
Diagnostic value of myocardial stress detection based on feature tracking MRI in patients with acute myocardial infarction
Introduction
Due to the inherent characteristics of magnetic resonance imaging (MRI), it has been the only non-invasive method that can be used for three-dimensional (3D) evaluation of myocardial activity with various parameters in recent years (1). Delayed-enhancement MRI (DE-MRI) is rapidly emerging as an outstanding and effective means of assessing cardiac activity, where patients can be examined at rest without being exposed to radiation (2). The delayed enhancement mechanism of DE-MRI in acute myocardial infarction (AMI) is different from that in old myocardial infarction. The enhancement mechanism of AMI is closely related to the loss of cell integrity and cell necrosis, while in old myocardial infarction, the volume of extracellular distribution of contrast agent increases mainly due to the formation of collagen removal marks. MRI is widely used in clinic (3-5). In this study, we aimed to determine the application of DE-MRI in evaluating myocardial activity. Direct viable myocardium is defined as the presence of cellular metabolism, intact membrane, and potential contractility reserve, and contractility enhancement response to positive inotropic drugs, including normal myocardium and reversely impaired myocardium (depressed and hibernating myocardium) (6). The histological spectrum of inactivated myocardium has been found to vary from cellular dedifferentiation to degeneration, with contractility and loss of cytoskeletal proteins. Loss of myocardial activity is defined as myocardial infarction. Serum markers such as creatine kinase and troponin are highly effective in infarcted myocardium but have limitations. For example, there is a finite time window for enzyme detection, which cannot diagnose old myocardial infarction and cannot be located. Further, the sensitivity of electrocardiogram (ECG) is not suitable for this type of detection (7). Due to increased awareness and awareness, the incidence of stress cardiomyopathy has been increasing (15 to 30 cases per 100 000 people per year) because underdiagnosis may exist and the actual incidence is unknown.
Obesity is highly correlated with cardiovascular disease and is an important risk factor for coronary atherosclerosis (8). Epicardial adipose tissue is a special form of visceral adipose tissue, which deposits in epicardial tissue, and encases cardiac muscle and the crucial cardiac vessels. It not only reflects the deposition of visceral fat in the heart, it is an active endocrine organ. Stress cardiomyopathy, an acute reversible heart failure syndrome, was initially considered a benign disease because of its self-limiting clinical course, but is now associated with serious complications such as ventricular arrhythmias, systemic thromboembolism, and cardiogenic shock. In the past, stress cardiomyopathy was thought to be mostly benign, but current studies (9,10) have found that the prognosis of stress cardiomyopathy is not as good as imagined, and 3%-5% of patients will suffer from severe cardiogenic shock, heart failure that is difficult to correct, and even sudden death, which is comparable to the mortality of acute myocardial infarction.
Fat cells can secrete a variety of factors, mediate the local inflammatory response, which is closely related to the development of coronary atherosclerosis (11). Coronary angiography (CAG) is the gold standard for the diagnosis of AMI, but the procedure is traumatic and difficult to repeat in the follow-up of patients. Therefore, the exploration of non-invasive examinations for the diagnosis and prediction of AMI has been increasingly emphasized. Vascular echo tracking is a new non-invasive MRI technique which can be used to detect vascular endothelial dilation and arterial elasticity. It was found that epicardial adipose thickness [hematopoietic stem cell transplantation (HSCT) adipose thickness] could be an independent predictor of atherosclerotic plaque vulnerability in patients with AMI (12). Therefore, this study aimed to investigate the predictive value of myocardial stress measured by MRI in AMI. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-973/rc).
Methods
Participants
A total of 120 patients hospitalized in the First Affiliated Hospital of Hainan Medical University from January 2018 to June 2021 were retrospectively selected, all of whom underwent coronary intracardiac MRI [Intravascular ultrasound (IVUS)] examination for chest pain, angina pectoris, and myocardial infarction due to unknown causes, including 68 males and 52 females. Participant ages ranged from 39 to 81 years, with an average of 63.5±6.4 years. The inclusion criteria were as follows: (I) adult patients admitted to hospital for the first time due to unexplained chest pain, angina pectoris, and myocardial infarction; (II) the clinical history was provided by the patient and the data were complete. Patients with diabetes, cardiac dysfunction [left ventricular ejection fraction (LVEF) <45%], organic heart valvular disease, arrhythmia, tumor, and hepatic and renal dysfunction were excluded. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Hainan Medical University (No. 20220062). Individual consent for this retrospective analysis was waived.
