
Identification of tumor antigens and immune subtypes in lung squamous cell carcinoma for mRNA vaccine development
Introduction
Lung cancer is the leading cause of cancer-related death (1,2). The emergence of targeted therapy and immunotherapy in recent years has greatly improved the overall survival (OS) of patients; however, drug resistance and the low expression of immune checkpoints are still key issues that need to be solved (3). The mortality rate of lung squamous cell carcinoma (LUSC) patients continues to increase due to a lack of effective targeted drugs (3,4). Drug resistance in lung cancer is mainly caused by the heterogeneity of cancer cells and metabolic reprogramming-driven microenvironment adaptation phenotypes (5). Thus, it is very important to focus on the development of individualized treatment strategies and to find new treatments for lung cancer to address existing problems.
Cancer vaccines and chimeric antigen receptor T (CAR-T) cell therapy are emerging hot topics in cancer treatment. Cancer vaccines mainly activate the immune system of cancer patients to recognize and remove cancer cells by vaccinating specific antigens of cancer, while CAR-T therapy mainly enhances the anti-tumor immunity of patients by engineering the T cells extracted from the patients themselves and expanding them before transfusion (6-9). Cancer vaccines have received much attention in recent years due to their economical nature, and clinical studies have been conducted in the treatment of a variety of cancers (10-14). Cancer vaccines are mainly divided into messenger ribonucleic acid (mRNA) vaccines, peptide vaccines, and lentiviral vaccines, and their main difference is the different vectors that deliver the cancer antigens (6,15).
The mRNA tumor vaccine is generally prepared by using the template mRNA of translated proteins and injected into the body to synthesize specific antigenic proteins as “targets” through the protein synthesis system of human cells to induce an immune response to the "targets" and then target the tumor cells. mRNA vaccines have attracted much attention in the study of a variety of cancer vaccines because of their easy synthesis and economic advantages (16). mRNA vaccines do not integrate into the genome and can also be degraded by mRNA enzymes in vivo with good long-term safety (9,17), and their safety has also been confirmed in the process of counteracting coronavirus disease 2019 (18). Meanwhile, mRNA has the limitations of causing strong immune response and high storage conditions. However, to date no relevant studies on mRNA cancer vaccines in the treatment of LUSC have been conducted.
In this article, we used bioinformatics methods based on the specificity and immunogenicity of the vaccine to identify potential LUSC tumor antigens and revealed the immune landscape of patients with LUSC through a subsequent analysis, identified the characteristics of the population suitable for mRNA vaccination, provided a new perspective for the development of mRNA vaccines for LUSC, and emphasized the importance of individualized therapy. We present the following article in accordance with the STREGA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1113/rc).
Methods
Acquisition of public data on LUSC
The LUSC transcriptome and mutation data used in this study were downloaded from University of California, Santa Cruz website (https://xena.ucsc.edu/) [cohort: GDC The Cancer Genome Atlas (TCGA)-LUSC]. High-throughput sequence (HTSeq)-Fragments per kilobase of exon model per million mapped fragments (FPKM), somatic mutation data, and sample clinical information were downloaded (19). Masked copy number segment data for LUSC were downloaded from TCGA (https://portal.gdc.cancer.gov/). Genes associated with OS in LUSC were obtained from the Gene Expression Profiling Interactive Analysis 2 (GEPIA 2; http://gepia2.cancer-pku.cn/#survival) database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Identification of potential tumor antigens
A copy number variation analysis was performed using genomic identification of significant targets in cancer (GISTIC) 2.0 software (20). The human genome reference consortium human build 38 (GRCh38) was used as the reference genome, and the significance threshold was set at 0.01. Software default values were used for the remaining parameters. The genomic localization of all significantly amplified genes was visualized using the “RCircos” R package. The functional enrichment analysis of genes with significantly amplified copy numbers was performed using the “clusterprofiler” R package (21). All the mutated genes were visualized using the “maftools” R package (22). Venn diagrams identifying the mutations and amplified genes associated with OS in patients with LUSC were plotted by online tools (http://www.ehbio.com/test/venn/#/).
Identification of LUSC tumor antigens
To identify LUSC tumor antigens with higher immunogenicity, we analyzed the correlations of all potential tumor antigens with the level of infiltration of the 3 antigen-presenting cells using the tumor immune estimation resource (TIMER) online tool (23) (https://cistrome.shinyapps.io/timer/). Survival curves showing the expression of the potential tumor antigens were plotted using GEPIA 2 (24). In addition, the cohorts were divided into high and low groups using the expression of the 2 tumor antigens separately and analyzed for functional enrichment using the “gene set variation analysis (GSVA)” R package (25).
