase mortality in patients ≤3 months of age. It is recommended to perform surgery in children who have symptoms of congestive heart failure at an age of 3–6 months (9). This recommendation is supported by Delmo Walter et al., who pointed out that surgery in younger patients can increase mortality, as their LAVV is relatively fragile and easy to tear, and the surgical exposure during surgery is limited (10). In our cohort, eight patients died after surgery, including four who were ≤3 months of age. The univariable analysis depicted a higher mortality in patients aged ≤3 months (Table 4, P=0.009), but this change was not statistically significant in the multivariable analysis (Table 4, P=0.222). This result was inconsistent with that reported by Ramgren et al. (11), which might be due to the fact that the surgeries in the younger patients were quite difficult, but getting part of these sick patients improved and survived. Fong et al. found decreased postoperative mortality over time and a better late survival in patients ≤3 months of age (12). Even for the complex CAVSD repair, complete repair performed on patients ≤3 months of age and weighing ≤3.5 kg was successful, with a 20-year survival rate of 92.0% and 83.8%, respectively, as reported by Buratto et al. and Goutallier et al. (3,13). On the basis of valvular morphology, individualized surgical strategies can be designed to improve the outcomes of AVSD patients.
In the present study, the multivariable Cox regression analysis revealed severe pulmonary hypertension before surgery as an independent risk factor for postoperative mortality (HR =12.139, P=0.006). This finding was also supported by the Kaplan-Meier survival plot (Figure 4, P=0.0017). In our cohort, five of the eight patients who died had pre-operative severe pulmonary hypertension, most of whom also presented with severe LAVV regurgitation. It is also worth noting that pre-operative severe pulmonary hypertension and surgical stimulation may induce intra-operative pulmonary vasospasm, which might increase pulmonary circulatory resistance and, therefore, lead to pulmonary hypertensive crisis (2). In our study, incidence of pulmonary hypertensive crisis was significantly higher in the C-group (7/67, 10.4%) than in the T-group (1/26, 3.8%) and P-group (2/57, 3.5%). Preoperative and postoperative management should be further optimized to prevent pulmonary hypertensive crisis, especially in emergencies.
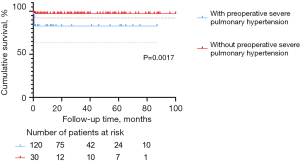
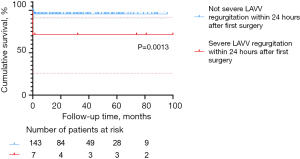
According to the multivariable Cox regression analysis and Kaplan-Meier survival plot, severe LAVV regurgitation within 24 hours after the first surgery remains a major life-threatening event (Table 4, P=0.023; Figure 5, P=0.0013). Postoperative severe LAVV regurgitation may aggravate pulmonary hypertension, thus leading to cardiac insufficiency, and even early death. Therefore, great caution should be taken in placing traction sutures to achieve good exposure of the surgical field, as well as pulling the valve to avoid severe LAVV regurgitation due to valve rupture. In addition, the operator should repair the valve as delicately as possible to reduce the degree of regurgitation to below moderate levels. Close monitoring of LAVV regurgitation is also required after surgery. As shown in Table 2 and Figure 1, the in-hospital mortality in the C-group was significantly higher than in the other groups, which corroborates the findings by Schleiger et al. (2). This difference in mortality could be attributed to the following reasons. First, surgical repair of CAVSD was more technically challenging than TAVSD and PAVSD, resulting in longer CPB time (P=0.011), cross-clamp time (P<0.001), postoperative mechanical ventilation time (P<0.001), LOS in ICU (P<0.001), and postoperative LOS in the hospital (P<0.001). Moreover, complications in seven CAVSD patients who died were much more complex and intractable, characterized by respiratory distress (5/7), pulmonary hypertensive crisis (2/7), arrhythmia (2/7), infections (2/7), delayed chest closure (1/7), and acute renal failure requiring peritoneal dialysis/hemodialysis (1/7). These findings are in line with those reported by Jacobs, who analyzed 2,882 AVSD operations in the STS Congenital Heart Surgery Database (14). Therefore, early postoperative care should be focused on multiple organ dysfunction and CAVSD complications.