Examination of risk factors related to AMI
Participant medical histories were collected; waist circumference, blood pressure, weight, and height were measured; body mass index (BMI) was calculated; fasting venous blood was drawn; and fasting blood glucose, blood lipids, and insulin concentrations were measured.
MRI to measure myocardial stress
The GE Vivid 7 MRI imaging system (GE Healthcare, Chicago, IL, USA) was used, with a probe frequency of 1.7–3.4 MHz, operated by the same MRI physician. The participants were placed in left decubitus position and examined on the left long axis of the heart beside the sternum. The aortic valve ring was used as the positioning marker, and the sampling line was perpendicular to the free wall of the right ventricle. The fat thickness between the free wall of the right ventricle and the pericardium visceral layer was measured at the end of ventricular diastole, and 5 cardiac cycles were recorded. The standard parasternal short axial section of left ventricular papillary muscle was selected, the sampling line was perpendicular to the interventricular septum, and the fat thickness between the free wall of the right ventricle and the visceral layer of the pericardium was measured at the end of ventricular diastolic stage. A total of 5 cardiac cycles were recorded, the thickness of myocardial stress force in 10 cardiac cycles was measured, and the average value was taken.
IVUS examination
We performed CAG was performed with at least 3 individual sites to show the target lesion and the left main trunk. The IVUS examination was performed after the end of angiography; 3,000 U heparin was added, a 0.014 inch guide wire was sent to the distal end of the coronary artery, 0.2 mg nitroglycerin was injected into the coronary artery, and the MRI catheter was sent along the guide wire to the distal end of the lesion more than 1 cm. Intravascular MRI was conducted using a 2.9–3.5 F 60 MHz monorail mechanical probe (Boston Scientific Corporation, Boston, MA, USA). All MRI images captured by automatic retreat, the retreat speed is 0.5 mm/s. The MRI began at a location more than 1 cm away from the distal end of the target lesion and ended at the coronary artery opening (8).
The cross-sectional area measured by IVUS included the lumen area, plaque area, and total cross-sectional area (sum of vascular lumen area and plaque area, unit mm2), area stenosis rate = plaque area/total cross-sectional area ×100%.
Diagnostic criteria
(I) The severity of coronary artery disease was assessed quantitatively by the Gensini scoring method according to the results of intravascular MRI examination and multiplied by the different coefficients of the coronary artery segment where the disease was located. The final total CAG score was the sum of the scores of each segment. (II) The diameter of coronary artery stenosis ≥50%, the number of main coronary artery lesions involved was the number of lesions, divided into 0, 1, 2, and 3 lesions; when the left main trunk was involved, the left anterior descending branch and left circumrotatory branch were involved simultaneously. (III) A diagnosis of AMI was made when ≥50% of the coronary artery stenosis diameter involved major coronary artery branches.
The degree of coronary artery lesions was graded as follows: mild lesions, single vessel lesions with an area stenosis rate of 50–70%; moderate lesions, single or 2-vessel lesions with an area stenosis rate of >70%; and severe lesions, 3-vessel lesions or a left main vessel lesion.
Statistical analysis
The software SPSS 23.0 (IBM Corp., Armonk, NY, USA) was used; χ2 test was used for inter-group comparison; mean ± standard deviation was used for measurement data; independent sample t-test was used for inter-group comparison. It is necessary to do univariate comparisons and multiple logistic regression analysis. Analysis of variance (ANOVA) was used for inter-group comparison; and linear regression analysis and logistic regression analysis were used for correlation analysis. A receiver operating characteristic (ROC) curve was used to evaluate the diagnostic efficacy of myocardial stress on AMI. The receiver operating characteristic (ROC) curve can easily detect the ability to identify performance at any threshold value and select the best diagnostic cutoff value. AUC is the area under the ROC curve. Since the range of true positive rate and false positive rate is [0, 1], the maximum AUC =1, and P<0.05 indicated a statistically significant difference (two-sided).