Identification of LUSC immune subtypes
All the immune-related genes were obtained from the IMMPORT database (https://www.immport.org/shared/). Immune-related gene expression matrices from the TCGA-LUSC cohort were extracted and subjected to consensus clustering using the “ConsensusClusterPlus” R package. The optimal number of clusters was determined to be 5 using the elbow method. The HALLMARK gene set used for the gene set enrichment analysis (GSEA) of patients in the 5 clusters was obtained from the MSigDB database (http://www.gsea-msigdb.org/gsea/downloads.jsp).
Immune landscape analysis among clusters
Immune-stromal scores were calculated using the R package “ESTIMATE”. The calculation of tumor purity was based on a previous publication (26). Tumor purity = cos (0.6049872018+0.0001467884× ESTIMATE score). A GSVA algorithm enrichment analysis was performed using 28 immune cell gene sets from previous publications (27). The immune checkpoints and immunogenic cell death modulators genes have been referred to in previously published articles (28).
Mutational landscape of LUSC immune subtypes
The tumor mutation burden (TMB) was calculated using the R package “maftools”. The mutated driver genes were identified using MutsigCV software (29) and visualized using the R package “maftools”.
Weighted gene co-expression network analysis (WGCNA)
The “WGCNAR” package (30) was used for the WGCNA with a soft threshold set at 50 to exclude outlying samples. In total, 6 gene co-expression modules were ultimately identified, and we calculated the correlations of the modules with the phenotypes of suitable vaccinated mRNA cancer vaccines from the above-mentioned immune landscape analysis. Finally, a univariate Cox regression analysis was performed of suitable vaccinations using genes in the most relevant modules to identify potential prognostic biomarkers after vaccination.
Drug sensitivity analysis
The drug sensitivity analysis was performed using the R package “pRRophetic” (31). In the analysis, we used the (genomics of drug sensitivity in cancer) GDSC database as the reference data to construct the model and performed an analysis of the sensitivity of multiple anti-cancer drugs on the 2 TCGA-LUSC subtypes that we had identified.
Statistical analysis
R software (version 4.1.1) (http://www.r-project.org/) and its corresponding R packages were used for statistical data analysis. Spearman correlation analysis was used to analyze the correlation between antigen-presenting cells and gene expression. A log-rank test was used to compare K-M curves for DFS and OS analysis. Kruskal-Wallis signed-rank test was used to compare gene expression, ESTIMATE scores, and TMB between multiple groups. Wilcoxon signed-rank test was used to compare the IC50 of anti-cancer drugs between two groups.
Results
Screening of potential tumor antigens in LUSC
To search for characteristic LUSC tumor antigens, we started with genomic variant events. First, regions of chromosomal copy number amplification in LUSC were identified by GISTIC software (see Figure 1A). We used all the copy number amplified genes as candidates for LUSC tumor antigens and plotted the chromosomal localization of partial genes (see Figure 1B). A Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of all the copy number amplified genes showed their involvement in the regulation of cancer-related pathways and immune-related pathways (see Figure 1C). In addition, as genetic mutations play an important role in tumor development and progression, we also included all genes with mutations as candidates (see Figure 1D). All the LUSC tumor antigen candidates were intersected with genes related to patient survival obtained from the GEPIA database, and ultimately, 14 potential tumor antigens related to LUSC prognosis were obtained for the downstream analysis (see Figure 1E).

Identification of LUSC tumor antigens
The key mechanism of mRNA vaccines lies in the activation of adaptive immunity by recognition by antigen-presenting cells (9). Thus, we used the correlation between the expression of potential tumor antigens and the infiltration level of the 3 antigen-presenting cells to screen for potential mRNA vaccine antigens. Using the TIMER online tool (https://cistrome.shinyapps.io/timer/), we identified 2 genes [i.e., bone morphogenetic protein 5 (BMP5) and claudin 5 (CLDN5)] in the 14 candidate tumor antigens whose expression presented a positive correlation with the level of infiltration of the antigen-presenting cells (see Figure 2A,2B). In addition, patients with high expression of these genes had worse OS and disease-free survival (see Figure 2C-2F). After grouping patients according to the expression of these 2 genes, the GSEA-based KEGG pathway enrichment analysis showed that the high expression group of these 2 genes had enriched immune-related pathways, while the low expression group had a more active cell cycle (see Figure 2G,2H). Taken together with the above results, while the high expression of BMP5 and CLDN5 positively regulates immune-related pathways, the high expression of tumor cells is associated with a worse prognosis. Thus, mRNA vaccines developed based on these 2 genes to exogenously vaccinate patients can be recognized by antigen-presenting cells and promote tumor immunity, while avoiding the worse prognosis brought about by the high expression of the tumor cells themselves.