LAVV re-operation
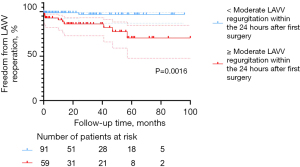
The LAVV re-operation rate increases as LAVV function decreases. The main factor affecting LAVV re-operation is LAVV regurgitation. At the final follow-up, the percentage of patients with severe LAVV regurgitation was higher in the C-group (n=16, 23.9%) than in the T-group (n=4, 15.4%) and P-group (n=7, 12.3%). The re-operation rate of LAVV was 8.0% (12/150) in the entire cohort, with seven patients in the C-group, three in the T-group, and two in the P-group. This result is in accordance with finding by Mery et al. and Airaksinen et al. (4,5). According to the multivariable Cox regression analysis, ≥ moderate LAVV regurgitation within 24 hours after surgery (P=0.014) was an independent risk factor for LAVV re-operation, which is consistent with the conclusion drawn by Fong et al. (12). The finding was also displayed in the Kaplan-Meier survival plot (Figure 6, P=0.0016). Interestingly, there was no significant difference in freedom from LAVV re-operation among the three groups (P=0.2921, Figure 3), suggesting approximate LAVV re-operation rates of different AVSD types. As suggested by Sarısoy et al., this phenomenon may have arisen from the low incidence of Down syndrome in the T-group and P-group (15-17). In patients without Down syndrome, the morphology of the LAVV is more dysplastic and no redundant valve tissues can be used for LAVV reconstruction (15,16), which may lead to residual regurgitation. During our follow-up period, five patients with TAVSD or PAVSD who underwent LAVV re-operation did not have Down syndrome. Our conclusion was also supported by previous findings of Lange et al. (15), who reported a higher rate of freedom from LAVV re-operation in the group with Down syndrome (82%±2.9%) than in the group without Down syndrome (72%±5.3%) during 20 years of follow-up (P=0.004).
Surgical era [2017–2021] was not an independent risk factor for LAVV re-operation in the univariable Cox regression analysis (P=0.111, Table 5). In recent years, annuloplasty has been conventionally adopted in our center. In the present study, 15 of 16 patients receiving annuloplasty did not have to undergo LAVV re-operation during the follow-up. In these 16 patients, nine had severe LAVV regurgitation before surgery. At the last follow-up after annuloplasty, 11 patients had mild or moderate LAVV regurgitation, which demonstrates the good outcomes of this technique. According to Komoda et al. and Fundarò et al., the application of annuloplasty, especially Wooler annuloplasty, can substantially reduce the risk of LAVV regurgitation, which was confirmed by our statistics (18,19).
Impact of pulmonary hypertension on children of different intervals of age
The proportion of patients with severe pulmonary hypertension before surgery was highest in patients under 3 months of age, which may be related to the large left-to-right shunt, LAVV regurgitation, and the unreduced physiological pulmonary hypertension. It was also interesting to note that the number of patients with pre-operative severe pulmonary hypertension had a bimodal distribution, mostly in those under 6 months and beyond 12 months of age (Table 6). Those with pre-operative severe pulmonary hypertension in the two age distributions were more likely to face postoperative pulmonary hypertensive crisis (n=6) and progress to death (n=5), compared with none between 6–9 and 9–12 months of age. This indicates that, due to the evolution of hemodynamics, more positive measures should be taken for children under 6 months and beyond 12 months of age to avoid poor prognosis.
Limitations
The most important limitation of this study lies in that it was a retrospective study carried out in a single center with a limited number of patients. The number of patients ≤3 months of age and weighing ≤4 kg was relatively small, which challenges risk factor analysis, and the results from the analysis might have deviations.
Conclusions
Mortality was higher after surgical repair in CAVSD patients, while freedom from re-operation and LAVV re-operation rates were similar among different AVSD types. Severe pulmonary hypertension before surgery and severe LAVV regurgitation within 24 hours after the first surgery were risk factors for mortality. ≥ Moderate LAVV regurgitation within 24 hours after the first surgery was a risk factor for LAVV re-operation.