Results
Comparison of basic data and laboratory and auxiliary examination results
According to the MRI results of the coronary vessels, patients with stenosis rate >50% were diagnosed with AMI. Among the 120 patients enrolled, 77 were in the AMI group, including 43 males and 34 females. In the non-AMI group, there were 43 cases, including 25 males and 18 females. The BMI, waist circumference, low density lipoprotein, diastolic pressure, and myocardial stress in AMI group were significantly higher than those in non-AMI group (all P<0.05), but there were no significant differences in other indexes (all P>0.05) (Table 1).
Table 1
| Indicators | Non-AMI group (n=43) | AMI group (n=77) | t/χ2 | P value |
|---|---|---|---|---|
| Gender | χ2=0.059 | 0.808 | ||
| Male | 25 | 43 | ||
| Female | 18 | 34 | ||
| Age (years) | 66.1±5.9 | 65.6±6.2 | t=0.431 | 0.667 |
| BMI (kg/m2) | 22.8±2.8 | 25.9±3.1 | t=5.434 | <0.001 |
| Waist circumference (cm) | 83.2±5.8 | 87.1±5.4 | t=3.694 | <0.001 |
| Systolic blood pressure (mmHg) | 141.7±21.3 | 144.2±20.8 | t=0.626 | 0.535 |
| Diastolic blood pressure (mmHg) | 79.4±7.8 | 85.6±8.4 | t=3.976 | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.24±0.78 | 5.04±0.81 | t=1.314 | 0.191 |
| Total cholesterol (mmol/L) | 5.61±0.67 | 5.78±0.83 | t=0.473 | 0.637 |
| Triacylglycerol (mmol/L) | 1.97±0.43 | 2.11±0.54 | t=1.460 | 0.147 |
| Low density lipoprotein (mmol/L) | 2.72±0.56 | 3.21±0.64 | t=4.483 | <0.001 |
| High-density lipoprotein (mmol/L) | 1.05±0.32 | 0.96±0.24 | t=1.743 | 0.084 |
| Fasting insulin (mU/L) | 7.01±0.89 | 6.97±0.94 | t=0.228 | 0.820 |
| Myocardial stress (mm) | 4.97±0.56 | 6.55±0.62 | t=13.848 | <0.001 |
AMI, acute myocardial infarction; BMI, body mass index.
Comparison of myocardial stress in different degrees of coronary artery disease
The myocardial stress of patients with mild, moderate, and severe AMI was significantly thicker than that of those in the non-AMI group, and the difference was statistically significant (F=22.346, P<0.001). In the AMI group, the value of myocardial stress increased gradually with the aggravation of mild, moderate, and severe lesions, and the difference between the groups was statistically significant (F=10.283, P<0.001) (Table 2).
Table 2
| Grouping | Cases | Myocardial stress (1/s) |
|---|---|---|
| Non-AMI group | 43 | 4.97±0.56 |
| AMI group | ||
| Mild lesions | 21 | 5.68±0.62 |
| Moderate lesions | 40 | 6.67±0.58 |
| Severe lesions | 16 | 7.49±0.67 |
AMI, acute myocardial infarction.
Correlation between myocardial stress and coronary artery stenosis rate
In 77 patients with AMI, myocardial stress was positively correlated with the percentage of coronary artery stenosis rate (R=0.405, P<0.001), and the greater the myocardial stress was, the higher the coronary artery stenosis rate was (Figure 1).
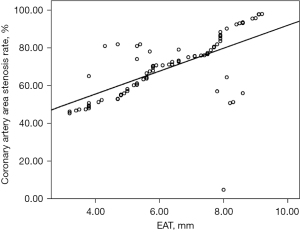
Correlation between coronary artery disease score and myocardial stress
There was significant positive correlation between CAD and myocardial stress in patients with coronary artery disease (R=0.643, P<0.05). The myocardial stress was divided into quartiles: 1stQ group, 2ndQ group, 3rdQ group, and 4thQ group. The comparison of CAD values among all groups showed statistically significant differences (P<0.05) (Figure 2).