Immune subtypes in patients with lung adenocarcinoma
To identify a potentially beneficial population for mRNA vaccination, we used immune-related genes to identify the different immune statuses of patients with lung adenocarcinoma by consensus clustering. Using the elbow method, we ultimately identified 5 lung adenocarcinomas with different immune statuses (see Figure 3A-3C). In addition, a survival analysis of these patients with different immune statuses showed that Clusters C and D had a poor prognosis (see Figure 3D). To understand the tumor statuses of different patients, we performed an enrichment analysis of the HALLMARK gene set using the GSVA algorithm. The results showed that Cluster D was significantly enriched in multiple carcinogenic pathways, which may be responsible for the poor prognosis of cluster D (see Figure 3E). Finally, we also checked the expression of the 2 potential tumor antigens identified above that could be used for mRNA vaccine development. The results showed that BMP5 and CLDN5 were highly expressed in stage I and Cluster B, which suggests that mRNA vaccines developed based on our identified tumor antigens may have potentially better benefits for early stage and Cluster B patients than other patients (see Figure 3F,3G).

Immune cell infiltration landscape of different immune subtypes
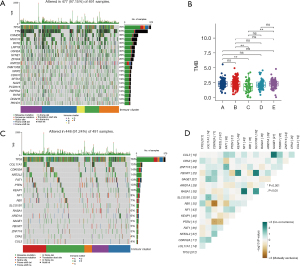
To identify the immune landscape between immune subtypes, we evaluated the immune-stromal scores of each cluster using the ESTIMATE algorithm. In the results, the higher scores of immune and stromal cells in Clusters B, C, and D, and the lower tumor purity predicted a high level of immune cell infiltration in the tumor microenvironment (see Figure 4A-4D). To quantify different immune cells in the tumor microenvironment, we conducted a GSVA enrichment analysis using gene sets of 28 immune cells previously published (27). After comparing the immune cell enrichment scores of different clusters, we also observed that Clusters B, C, and D had significantly higher immune cell infiltration than Clusters A and E (see Figure 4E). Thus, Clusters B, C, and D are immune “Hot” subtypes, while Cluster A and E are immune “Cold” subtypes (32), and patients in Clusters B, C and D may have better immunogenicity to mRNA vaccinations. In addition, we also compared the expression of immune checkpoints and immunogenic cell death modulator genes in each cluster (see Figure 4F). These genes were highly expressed in Clusters B, C, and D, which indicates that patients from these clusters may also have better sensitivity to immunotherapy, targeted therapy, or chemotherapy than other patients (see Figure 4G,4H). In conclusion, our findings reflect the diversity of tumor treatment for lung adenocarcinoma, and treatments should be precisely individualized for individuals to obtain better benefits.

Identification of mutation landscapes of different clusters
To identify the different mutational landscapes, we visualized the top 20 mutation genes of the TCGA-LUSC cohort and calculated the TMB for each sample (see Figure 5A,5B). We found that among all the clusters, only Cluster C had a lower TMB, which suggests that the development of a mutation-based gene-targeted therapy strategy for Cluster C will be limited, but the development of mRNA vaccines may provide a new therapeutic method for Cluster C. We also identified driver mutation genes in LUSC using the MutsigCV algorithm (29) and investigated the correlations among them (see Figure 5C). Overall, most of the mutations that occurred between them were independent, but there was a significant mutual exclusion of mutations in cyclin dependent kinase inhibitor 2A (CDKN2A) and retinoblastoma protein transcriptional corepressor 1 (RB1), while there was significant co-occurrence of mutations in neurofibromin 1 (NF1) and RAS P21 protein activator 1 (RASA1) (see Figure 5D).
