
Intestinal barrier dysfunction is involved in the development of systemic inflammatory responses and lung injury in type A aortic dissection: a case-control study
Introduction
The annual incidence of aortic dissection is approximately 3.5/100,000 (1), equating to an estimated 50,000 new cases every year in China. Type A aortic dissection (TAAD) is the most common aortic catastrophe, with an extremely high lethality rate of 1–2% per hour after symptom onset and 74% within 2 weeks in untreated patients (2). Acute lung injury is a serious complication of acute TAAD, which occurs in approximately 50% of patients and can be life-threatening in severe cases (3-5). Some studies have revealed the significant involvement of systemic inflammatory responses in the development of acute lung injury in patients with acute TAAD (3-11). It was generally believed that long-term chronic inflammation in the aortic wall, contributing greatly to aortic wall remodeling (similar to the main histological finding described in aortic aneurysm), rapidly progresses to acute systemic inflammatory responses when acute aortic dissection occurs (5,12-15). Recently, a study found that intestinal barrier dysfunction can occur in patients with acute TAAD and may be important for the development of systemic inflammatory responses and acute lung injury (16). However, gaps remain in our knowledge as to whether the levels of systemic inflammatory responses and intestinal barrier dysfunction are different between patients with acute TAAD and non-acute aortic diseases, such as aortic aneurysm and non-acute TAAD, in which acute lung injury rarely occurs. In this study, we investigated intestinal injury, lung injury, and systemic inflammatory responses in patients with acute TAAD, non-acute TAAD, and aortic root aneurysm (ARA), and their relationships with each other, in order to explore the mechanism underlying intestinal injury, lung injury, and systemic inflammatory responses in patients with TAAD. We believe that this study may be helpful for the development of future therapeutic strategies for acute lung injury in patients with acute TAAD, regarding to the protection for intestinal barrier function. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1122/rc).
Methods
Study population and sample collection
This was a single-center, observational case-control study. From January 2016 to April 2016, 42 patients with TAAD and 36 patients with ARA were admitted to our institution. TAAD and ARA were diagnosed based on clinical symptoms, echocardiography, and aortic computed tomography angiography. TAAD was defined as an intimal tear and double lumen separated by the septum involving the ascending aorta, and ARA was defined as an aortic root of greater than 4.5 cm in diameter (1). The acute phase of dissection was defined as the time from symptom onset to admission within 14 days. The exclusion criteria included renal insufficiency, autoimmune diseases, lung diseases, gastrointestinal diseases, liver diseases, or malperfusion of the heart, brain, gastrointestinal tract, liver, pancreas, kidney, or limbs. Of the patients admitted, 5 patients refused to participate in the study, and 7 patients met at least 1 of the exclusion criteria. The remaining 36 patients with TAAD and 30 patients with ARA were enrolled in this study. Of the 36 patients with TAAD, 18 had acute TAAD (≤14 days), while the remaining 18 patients had non-acute (>14 days) TAAD. Blood samples were collected on admission, from which serum samples were obtained after centrifugation and stored at −80 ℃. Serum samples pertaining to TAAD and ARA were always analyzed together in the same batch and laboratory personnel were unable to distinguish among TAAD and ARA samples. In order to reduce selection bias, all cases of TAAD and ARA were reviewed by 2 experienced cardiologists, using diagnostic criteria and exclusion criteria, to ensure appropriate inclusion of cases. Disagreements were noted and discussed between reviewers. The minimum sample size that we needed was 9 cases for each group, with 80% power and 5% significance. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. GZR 046), and written informed consent was obtained from all patients and/or immediate relatives.
Data collection and measurements
Baseline study population characteristics
Patient data were obtained by reviewing medical records, including age, sex, history of smoking, hypertension, diabetes, coronary heart disease, and cerebral infarction. Admission biochemical measurements were also obtained, including creatinine (Cr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), D-dimer, and lactic acid levels.