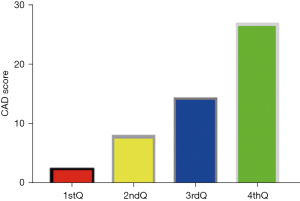
Logistic regression analysis of factors influencing the occurrence of AMI
The factors with statistically significant differences were assigned as follows: BMI (<24 kg/m2 =0, 24–27 kg/m2 =1, ≥28 kg/m2 =2); waist circumference (<80 cm =0, 80–84 cm =1, 85–89 cm =2, 90–94 cm =3, ≥95 cm =4); diastolic blood pressure (<90 mmHg =0, 90–99 mmHg =1, 100–109 mmHg =2, 110–119 mmHg =3, ≥120 mmHg =4); low density lipoprotein (<3.15 mmol/L =0, ≥3.15 mmol/L =1); and myocardial stress (as a percentage: <25% =0, 25–49% =1, 50–74% =2, ≥75% =3). Binary logistic regression analysis was performed on the above factors and the corresponding variables (non-AMI group =0, AMI group =1), and the results are shown in Table 3. After logistic regression was used to balance other factors, there was statistically significant difference in myocardial stress between the AMI group and the non-AMI group (P<0.05).
Table 3
| Factors | Wald | OR (95% CI) | P value |
|---|---|---|---|
| BMI | 4.387 | 3.345 (1.472–4.528) | 0.019 |
| Waist circumference | 0.614 | 1.627 (0.983–2.146) | 0.578 |
| Low density lipoprotein | 5.242 | 3.704 (2.564–6.182) | 0.034 |
| Diastolic blood pressure | 4.762 | 1.782 (1.343–3.546) | 0.038 |
| Myocardial stress | 5.128 | 2.043 (1.712–7.513) | 0.015 |
OR, odds ratio; CI, confidence interval; BMI, body mass index.
Diagnostic efficacy of myocardial stress in AMI
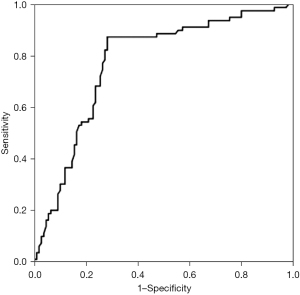
The area under the ROC curve (AUC; Figure 3) was 0.834 [95% confidence interval (CI): 0.714 to 0.925, P<0.01]. The sensitivity and specificity of myocardial stress >5.15 mm in the diagnosis of AMI were 83.5% and 68.6%.
Discussion
At present, the gold standard for the diagnosis of AMI is CAG or IVUS (13). However, due to its associated trauma, radioactivity, and high costs, it cannot be used as a routine screening method (14). Therefore, the search for various non-invasive diagnostic indicators has always received clinical attention. In this study, myocardial stress as measured by MRI was a risk factor for AMI, along with BMI, low density lipoprotein cholesterol, and diastolic blood pressure. Relevant studies (15-19) have found that systolic blood pressure, diastolic blood pressure, low density lipoprotein, and fasting blood glucose are significantly increased with each one standard deviation increase of myocardial stress, although high density lipoprotein cholesterol is decreased, and the risk of diabetes, hypertension, and metabolic syndrome is increased by 1.56–2.13 times, which is consistent with the results of this study. In this study, multiple risk factors for AMI were analyzed by binary logistic regression, and the results showed that Wald =5.057, P=0.028, suggesting that myocardial stress is an independent risk factor for AMI, and the accuracy of predicting AMI is also high (20,21).