WGCNA
To identify the genes associated with suitable vaccination clusters, we performed the module identification of all immune-related genes by WGCNA. The outlier samples were first sieved out by clustering the samples, and an optimal soft threshold of 8 was determined by connectivity (see Figure 6A-6C). Ultimately, we identified 5 modules in all the immune-related genes, and merged Clusters B, C, and D as the “Suitable” group and Clusters A and E as the “Not-suitable” group. After calculating the association between the modules and phenotypes, we found that the population suitable for mRNA vaccination had the highest correlation coefficient with the “brown” module (see Figure 6D,6E). In addition, in a prognostic univariate Cox analysis in a suitable vaccinated population, we found 2 genes in “brown” module with P values <0.05 and a hazard ratio >1 [i.e., immunoglobulin heavy variable 7-81 (IGHV7-81) and immunoglobulin kappa variable 2-40 (IGKV2-40)] (see Figure 6F). These 2 genes were previously reported to be immunoglobulin component related genes (33). Thus, these 2 genes may be used as vaccine response biomarkers in LUSC patients suitable for mRNA vaccination.

Finally, we performed a drug sensitivity analysis of commonly used clinical treatments for samples suitable and unsuitable for mRNA vaccination and observed that patients unsuitable for mRNA vaccination were more sensitive to 2 chemotherapeutic agents (i.e., cisplatin, and etoposide) (see Figure 6G,6H). In addition, in relation to the current epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) commonly used in clinical practice, there was no significant difference in the sensitivity of gefitinib between the 2 groups, but there was a significant difference in the sensitivity of erlotinib between the 2 groups. We speculated that this may be due to the different EGFR mutation landscapes between the 2 groups (see Figure 6I,6J). Thus, the treatment of LUSC should focus on the development of an individualized treatment strategy.
Discussion
As an emerging immunotherapy, cancer vaccines have attracted much attention from researchers (6,9). In addition, because mRNA vaccines do not integrate into the genome avoiding mutations generated during genome integration and can be degraded by abundant mRNA enzymes in the body, they are also easier to prepare than peptide vaccines (16,17,34). Thus, mRNA vaccines have potential clinical application value because of their easy preparation and long-term safety (16,17,34). The main mechanism of action of cancer vaccines is that tumor antigens delivered by various carriers can be recognized by antigen-presenting cells and induce the body to produce immune memory to activate the immune system to produce anti-tumor effects (35,36). Thus, it is essential to search for tumor antigens with good specificity and immunogenicity for vaccine development in different cancers.
In this study, we screened mutant and copy number amplified genes associated with the prognosis of patients with LUSC to identify potential specific tumor antigen candidates for squamous cell carcinoma of the lung, and further analyzed their expression in correlation with the level of infiltration of 3 common antigen-presenting cells to determine their immunogenicity. Ultimately we identified 2 potential tumor antigens (i.e., BMP5 and CLDN5) that could be used for the development of mRNA vaccines for LUSC.
It has been reported that BMP5 is associated with clinical prognosis and plays a role as a tumor suppressor in patients with a variety of cancers (37-39). However, there are no relevant studies on BMP5 in LUSC. In our study, we found that patients with high BMP5 expression had a worse prognosis. Additionally, the activity of cell cycle-related pathways in patients in the high BMP5 expression group revealed the multiple role of BMP5 in cancer. In addition, BMP5 expression is associated with the infiltration of a variety of antigen-presenting cells in LUSC. Thus, due to its good immunogenicity, BMP5 can be used as a potential mRNA vaccine tumor antigen to block BMP5 targets by inducing immune memory.
In a previous study, CLDN5 was identified as a diagnostic biomarker for lung adenocarcinoma and LUSC (40). Additionally, CLDN5 can inhibit the cell cycle G1-S transition by decreasing the expression of cyclin D1 in LUSC cells (41). However, to date, no anti-tumor immune-related studies of CLDN5 in LUSC appear to have been conducted. Our study showed the good specificity and immunogenicity of CLDN5 as a potential LUSC tumor antigen, which could potentially be developed as a mRNA vaccine.
Individualized treatment is essential in overall cancer treatment (42,43), and different immune subtypes can reflect the current immune status of patients, and are informative for the prediction of treatment responsiveness (44,45). In this study, we clustered the entire TCGA-LUSC cohort using all the immune-related genes and identified 5 immune subtypes. A further analysis showed that different immune subtypes had different survival states, different activation states of cancer-related pathways, and different mutational landscapes. Ultimately, we identified subtypes with abundant immune cell infiltration in the tumor microenvironment as potential markers of good responsiveness to mRNA vaccination, and further identified predictive potential benefits after vaccination by a WGCNA analysis. Through anti-cancer drug sensitivity analysis, we also found that patients who are not suitable for mRNA vaccine therapy may respond better to chemotherapy regimens based on Cisplatin, Etoposide and molecularly targeted drug regimens based on Erotinib.
