Retrospectively analyze and compare the efficacy and safety of thoracoscopic-assisted Nuss repair of pectus excavatum under intubation anesthesia and non-intubation anesthesia
Introduction
Pectus excavatum (PE) is the most common congenital chest wall malformation, accounting for 90% of all anterior chest wall malformations (1,2). Surgical repair of chest depression has always been the most effective treatment for PE (3). Previous methods of PE repair included Ravitch surgery (4), sternal costal cartilage lifting surgery (5), and sternal turnover surgery (6), all of which were invasive, had more complications, and had a high recurrence rate for patients (7). In 1998, Professor Nuss et al. (8,9) reported a minimally invasive and easy-to-master technique for repairing chest wall depression malformation from the back of the sternum with a pectus bar, characterized by minimal trauma, a short operation time, and fast postoperative recovery. Since then, with some refinements, minimally invasive repair of PE (MIRPE, also known as the Nuss procedure) has been primarily used in the surgical repair of PE (10).
Endotracheal intubation combined with muscle relaxant general anesthesia is the standard anesthesia used in the Nuss procedure repair of PE (11). However, adverse events arising from intubation-related injuries, such as an increased risk of pneumonia, impaired cardiac function, postoperative nausea and vomiting, and hoarseness, have been associated with endotracheal intubation (12,13). It has been reported (14,15) that surgical treatment under non-tracheal intubation anesthesia has the advantages of less trauma, less anesthetic dose, less postoperative complications, and fast recovery. In non-tracheal intubation anesthesia in the Nuss procedure repair of PE, the use of laryngeal mask instead of tracheal intubation will not damage the tracheal mucosa of patients, and patients in the spontaneous breathing state close to the normal physiological state of the lung. In addition, non-intubation anesthesia without the use of muscle relaxants has little effect on the diaphragm, digestive system and limb muscle function. Therefore, this study aims to investigate whether thoracoscopic-assisted Nuss procedure repair of pectus excavatum under non-intubated anesthesia has the advantages of reducing intraoperative risks, postoperative complications and accelerating postoperative recovery of patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1150/rc).
Methods
Study population and data collection
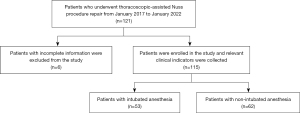
A total of 121 PE patients who underwent thoracoscopic-assisted Nuss procedure repair in the Department of Thoracic Surgery of Yunnan First People’s Hospital from January 2017 to January 2022 were eligible for inclusion. Six patients with incomplete information were excluded; hence, 115 PE patients were finally included in this study. All patients completed preoperative examinations, such as routine blood tests, biochemical blood index, chest CT, electrocardiogram, and color doppler echocardiography. Patients were randomly divided into intubation anesthesia (IN) group and non-intubation anesthesia (N-IN) group before surgery according to different anesthesia methods as long as they met the operation and anesthesia guidelines. Then the same surgical team performed the same thoracoscopic-assisted Nuss repair procedure. Chest X-rays were performed on the first and third postoperative days, and routine blood and biochemical examinations were performed on the third postoperative day. No postoperative complications, such as fever or infection, were found, and the patients were discharged after recovery. According to the different anesthesia methods, 53 patients in the IN group and 62 patients in the N-IN group were finally included in this study. Preoperative, intraoperative, and postoperative indicators were recorded and retrospectively analyzed (Figure 1).
Ethical approval for the study was granted by the Ethics Committee of The First People’s Hospital of Yunnan Province (No. KHLL2022-KY012). In addition, written informed consent was obtained from all patients who participated in the study. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
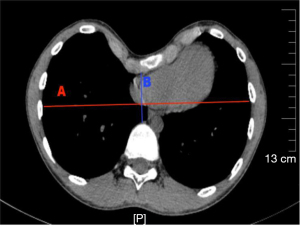
We compared the relevant indicators recorded in the IN and N-IN groups. Their baseline clinical characteristics and preoperative indicators are shown in Table 1. There was no significant difference in the baseline clinical characteristics between the two groups, which was comparable. The Haller index is the transverse diameter of the chest at the lowest depression divided by the distance from the lowest depression to the anterior vertebral body as measured by chest CT (Figure 2). Anesthesia intubation time refers to the time the anesthesiologist takes to accurately insert the endotracheal intubation or laryngeal mask (China Zhejiang Dawning Technology Co. Ltd., China) and fix the patient’s position once they lose consciousness. The operative time was recorded from the first skin incision to the final skin suturing. Intraoperative blood loss was measured by the volume of all fluid in the container connected to the aspirator during surgery minus the fluid irrigating the chest. In addition, the mean intraoperative oxygen saturation, heart rate, blood pressure, and End-Tidal Carbon Dioxide (ETCO2) values, and whether arrhythmias occurred were recorded.