Systemic inflammatory response levels
Admission white blood cell (WBC) parameters, including WBC count, neutrophil count, and neutrophil percentage, were obtained by reviewing medical records. Admission inflammatory cytokines [interleukin (IL)-6, IL-8, tumor necrosis factor α (TNF-α), C-reactive protein (CRP), and histamine (HIS)] were measured in serum collected as described above using the enzyme-linked immunosorbent assay technique (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions.
Lung injury, intestinal injury, and bacteremia levels
Lung injury extent was determined by reviewing the admission PaO2-FiO2 ratio in the medical records. The level of intestinal injury was assessed by measuring diamine oxidase (DAO) activity and the concentration of intestinal fatty acid binding protein (iFABP), and bacteremia level was determined by measuring peptidoglycan (PGN) concentration in serum collected as described above. DAO activity was determined by spectrophotography (Sigma-Aldrich, Louis, MO, USA), and iFABP and PGN were measured using enzyme-linked immunosorbent assays (R&D Systems), according to the manufacturer’s instructions.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and the tests of normality of continuous outcomes were performed. Independent sample t-tests or t'-tests were used for comparisons between the TAAD group and ARA group and between the acute TAAD subgroup and non-acute TAAD subgroup. Analyses of covariance were used to adjust for potential confounders in baseline characteristics, if necessary. Qualitative variables are expressed as rates, and chi-square tests, corrected chi-square tests, or Fisher’s exact probability tests were used for comparisons between 2 groups. Correlations were assessed by calculating Pearson correlation coefficient values. Sample size calculations were performed based on coefficient of variation with 80% power and 5% significance. There were some missing values in the variables of D-dimer, lactic acid, and PaO2-FiO2 ratio and no missing values in the other variables in this study, which may not affect the main outcomes of this study regarding systemic inflammatory responses, intestinal injury, bacteremia, and their relationships. These missing values, including 2 for D-dimer in ARA, 6 for lactic acid in TAAD, 9 for lactic acid in ARA, 5 for PaO2-FiO2 ratio in TAAD, and 9 for PaO2-FiO2 ratio in ARA not to be tested owing to the physician’s prescribing preference and recorded in medical records, can be regarded as ignorable according to the assumption of missing at random. Two-sided P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS 22.0 for Windows (IBM SPSS, Armonk, NY, USA).
Results
Study population demographic characteristics
A total of 66 individuals were included in the study, and their baseline characteristics are summarized in Table 1. Patients in the TAAD group were younger and had higher serum Cr levels on admission than those in the ARA group and no significant differences were observed in sex, medical history, or other clinical variables between the two groups. Patients in the acute TAAD subgroup had higher serum Cr levels than those in the non-acute subgroup and no significant differences were observed in age, sex, medical history, or other clinical variables between the two subgroups (Table 2). Analyses of covariance were used for comparisons in the levels of systemic inflammatory response and the levels of lung injury, intestinal injury, and bacteremia between the TAAD group and ARA group and between the acute TAAD subgroup and non-acute TAAD subgroup to adjust for potential confounders in baseline characteristics, including age and serum Cr levels.