The CAG to determine degree of coronary artery lesions has important value, but it has been shown that only the vascular lumen long axis of the CAG 2D images can estimate the degree of relatively narrow lumen, particularly for heart disease, making it vulnerable to the projection (22-24). The angle and the reference section of the CAG influence images of the blood vessels, and cannot provide valuable information regarding the traits of coronary artery lesions. These aspects are exactly the strengths of IVUS, which can reflect not only the changes of vascular lumen, but also the cross-sectional structure, thickness, morphology, and plaque characteristics of vessels including plaques. For early coronary artery disease, CAG may be negative, but IVUS can accurately detect the extent and characteristics of the disease. For lesions with coronary artery stenosis <50%, or even lesions with normal CAG, especially for patients with ischemia symptoms, IVUS often has important positive findings, which compensates for the underestimation and false negative phenomenon of lesions by CAG. In this study, IVUS was used to evaluate the degree of lesion vascular lumen stenosis and to calculate the area stenosis rate of the coronary artery, thus improving the diagnostic accuracy. This study showed that myocardial stress was positively correlated with the stenosis rate of coronary artery lesions (25). With the increase of myocardial stress, the severity of coronary artery lesions became worse, and the total Gensini score of coronary artery lesions increased. The results showed that with the increase of epicardial adipose tissue thickness, the incidence of AMI gradually increased. The incidence of AMI was 66% in the group with the lowest value of myocardial stress, and 94% in the group with the highest value. The Gensini score of all patients with AMI was 49.5 in the high-value group and 28.1 in the low-value group (26). The degree of coronary artery stenosis gradually increased with the increase of epicardial fat thickness, and the difference was significant, which was similar to previous results (27).
The ROC curve takes the maximum point of Youden index value as the diagnostic cut-off point, and the more the AUC is greater than 50%, the greater its predictive value will be. In this study, the AUC for predicting the presence of AMI is 0.834, and the thickness of myocardial stress >5.15 mm is used as the cut-off value to predict AMI. The sensitivity and specificity were 83.5% and 68.6%, respectively. This study showed that MRI measurement of myocardial stress was closely related to coronary artery stenosis detected by MRI and the myocardial stress increased gradually with the aggravation of the lesion degree. As MRI detection of myocardial stress can better predict the existence and lesion degree of AMI, it is non-invasive, cheap and easy to operate, especially in hospitals that cannot carry out CAG and IVUS, MRI detection of myocardial stress can be used as one of the collaborative methods for non-invasive diagnosis of AMI.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-973/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-973/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-973/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Hainan Medical University (No. 20220062). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scally C, Abbas H, Ahearn T, et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019;139:1581-92. [Crossref] [PubMed]
- Jiménez-Méndez C, Cecconi A, Vera A, et al. Concomitant acute myocardial infarction and stress cardiomyopathy. Coron Artery Dis 2021;32:261-2. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J 2021;42:1866-78. [Crossref] [PubMed]
- Manolis AS, Manolis AA, Manolis TA, et al. COVID-19 and Acute Myocardial Injury and Infarction: Related Mechanisms and Emerging Challenges. J Cardiovasc Pharmacol Ther 2021;26:399-414. [Crossref] [PubMed]
- Santer D, Nagel F, Gonçalves IF, et al. Tenascin-C aggravates ventricular dilatation and angiotensin-converting enzyme activity after myocardial infarction in mice. ESC Heart Fail 2020;7:2113-22. [Crossref] [PubMed]
- Pezel T, Garot P, Kinnel M, et al. Prognostic Value of Vasodilator Stress Perfusion Cardiovascular Magnetic Resonance in Patients With Prior Myocardial Infarction. JACC Cardiovasc Imaging 2021;14:2138-51. [Crossref] [PubMed]