Cancer vaccines are now considered to have potential clinical applications; however, there are still some problems to be solved in the development of vaccines (9). For example, the discovery of cancer antigens requires in-depth study and clinical validation by researchers, and the selection of vaccine vectors is related to the efficacy and long-term safety of cancer vaccines (46,47), and further studies are needed. However, the unique advantages of mRNA vaccines also make them an excellent prospect for application, and the development of LUSC mRNA vaccines based on BMP5, CLDN5 or other potential antigens will enrich the treatment options for LUSC patients.
Since our study is a bioinformatics-based study, the lack of experimental validation is a shortcoming of this paper. We have added a description in the discussion section to point out our shortcomings.
In conclusion, in this article, we identified 2 potential tumor antigens by bioinformatics methods that can be used for mRNA vaccine development in LUSC and analyzed the immune landscape of patients with LUSC, providing a new perspective for mRNA vaccine development in LUSC. There have been related studies on mRNA vaccine development in other cancers (28,48-52); however, this is the first study on mRNA vaccine development in LUSC. In addition, drug sensitivity analyses of populations with different immune subtypes also emphasize the importance of individualized treatment.
Acknowledgments
The survival analysis in this article used the GEPIA2 online tool (http://gepia2.cancer-pku.cn). The correlation analysis between gene expression and immune cell infiltration was performed using the TIMER online tool (https://cistrome.shinyapps.io/timer/).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STREGA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1113/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1113/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 2018;17:38. [Crossref] [PubMed]
- Yuan H, Liu J, Zhang J. The Current Landscape of Immune Checkpoint Blockade in Metastatic Lung Squamous Cell Carcinoma. Molecules 2021;26:1392. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- DeMaria PJ, Bilusic M. Cancer Vaccines. Hematol Oncol Clin North Am 2019;33:199-214. [Crossref] [PubMed]
- Feins S, Kong W, Williams EF, et al. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol 2019;94:S3-9. [Crossref] [PubMed]
- Khan P, Siddiqui JA, Lakshmanan I, et al. RNA-based therapies: A cog in the wheel of lung cancer defense. Mol Cancer 2021;20:54. [Crossref] [PubMed]
- Xu S, Yang K, Li R, et al. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int J Mol Sci 2020;21:6582. [Crossref] [PubMed]
- Snook AE, Baybutt TR, Xiang B, et al. Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients. J Immunother Cancer 2019;7:104. [Crossref] [PubMed]
- Sahin U, Oehm P, Derhovanessian E, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020;585:107-12. [Crossref] [PubMed]
- Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547:217-21. [Crossref] [PubMed]
- Charles J, Chaperot L, Hannani D, et al. An innovative plasmacytoid dendritic cell line-based cancer vaccine primes and expands antitumor T-cells in melanoma patients in a first-in-human trial. Oncoimmunology 2020;9:1738812. [Crossref] [PubMed]
- Cafri G, Gartner JJ, Zaks T, et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest 2020;130:5976-88. [Crossref] [PubMed]
- Jahanafrooz Z, Baradaran B, Mosafer J, et al. Comparison of DNA and mRNA vaccines against cancer. Drug Discov Today 2020;25:552-60. [Crossref] [PubMed]
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 2018;17:261-79. [Crossref] [PubMed]
- Sullenger BA, Nair S. From the RNA world to the clinic. Science 2016;352:1417-20. [Crossref] [PubMed]
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-15. [Crossref] [PubMed]
- Goldman MJ, Craft B, Hastie M, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol 2020;38:675-8. [Crossref] [PubMed]
- Mermel CH, Schumacher SE, Hill B, et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol 2011;12:R41. [Crossref] [PubMed]
- Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284-7. [Crossref] [PubMed]
- Mayakonda A, Lin DC, Assenov Y, et al. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res 2018;28:1747-56. [Crossref] [PubMed]