Table 1
| Clinical variable | TAAD | ARA | P |
|---|---|---|---|
| Number, n | 36 | 30 | |
| Age, years, mean ± SD | 45.25±11.65 | 51.57±12.76 | 0.040 |
| Men, n (%) | 31 (86.1) | 25 (83.3) | 0.754 |
| Smoker, n (%) | 16 (44.4) | 13 (43.3) | 0.928 |
| Hypertension, n (%) | 21 (58.3) | 13 (43.3) | 0.225 |
| Diabetes, n (%) | 0 | 1 (3.3) | 0.455 |
| Coronary heart disease, n (%) | 0 | 2 (6.7) | 0.203 |
| Cerebral infarction, n (%) | 0 | 2 (6.7) | 0.203 |
| Serum measurements, mean ± SD | |||
| Cr, μmol/L | 103.21±52.26 | 80.03±16.75 | 0.016 |
| ALT, U/L | 38.39±44.03 | 24.57±13.85 | 0.082 |
| AST, U/L | 34.61±32.35 | 24.03±9.38 | 0.069 |
| D-dimer, mmol/L | 3,550.67±11,518.63 | 404.36±1,274.59 | 0.156 |
| Lactic acid, ng/mL | 1.72±1.12 | 1.85±0.56 | 0.630 |
TAAD, type A aortic dissection; ARA, aortic root aneurysm; SD, standard deviation; Cr, creatinine; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 2
| Clinical variable | Acute TAAD | Non-acute TAAD | P |
|---|---|---|---|
| Number, n | 18 | 18 | |
| Age, years, mean ± SD | 47.00±11.11 | 43.50±12.21 | 0.375 |
| Men, n (%) | 17 (94.4) | 14 (77.8) | 0.338 |
| Smoker, n (%) | 8 (50.0) | 8 (50.0) | 1.000 |
| Hypertension, n (%) | 11 (61.1) | 10 (55.6) | 1.000 |
| Diabetes, n (%) | 0 | 0 | – |
| Coronary heart disease, n (%) | 0 | 0 | – |
| Cerebral infarction, n (%) | 0 | 0 | – |
| Serum measurements, mean ± SD | |||
| Cr, μmol/L | 122.62±66.43 | 83.80±20.30 | 0.028 |
| ALT, U/L | 31.17±25.39 | 45.61±56.89 | 0.332 |
| AST, U/L | 31.00±19.86 | 38.22±41.63 | 0.511 |
| D-dimer, mmol/L | 6,469.11±15,954.76 | 632.22±755.85 | 0.139 |
| Lactic acid, ng/mL | 1.99±1.36 | 1.37±0.57 | 0.102 |
TAAD, type A aortic dissection; SD, standard deviation; Cr, creatinine; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Systemic inflammatory response levels
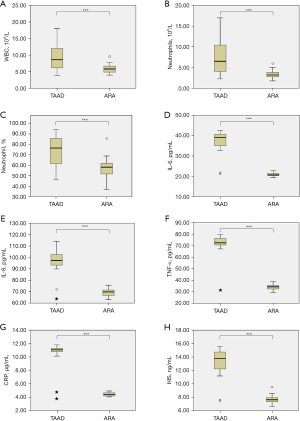
In patients with TAAD, WBC count [(9.70±4.05)×109/L vs. (5.88±1.2)×109/L, P<0.001, Figure 1A], neutrophil count [(7.65±4.27)×109/L vs. (3.40±0.97)×109/L, P<0.001, Figure 1B], and neutrophil percentage [(74.73±13.42)% vs. (57.67±9.45)%, P<0.001, Figure 1C] were significantly elevated compared to those in the ARA group. Serum levels of IL-6 (37.48±4.87 vs. 20.90±0.92 pg/mL, P<0.001, Figure 1D), IL-8 (97.15±9.11 vs. 69.46±3.17 pg/mL, P<0.001, Figure 1E), TNF-α (71.32±10.35 vs. 33.90±2.27 pg/mL, P<0.001, Figure 1F), CRP (10.67±1.62 vs. 4.43±0.26 µg/mL, P<0.001, Figure 1G), and HIS (13.29±1.88 vs. 7.63±0.58 ng/mL, P<0.001, Figure 1H) were significantly higher in patients with TAAD than in those with ARA.
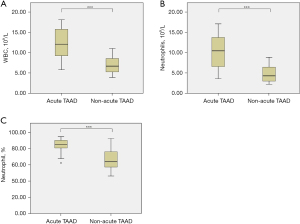
In patients with acute TAAD, WBC count [(12.35±3.83)×109/L vs. (7.05±2.08)×109/L, P<0.001, Figure 2A], neutrophil count [(10.48±4.03)×109/L vs. (4.82±2.07)×109/L, P<0.001, Figure 2B], and neutrophil percentage [(82.88±9.13)% vs. (66.58±12.12)%, P<0.001, Figure 2C] were significantly elevated compared to those in patients with non-acute TAAD. No significant differences in IL-6 (38.29±2.90 vs. 36.67±6.25 pg/mL, P=0.330), IL-8 (98.27±4.94 vs. 96.02±12.00 pg/mL, P=0.468), TNF-α (73.72±3.86 vs. 68.92±13.91 pg/mL, P=0.168), CRP (11.13±0.42 vs. 10.22±2.19 µg/mL, P=0.099), or HIS (13.53±1.41 vs. 13.04±2.28 ng/mL, P=0.448) were observed between the acute and non-acute TAAD groups.