Acknowledgments
We would like to thank Medjaden Inc for the help in polishing our paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-790/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-790/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-790/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-790/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Board of the Children’s Hospital of Fudan University [No. (2022)132]. The need for patient consent was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rastelli G, Kirklin JW, Titus JL. Anatomic observations on complete form of persistent common atrioventricular canal with special reference to atrioventricular valves. Mayo Clin Proc 1966;41:296-308. [PubMed]
- Schleiger A, Miera O, Peters B, et al. Long-term results after surgical repair of atrioventricular septal defect. Interact Cardiovasc Thorac Surg 2019;28:789-96. [Crossref] [PubMed]
- Goutallier CS, Buratto E, Schulz A, et al. Repair of complete atrioventricular septal defect between 2 and 3.5 kilograms: Defining the limits of safe repair. J Thorac Cardiovasc Surg 2022;164:1167-75. [Crossref] [PubMed]
- Mery CM, Zea-Vera R, Chacon-Portillo MA, et al. Contemporary Outcomes After Repair of Isolated and Complex Complete Atrioventricular Septal Defect. Ann Thorac Surg 2018;106:1429-37. [Crossref] [PubMed]
- Airaksinen R, Mattila I, Jokinen E, et al. Complete Atrioventricular Septal Defect: Evolution of Results in a Single Center During 50 Years. Ann Thorac Surg 2019;107:1824-30. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Hoohenkerk GJ, Bruggemans EF, Rijlaarsdam M, et al. More than 30 years' experience with surgical correction of atrioventricular septal defects. Ann Thorac Surg 2010;90:1554-61. [Crossref] [PubMed]
- Ginde S, Lam J, Hill GD, et al. Long-term outcomes after surgical repair of complete atrioventricular septal defect. J Thorac Cardiovasc Surg 2015;150:369-74. [Crossref] [PubMed]
- Atz AM, Hawkins JA, Lu M, et al. Surgical management of complete atrioventricular septal defect: associations with surgical technique, age, and trisomy 21. J Thorac Cardiovasc Surg 2011;141:1371-9. [Crossref] [PubMed]
- Delmo Walter EM, Ewert P, Hetzer R, et al. Biventricular repair in children with complete atrioventricular septal defect and a small left ventricle. Eur J Cardiothorac Surg 2008;33:40-7. [Crossref] [PubMed]
- Ramgren JJ, Nozohoor S, Zindovic I, et al. Long-term outcome after early repair of complete atrioventricular septal defect in young infants. J Thorac Cardiovasc Surg 2021;161:2145-53. [Crossref] [PubMed]
- Fong LS, Betts K, Ayer J, et al. Predictors of reoperation and mortality after complete atrioventricular septal defect repair. Eur J Cardiothorac Surg 2021;61:45-53. [Crossref] [PubMed]
- Buratto E, Hu T, Lui A, et al. Early repair of complete atrioventricular septal defect has better survival than staged repair after pulmonary artery banding: A propensity score-matched study. J Thorac Cardiovasc Surg 2021;161:1594-601. [Crossref] [PubMed]
- Jacobs JP, Jacobs ML, Mavroudis C, et al. Atrioventricular septal defects: lessons learned about patterns of practice and outcomes from the congenital heart surgery database of the society of thoracic surgeons. World J Pediatr Congenit Heart Surg 2010;1:68-77. [Crossref] [PubMed]
- Lange R, Guenther T, Busch R, et al. The presence of Down syndrome is not a risk factor in complete atrioventricular septal defect repair. J Thorac Cardiovasc Surg 2007;134:304-10. [Crossref] [PubMed]
- Al-Hay AA, MacNeill SJ, Yacoub M, et al. Complete atrioventricular septal defect, Down syndrome, and surgical outcome: risk factors. Ann Thorac Surg 2003;75:412-21. [Crossref] [PubMed]
- Sarısoy Ö, Ayabakan C, Tokel K, et al. Long-term outcomes in patients who underwent surgical correction for atrioventricular septal defect. Anatol J Cardiol 2018;20:229-34. [Crossref] [PubMed]
- Komoda T, Huebler M, Berger F, et al. Growth of mitral annulus in the pediatric patient after suture annuloplasty of the entire posterior mitral annulus. Interact Cardiovasc Thorac Surg 2009;9:354-6. [Crossref] [PubMed]
- Fundarò P, Tartara PM, Villa E, et al. Mitral valve repair: is there still a place for suture annuloplasty? Asian Cardiovasc Thorac Ann 2007;15:351-8. [Crossref] [PubMed]