- Li T, Fan J, Wang B, et al. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res 2017;77:e108-10. [Crossref] [PubMed]
- Tang Z, Kang B, Li C, et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res 2019;47:W556-60. [Crossref] [PubMed]
- Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 2013;14:7. [Crossref] [PubMed]
- Yoshihara K, Shahmoradgoli M, Martínez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013;4:2612. [Crossref] [PubMed]
- Charoentong P, Finotello F, Angelova M, et al. Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep 2017;18:248-62. [Crossref] [PubMed]
- Huang X, Zhang G, Tang T, et al. Identification of tumor antigens and immune subtypes of pancreatic adenocarcinoma for mRNA vaccine development. Mol Cancer 2021;20:44. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [Crossref] [PubMed]
- Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008;9:559. [Crossref] [PubMed]
- Geeleher P, Cox N, Huang RS. pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS One 2014;9:e107468. [Crossref] [PubMed]
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18:197-218. [Crossref] [PubMed]
- Lefranc MP. Immunoglobulin and T Cell Receptor Genes: IMGT(®) and the Birth and Rise of Immunoinformatics. Front Immunol 2014;5:22. [Crossref] [PubMed]
- Kim J, Eygeris Y, Gupta M, et al. Self-assembled mRNA vaccines. Adv Drug Deliv Rev 2021;170:83-112. [Crossref] [PubMed]
- Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer 2021;20:41. [Crossref] [PubMed]
- Grunwitz C, Kranz LM. mRNA Cancer Vaccines-Messages that Prevail. Curr Top Microbiol Immunol 2017;405:145-64. [Crossref] [PubMed]
- Tremblay M, Viala S, Shafer ME, et al. Regulation of stem/progenitor cell maintenance by BMP5 in prostate homeostasis and cancer initiation. Elife 2020;9:54542. [Crossref] [PubMed]
- Karim MA, Samad A, Adhikari UK, et al. A Multi-Omics Analysis of Bone Morphogenetic Protein 5 (BMP5) mRNA Expression and Clinical Prognostic Outcomes in Different Cancers Using Bioinformatics Approaches. Biomedicines 2020;8:19. [Crossref] [PubMed]
- Chen E, Yang F, He H, et al. Alteration of tumor suppressor BMP5 in sporadic colorectal cancer: a genomic and transcriptomic profiling based study. Mol Cancer 2018;17:176. [Crossref] [PubMed]
- Paschoud S, Bongiovanni M, Pache JC, et al. Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol 2007;20:947-54. [Crossref] [PubMed]
- Akizuki R, Shimobaba S, Matsunaga T, et al. Claudin-5, -7, and -18 suppress proliferation mediated by inhibition of phosphorylation of Akt in human lung squamous cell carcinoma. Biochim Biophys Acta Mol Cell Res 2017;1864:293-302. [Crossref] [PubMed]
- Vormehr M, Türeci Ö, Sahin U. Harnessing Tumor Mutations for Truly Individualized Cancer Vaccines. Annu Rev Med 2019;70:395-407. [Crossref] [PubMed]
- Jackson SE, Chester JD. Personalised cancer medicine. Int J Cancer 2015;137:262-6. [Crossref] [PubMed]
- Pan Y, Han H, Labbe KE, et al. Recent advances in preclinical models for lung squamous cell carcinoma. Oncogene 2021;40:2817-29. [Crossref] [PubMed]
- Li B, Cui Y, Nambiar DK, et al. The Immune Subtypes and Landscape of Squamous Cell Carcinoma. Clin Cancer Res 2019;25:3528-37. [Crossref] [PubMed]
- Oberli MA, Reichmuth AM, Dorkin JR, et al. Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Lett 2017;17:1326-35. [Crossref] [PubMed]
- Midoux P, Pichon C. Lipid-based mRNA vaccine delivery systems. Expert Rev Vaccines 2015;14:221-34. [Crossref] [PubMed]
- Zhou Q, Yan X, Zhu H, et al. Identification of three tumor antigens and immune subtypes for mRNA vaccine development in diffuse glioma. Theranostics 2021;11:9775-90. [Crossref] [PubMed]
- Zheng X, Xu H, Yi X, et al. Tumor-antigens and immune landscapes identification for prostate adenocarcinoma mRNA vaccine. Mol Cancer 2021;20:160. [Crossref] [PubMed]
- Ye L, Wang L, Yang J, et al. Identification of tumor antigens and immune subtypes in lower grade gliomas for mRNA vaccine development. J Transl Med 2021;19:352. [Crossref] [PubMed]
- Ye L, Wang L, Yang J, et al. Identification of Tumor Antigens and Immune Landscape in Glioblastoma for mRNA Vaccine Development. Front Genet 2021;12:701065. [Crossref] [PubMed]
- Huang X, Tang T, Zhang G, et al. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol Cancer 2021;20:50. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