Levels of lung injury, intestinal injury, and bacteremia
The PaO2-FiO2 ratio was significantly lower in patients with TAAD than in those with ARA (365.35±146.47 vs. 447.86±70.80 mmHg, P=0.01, Figure 3A). Moreover, the PaO2-FiO2 ratio was significantly lower (273.39±98.04 vs. 492.69±98.80 mmHg, P<0.001, Figure 3B) in patients with acute TAAD compared to those with non-acute TAAD.
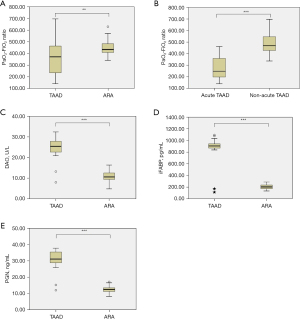
DAO activity (24.94±4.72 vs. 10.92±2.44 U/L, P<0.001, Figure 3C), iFABP (879.01±190.12 vs. 206.35±42.20 pg/mL, P<0.001, Figure 3D), and PGN (31.10±5.51 vs. 12.52±2.20 ng/mL, P<0.001, Figure 3E) were significantly elevated in patients with TAAD compared to those with ARA. No significant differences in DAO activity (25.61±3.29 vs. 24.27±5.83 U/L, P=0.402), iFABP (930.71±64.39 vs. 827.31±254.19 pg/mL, P=0.111), or PGN (32.27±3.63 vs. 29.92±6.80 ng/mL, P=0.205) were observed between patients with acute and non-acute TAAD.
Correlations among systemic inflammatory responses, lung injury, intestinal injury, and bacteremia
In patients with TAAD, significant positive correlations were detected between DAO activity and inflammatory cytokine levels, including IL-6 (r=0.56, P<0.001, Figure 4A), IL-8 (r=0.61, P<0.001, Figure 4B), TNF-α (r=0.71, P<0.001, Figure 4C), and CRP (r=0.68, P<0.001, Figure 4D). Furthermore, significant correlations were detected between iFABP and inflammatory cytokine levels, including IL-6 (r=0.72, P<0.001, Figure 4E), IL-8 (r=0.71, P<0.001, Figure 4F), TNF-α (r=0.90, P<0.001, Figure 4G), and CRP (r=0.89, P<0.001, Figure 4H).

In patients with TAAD, significant positive correlations were also found between DAO activity and PGN (r=0.52, P<0.001, Figure 5A) as well as between iFABP and PGN (r=0.74, P<0.001, Figure 5B).

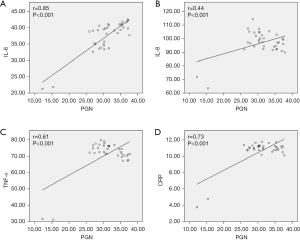
Significant positive correlations were found between PGN and several inflammatory cytokines, including IL-6 (r=0.85, P<0.001, Figure 6A), IL-8 (r=0.44, P<0.001, Figure 6B), TNF-α (r=0.61, P<0.001, Figure 6C), and CRP (r=0.73, P<0.001, Figure 6D), in patients with TAAD.
In addition, in patients with acute TAAD, serum PGN level and PaO2-FiO2 ratio value were significantly negatively correlated (r=−0.50, P=0.036, Figure 7).