Table 1
| Variable | N-IN group | IN group | P value |
|---|---|---|---|
| Age (years) | 8.69±4.21 | 9.00±3.82 | 0.525 |
| Sex (male/female) | 17 (27.4)/45 (72.6) | 7 (15.2)/46 (84.8) | 0.058 |
| Haller index | 3.44±0.18 | 3.99±0.19 | 0.088 |
| Preoperative electrocardiogram (normal/abnormal) | 28 (45.2)/34 (54.8) | 26 (49.1)/27 (51.9) | 0.677 |
| BMI (kg/m2) | 22.16±2.49 | 22.00±2.52 | 0.480 |
| ASA classification (I/II) | 30 (45.2)/32 (54.8) | 28 (45.2)/25 (54.8) | 0.635 |
| Preoperative WBC, ×109 | 6.37±1.62 | 6.54±1.06 | 0.09 |
| Preoperative NEUT, ×109 | 4.92±1.38 | 4.91±1.17 | 0.654 |
| Preoperative LYMPH, ×109 | 1.98±0.68 | 2.01±0.57 | 0.216 |
| Preoperative mean SBP (mmHg) | 107.26±7.09 | 104.70±7.53 | 0.071 |
| Preoperative mean DBP (mmHg) | 66.65±5.91 | 68.09±6.46 | 0.128 |
| Preoperative mean heart rate (min−1) | 76.94±9.71 | 77.32±12.59 | 0.532 |
Continuous data are presented as mean ± SD and categoric variables as number (frequency and/or %). P<0.05 is considered significant. Haller index is the transverse diameter of the chest at the lowest depression/the distance from the lowest depression to the anterior vertebral body as measured by chest CT. N-IN, non-intubation anesthesia; IN, intubation anesthesia; BMI, body mass index; ASA, Anesthesiologists; WBC, white blood cell; NEUT, neutrophil; LYMPH, lymphocyte; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; CT, computerized tomography.
Bedside chest X-rays (Figure 3) were performed on the first and third postoperative days, and the presence of any pectus bar displacement, lung infection, pneumothorax, or pleural effusion was recorded. The first postoperative ambulation time referred to the first time the patient could get out of bed independently after returning to the thoracic surgical care unit. Similarly, the time of postoperative first oral food and water intake and the time of postoperative first defecation were, respectively, the time when the patient tolerated a liquid diet or water without choking and the time of the first postoperative defecation after returning to the thoracic surgical care unit. Routine blood results, such as white blood cell count, neutrophils, and absolute lymphocyte count, were recorded on the third day after surgery.
Inclusion criteria
- Patients with pectus excavatum whose Haller index was greater than 3.25 as measured by chest CT;
- American Society of Anesthesiologists Standard (ASA) grade of ≤ II;
- Patients with complete information;
- The deformity causes poor body-image and psychosocial impairments for the patient.
Exclusion criteria
- The patient had contraindications for surgery, such as severe congenital heart disease;
- The patient was younger than 3 or older than 18 years;
- Body mass index (BMI) >30 kg/m2;
- Difficult airway management;
- The patient or authorized client did not consent to the Nuss repair.
Preoperative preparation
All patients underwent preoperative routine blood examination, X-ray, electrocardiogram, color doppler echocardiography, lung function, chest CT, and other examinations. In the patient’s natural standing position, the lowest point of the PE depression, the entry points of the pectus bar, and the incision sites from the axillary front to the midaxillary line were marked. Then, a soft ruler was used to measure the distance between the midaxillary line on both sides of the lowest point of the depression to evaluate the degree of depression and to ascertain the appropriate length of the pectus bar (Shenzhen Pty Medical Device Co. Ltd., China).
Anesthesia
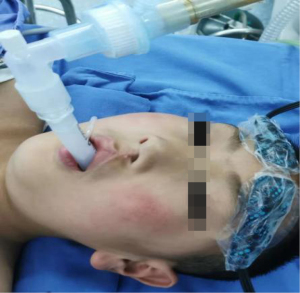
Indications for non-intubation anesthesia are as follows: (I) BMI ≤30 kg/m2; (II) there was no airway abnormality or foreseeable difficult airway; (III) the patient had no cardiovascular or cerebrovascular disease, asthma, or chronic obstructive pulmonary disease; (IV) there was no serious adhesion to the pleural cavity. Indications for intubation anesthesia are as follows: (I) the patient’s blood pressure was ≤140/90 mmHg; (II) laryngeal anatomy was normal or without laryngeal edema. Patients in both groups fasted for 8 h and abstained from drinking for 4 h before anesthesia (16). After the patients were admitted to the anesthesia operating room, intravenous access was opened, oxygen was inhaled, electrocardiogram (ECG), blood pressure, heart rate, ETCO2, and blood oxygen saturation were routinely monitored (17). After the anesthesiologist prepared the anesthesia-related drugs and equipment, the following drugs were administered through intravenous channels to induce anesthesia in the N-IN group patients: midazolam 0.1 mg/kg, etomidate 0.3 mg/kg, dexamethasone 10 mg, and atropine 0.5 mg. After loss of consciousness, disappearance of the eyelash reflex, and relaxation of the jaw were observed, the appropriate type of laryngeal mask was selected and inserted into the oral pharynx. The laryngeal mask was adjusted to the appropriate position and then inflated into the balloon, then the laryngeal mask was connected to the anesthesia machine to maintain a 30–40% oxygen concentration to keep the patient breathing spontaneously (Figure 4). The anesthesiologist performed the third and the fourth intercostal nerve blocks with 0.5% ropivacaine 1 mL and serratus anterior muscle blocks with 0.5% ropivacaine 20 mL under the guidance of an ultrasound probe. Propofol 10 mg/kg/h and remifentanil 0.4 µg/kg/h were pumped intravenously to maintain anesthesia, and the drug dosage was adjusted according to EEG consciousness depth and hemodynamics (18).