- Hedeer F, Ostenfeld E, Hedén B, et al. To what extent are perfusion defects seen by myocardial perfusion SPECT in patients with left bundle branch block related to myocardial infarction, ECG characteristics, and myocardial wall motion? J Nucl Cardiol 2021;28:2910-22. [Crossref] [PubMed]
- Inserte J, Barrabés JA, Aluja D, et al. Implications of Iron Deficiency in STEMI Patients and in a Murine Model of Myocardial Infarction. JACC Basic Transl Sci 2021;6:567-80. [Crossref] [PubMed]
- Meshref TS, Abd El-Aal RF, Ashry MA, et al. Impact of stress hyperglycemia on myocardial salvage in patients with ST-Elevation myocardial infarction: Cardiac magnetic resonance study. Indian Heart J 2020;72:462-5. [Crossref] [PubMed]
- Shah SA, Cui SX, Waters CD, et al. Nitroxide-enhanced MRI of cardiovascular oxidative stress. NMR Biomed 2020;33:e4359. [Crossref] [PubMed]
- Shi K, Yang MX, Xia CC, et al. Noninvasive oxygenation assessment after acute myocardial infarction with breathing maneuvers-induced oxygenation-sensitive magnetic resonance imaging. J Magn Reson Imaging 2021;54:284-9. [Crossref] [PubMed]
- Prokudina ES, Kurbatov BK, Maslov LN. Clinical Manifestation of Stressful Cardiomyopathy (Takotsubo Syndrome) and the Problem of Differential Diagnosis with Acute Myocardial Infarction. Kardiologiia 2020;60:777. [Crossref] [PubMed]
- Pezel T, Sanguineti F, Kinnel M, et al. Feasibility and Prognostic Value of Vasodilator Stress Perfusion CMR in Patients With Atrial Fibrillation. JACC Cardiovasc Imaging 2021;14:379-89. [Crossref] [PubMed]
- Seitz A, Wollert KC, Meyer GP, et al. Adenosine stress perfusion cardiac magnetic resonance imaging in patients undergoing intracoronary bone marrow cell transfer after ST-elevation myocardial infarction: the BOOST-2 perfusion substudy. Clin Res Cardiol 2020;109:539-48. [Crossref] [PubMed]
- Steen H, Montenbruck M, Kelle S, et al. Fast-Strain Encoded Cardiac Magnetic Resonance During Vasodilator Perfusion Stress Testing. Front Cardiovasc Med 2021;8:765961. [Crossref] [PubMed]
- Kochav JD, Kim J, Judd R, et al. Ischemia-Mediated Dysfunction in Subpapillary Myocardium as a Marker of Functional Mitral Regurgitation. JACC Cardiovasc Imaging 2021;14:826-39. [Crossref] [PubMed]
- De Rubeis G, Catapano F, Cundari G, et al. Cocaine Abuse: An Attack to the Cardiovascular System-Insights from Cardiovascular MRI. Radiol Cardiothorac Imaging 2019;1:e180031. [Crossref] [PubMed]
- Mangion K, Carrick D, Clerfond G, et al. Predictors of segmental myocardial functional recovery in patients after an acute ST-Elevation myocardial infarction. Eur J Radiol 2019;112:121-9. [Crossref] [PubMed]
- Arai AE, Schulz-Menger J, Berman D, et al. Gadobutrol-Enhanced Cardiac Magnetic Resonance Imaging for Detection of Coronary Artery Disease. J Am Coll Cardiol 2020;76:1536-47. [Crossref] [PubMed]
- Pezel T, Hovasse T, Kinnel M, et al. Prognostic value of stress cardiovascular magnetic resonance in asymptomatic patients with known coronary artery disease. J Cardiovasc Magn Reson 2021;23:19. [Crossref] [PubMed]
- Drakos S, Chatzantonis G, Bietenbeck M, et al. A cardiovascular magnetic resonance imaging-based pilot study to assess coronary microvascular disease in COVID-19 patients. Sci Rep 2021;11:15667. [Crossref] [PubMed]
- Park JJ, Kim SH, Kim MA, et al. Effect of Hyperglycemia on Myocardial Perfusion in Diabetic Porcine Models and Humans. J Korean Med Sci 2019;34:e202. [Crossref] [PubMed]
- Raj V, Pudhiavan A, Hrishikesh VJ, et al. Safety profile of adenosine stress cardiac MRI in a tertiary hospital in India. Indian J Radiol Imaging 2020;30:459-64. [Crossref] [PubMed]
- Arnold JR, McCann GP. Cardiovascular magnetic resonance: applications and practical considerations for the general cardiologist. Heart 2020;106:174-81. [Crossref] [PubMed]
- Yimcharoen S, Zhang S, Kaolawanich Y, et al. Clinical assessment of adenosine stress and rest cardiac magnetic resonance T1 mapping for detecting ischemic and infarcted myocardium. Sci Rep 2020;10:14727. [Crossref] [PubMed]
- Everaars H, van der Hoeven NW, Janssens GN, et al. Cardiac Magnetic Resonance for Evaluating Nonculprit Lesions After Myocardial Infarction: Comparison With Fractional Flow Reserve. JACC Cardiovasc Imaging 2020;13:715-28. [Crossref] [PubMed]
(English Language Editor: J. Jones)