Discussion
Acute lung injury, defined as PaO2-FiO2 ratio <300 mmHg, occurs in approximately 50% of patients with acute TAAD and can be life-threatening in severe cases (3-5). Preoperative hypoxemia can result in surgery delay, which increases the risk of aortic rupture, cardiac tamponade, or even death (17). Patients with acute TAAD and preoperative acute lung injury are at increased risk of postoperative acute respiratory distress syndrome and have prolonged intubation times and/or increased mortality rates after surgery (18). Preoperative acute lung injury can also result in more intraoperative blood loss, longer mechanical ventilation time, and a longer stay in the intensive care unit and hospital (4). Preoperative acute lung injury develops mainly in patients with acute TAAD, but rarely occurs in non-acute aortic diseases, such as aortic aneurysm and non-acute TAAD. In this study, we found that the PaO2-FiO2 ratio was significantly lower in patients with acute TAAD than in those with non-acute TAAD or ARA, and the incidence of PaO2-FiO2 ratio <300 mmHg was 66.67% (12/18) in patients with acute TAAD and 0 in non-acute TAAD or ARA. These findings indicated that significant lung injury developed during the acute period of TAAD and was alleviated after the acute phase, with lung function tending to normalize.
The definite pathogenesis of acute lung injury complicated by acute TAAD remains unclear. It is currently considered that systemic inflammatory reactions play important roles in the development of acute lung injury. IL-6, TNF-α, and CRP are common pro-inflammatory cytokines that have been reported as overexpressed in patients with aortic dissection (3-10). Studies found that inflammatory cytokines, including CRP, IL-6, and IL-8, might be associated with the development of acute lung injury in patients with acute TAAD (5,14,18-21), and WBC levels could be elevated in these patients (5,18). In this study, we found that various inflammatory response indicators, including WBC parameters and inflammatory cytokines, were activated in patients with TAAD and significantly higher than in those in patients with ARA. Furthermore, we found that the levels of WBC parameters, including WBC count, neutrophil count, and neutrophil percentage, were higher in patients with acute TAAD than in those with non-acute TAAD, but there were no significant differences in the levels of inflammatory cytokines between acute and non-acute TAAD. These findings indicated that the changes in WBCs and neutrophils were short-lived, tending to return to normal after the acute phase of TAAD, while the alterations in cytokine levels (including those of IL-6, IL-8, TNF-α, CRP, and HIS) were more persistent, remaining activated in the non-acute phase. The time course of changes in WBC and neutrophil parameters was consistent with lung injury occurrence and development, which may indicate that the pulmonary microcirculation is a natural reservoir of leukocytes that is prone to injury during the acute phase of TAAD.
Although the correlations between acute lung injury and systemic inflammatory reactions have been demonstrated repeatedly, the pathogenesis underlying the inflammatory response in patients with aortic dissection is still not completely understood. AAD is a systemic disease, and some organ damage often occurs before surgery, such as heart failure, neurological deficits, and renal, hepatic, and gastrointestinal dysfunction. This organ damage might participate in the development of systemic inflammatory reactions. In 2016, a study found that DAO activity was significantly increased in patients with acute TAAD, that DAO activity was strongly correlated with the levels of some inflammatory cytokines (including IL-6, TNF-α, and CRP), and that DAO activity was negatively correlated with the PaO2-FiO2 ratio (16). These results suggest that intestinal dysfunction plays an important role in the development of the systemic inflammatory response and lung injury in patients with acute TAAD. DAO and iFABP are specifically present in small intestinal mucosal cells, and serum DAO activity and iFABP levels reflect the integrity and degree of damage of the intestinal barrier and are established markers of intestinal barrier dysfunction (22-24). In this study, we found that DAO activity and iFABP concentration were higher in the TAAD group than in the ARA group, while there were no significant differences between the acute and non-acute TAAD subgroups. These findings indicate that the intestinal injury in patients with TAAD occurs and persists for an extended period, continuing after the acute stage. The time course of changes in DAO activity and iFABP concentration was consistent with that of inflammatory cytokines. PGN is a common component of both Gram-positive and Gram-negative bacteria, and it reaches measurable concentrations in serum during bacteremia (25,26). We found that serum PGN levels were increased in patients with TAAD, further suggesting intestinal mucosal damage and barrier dysfunction in these patients, resulting in intestinal bacterial translocation into the blood. In addition, we found that DAO activity and iFABP concentration were significantly correlated with IL-6, IL-8, TNF-α, CRP levels, and PGN concentration, while PGN levels were significantly correlated with levels of IL-6, IL-8, TNF-α, and CRP in the TAAD group. Based on these data, we speculate that the systemic inflammatory response caused by aortic dissection is the cause of intestinal injury, and that bacteremia caused by intestinal injury and intestinal barrier dysfunction may account for the persistence and lack of alleviation of the systemic inflammatory response after the acute stage. The present study also found that PGN concentration was significantly correlated with the PaO2-FiO2 ratio in the acute TAAD subgroup, suggesting that bacteremia caused by intestinal barrier dysfunction and intestinal bacterial translocation may contribute to the occurrence and development of lung injury. Therefore, intestinal injury, although not an initiating insult in pulmonary damage, might enhance lung permeability via an exacerbated inflammatory process.
The present study has several limitations. First, we could not obtain serial blood samples spanning the whole period of the natural progression of aortic dissection from the same patients with TAAD, who almost always require emergency surgery. Therefore, it is difficult to fully determine the dynamic changes in systemic inflammatory responses, intestinal injury, and lung injury and their relationship in the setting of TAAD. Second, although DAO, iFABP, and PaO2-FiO2 ratio are established as excellent indicators of the degree of intestine and lung injury, intestine and lung injury should ideally be evidenced by histological examinations. Nevertheless, this study is an important exploration focusing on the mechanisms and functional implications of systemic inflammatory responses, intestinal injury, and lung injury in patients with TAAD. Importantly, this is the first study to report differences in systemic inflammatory responses, intestinal injury, and lung injury between TAAD and ARA and between acute and non-acute TAAD, which will assist in a more comprehensive understanding of the mechanisms underlying systemic inflammatory responses in patients with TAAD and the resulting damage. It may also inform future studies aimed at optimizing therapeutic strategies for the attenuation of TAAD-related systemic inflammatory responses, regarding to the protection for intestinal barrier function.
Conclusions
In summary, our results highlight the underlying mechanisms and functional implications of intestinal barrier dysfunction in the development of systemic inflammatory responses and lung injury in TAAD. Systemic inflammatory responses in patients with TAAD may lead to lung and intestine injuries, and the latter may be involved in the development of systemic inflammatory responses and lung injury in these patients.
Acknowledgments
All authors thank Hualing Cai, Department of Cardiovascular Surgery, Beijing Anzhen Hospital, Beijing, China, for her kind assistance in blood sample collection, centrifugation, and storage.
Funding: This work was supported by Cultivation and Development Projects of Science and Technology Innovation Base.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1122/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1122/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1122/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (No. GZR 046), and written informed consent was obtained from all patients and/or immediate relatives.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Anesth Analg 2010;111:279-315. [Crossref] [PubMed]
- Mészáros I, Mórocz J, Szlávi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest 2000;117:1271-8. [Crossref] [PubMed]
- Pan X, Lu J, Cheng W, et al. Independent factors related to preoperative acute lung injury in 130 adults undergoing Stanford type-A acute aortic dissection surgery: a single-center cross-sectional clinical study. J Thorac Dis 2018;10:4413-23. [Crossref] [PubMed]
- Gao Z, Pei X, He C, et al. Oxygenation impairment in patients with acute aortic dissection is associated with disorders of coagulation and fibrinolysis: a prospective observational study. J Thorac Dis 2019;11:1190-201. [Crossref] [PubMed]
- Sugano Y, Anzai T, Yoshikawa T, et al. Serum C-reactive protein elevation predicts poor clinical outcome in patients with distal type acute aortic dissection: association with the occurrence of oxygenation impairment. Int J Cardiol 2005;102:39-45. [Crossref] [PubMed]
- Vrsalović M, Vrsalović Presečki A. Admission C-reactive protein and outcomes in acute aortic dissection: a systematic review. Croat Med J 2019;60:309-15. [Crossref] [PubMed]
- Hsieh WC, Henry BM, Hsieh CC, et al. Prognostic Role of Admission C-Reactive Protein Level as a Predictor of In-Hospital Mortality in Type-A Acute Aortic Dissection: A Meta-Analysis. Vasc Endovascular Surg 2019;53:547-57. [Crossref] [PubMed]
- Vrsalovic M, Zeljkovic I, Presecki AV, et al. C-reactive protein, not cardiac troponin T, improves risk prediction in hypertensives with type A aortic dissection. Blood Press 2015;24:212-6. [Crossref] [PubMed]
- Qin C, Gu J, Qian H, et al. Potential Mechanism of Post-Acute Aortic Dissection Inflammatory Responses: The Role of mtDNA from Activated Platelets. Cardiology 2016;135:228-35. [Crossref] [PubMed]
- Yuan SM. Profiles and Predictive Values of Interleukin-6 in Aortic Dissection: a Review. Braz J Cardiovasc Surg 2019;34:596-604. [Crossref] [PubMed]
- Ma M, Shi J, Feng X, et al. The elevated admission white blood cell count relates to adverse surgical outcome of acute Stanford type a aortic dissection. J Cardiothorac Surg 2020;15:48. [Crossref] [PubMed]
- Cifani N, Proietta M, Tritapepe L, et al. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: a review. Ann Med 2015;47:441-6. [Crossref] [PubMed]
- Zhao X, Bie M. Preoperative acute lung injury and oxygenation impairment occurred in the patients with acute aortic dissection. BMC Cardiovasc Disord 2022;22:129. [Crossref] [PubMed]
- Kashiwagi Y, Komukai K, Suzuki K, et al. Predictors of oxygenation impairment in medical treatment for type B acute aortic dissection. Heart Vessels 2018;33:1463-70. [Crossref] [PubMed]
- Wortmann M, Peters AS, Erhart P, et al. Inflammasomes in the Pathophysiology of Aortic Disease. Cells 2021;10:2433. [Crossref] [PubMed]
- Gu J, Hu J, Qian H, et al. Intestinal Barrier Dysfunction: A Novel Therapeutic Target for Inflammatory Response in Acute Stanford Type A Aortic Dissection. J Cardiovasc Pharmacol Ther 2016;21:64-9. [Crossref] [PubMed]
- Cheng Y, Jin M, Dong X, et al. Mechanism and early intervention research on ALI during emergence surgery of Stanford type-A AAD: Study protocol for a prospective, double-blind, clinical trial. Medicine (Baltimore) 2016;95:e5164. [Crossref] [PubMed]
- Guo Z, Yang Y, Zhao M, et al. Preoperative hypoxemia in patients with type A acute aortic dissection: a retrospective study on incidence, related factors and clinical significance. J Thorac Dis 2019;11:5390-7. [Crossref] [PubMed]
- Komukai K, Shibata T, Mochizuki S. C-reactive protein is related to impaired oxygenation in patients with acute aortic dissection. Int Heart J 2005;46:795-9. [Crossref] [PubMed]
- Zhao X, Bie M. Predictors for the development of preoperative oxygenation impairment in acute aortic dissection in hypertensive patients. BMC Cardiovasc Disord 2020;20:365. [Crossref] [PubMed]
- Duan XZ, Xu ZY, Lu FL, et al. Inflammation is related to preoperative hypoxemia in patients with acute Stanford type A aortic dissection. J Thorac Dis 2018;10:1628-34. [Crossref] [PubMed]
- Guo YY, Liu ML, He XD, et al. Functional changes of intestinal mucosal barrier in surgically critical patients. World J Emerg Med 2010;1:205-8. [PubMed]
- Zhang L, Fan X, Zhong Z, et al. Association of plasma diamine oxidase and intestinal fatty acid-binding protein with severity of disease in patient with heat stroke. Am J Emerg Med 2015;33:867-71. [Crossref] [PubMed]
- Montagnana M, Danese E, Lippi G. Biochemical markers of acute intestinal ischemia: possibilities and limitations. Ann Transl Med 2018;6:341. [Crossref] [PubMed]
- Lichtman SM. Bacterial [correction of baterial] translocation in humans. J Pediatr Gastroenterol Nutr 2001;33:1-10. [Crossref] [PubMed]
- Tsunooka N, Maeyama K, Hamada Y, et al. Bacterial translocation secondary to small intestinal mucosal ischemia during cardiopulmonary bypass. Measurement by diamine oxidase and peptidoglycan. Eur J Cardiothorac Surg 2004;25:275-80. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