Anesthesia induction and maintenance methods were the same for the IN group as the N-IN group, but the intercostal and serratus anterior nerve blocks were not performed in the IN group. After induction of anesthesia for patients in the IN group, the anesthesiologist injected vecuronium 0.1 mg/kg intravenously and, after the muscle relaxants took effect, placed the appropriate type of trachea using a visual laryngoscope and connected it to a ventilator.
After surgery, both groups of patients were sent to the Department of Anesthesiology resuscitation room. The airway device was removed if the patient met the criteria for removal, such as restored airway reflexes and normal spontaneous breathing with inhaled air oxygen saturation ≥95%. If the patient met the criteria for being transferred out of the anesthesia resuscitation room (Steward score >4, good state of consciousness, etc.), the patient was transferred from the anesthesia resuscitation room to the thoracic surgical care unit (19).
Surgery
The surgical methods of the two groups were identical, and the same surgical team performed the Nuss repair. All patients were placed in the supine double arm abduction position and close to the right side of the operating table. The thoracic surgeon evaluated the prepared pectus bar and the patient’s chest deformity (Figure 5) and reshaped the steel bar based on the assessment (Figure 6). The thoracic surgeon determined the lowest point of the patient’s sternal depression and made an incision of 1.5–3.0 cm in length from the right axillary front to the midaxillary line. The submuscular space was separated from the incision. Then vascular forceps were used to blunt through the intercostal muscle to enter the pleural cavity at the highest point of the right edge of the depression. Blunt expansion was performed to make a tunnel towards the edge of the sternal depression, which was the entry point of the pectus bar. A thoracoscopic sheath was inserted in the intercostal space below the right incision, and a 5 mm diameter thoracoscope was inserted. Under thoracoscopic visualization, the guiding instrument was inserted via a right incision through the chest wall tunnel, which penetrated through the intercostal margin of the depression into the right thoracic cavity and then slowly through the nadir of the sternum depression and the anterior mediastinum of the pericardium into the left thoracic cavity to the marker of the left margin of the depression. Special attention was paid to the gentle movement of the guiding instrument during its penetration to avoid damage caused by close contact with the heart and pericardium. In addition, the ECG waveform and heart rate were closely observed. If there was no apparent interference or fluctuation, there was no risk of heart injury.
After removal of the guiding instrument, the pectus bar that had been prepared in advance was placed along the tunnel, with the concave surface of the bar facing upward. After slowly maneuvering it into the appropriate position, the steel bar was rotated 180° and adjusted to the chest shape to achieve the desired effect. The pectus bar was fixed with a grooved plate on the right side and attached to the rib periosteum and chest wall tissue using dacron wire, leaving the left side unfixed. Then, the thoracoscope and thoracic cannula were removed, and a catheter was placed at the tunnel position of the thoracoscope cannula. The skin and subcutaneous tissues were sutured layer by layer, and after the lung was inflated to drain the gas in the thorax, the catheter was removed, and the skin was sutured, completing the thoracoscopic-assisted Nuss procedure repair for PE (20).
Statistical analysis
IBM SPSS Statistics 26.0 statistical software was used for the data analysis. If both groups met normality, the mean ± standard deviation (SD) was used for statistical description, and the T-test was used for comparison between groups. Otherwise, the median was used for statistical description, and the nonparametric test was used for comparison between groups. The categorical variables were expressed as percentages (%), and the Mann-Whitney U-test was used to examine the differences between the two groups. The test level was α=0.05, and P<0.05 was considered statistically significant.
Results
A total of 115 patients with PE who underwent thoracoscopic assisted NUSS repair were enrolled in this study, including 62 patients in the N-IN group and 53 patients in the IN group. There was no statistical significance between the two patient groups in age (8.69±4.21 vs. 9.00±3.82 years, P=0.525), gender (P=0.058), Haller index (3.44±0.18 vs. 3.99±0.19, P=0.088), preoperative electrocardiogram (P=0.677), BMI (22.16±2.49 vs. 22.00±2.52 kg/m2, P=0.480), ASA classification (I/II) (P=0.635), preoperative white blood cell (WBC) [(6.37±1.62)×109 vs. (6.54±1.06)×109, P=0.09], preoperative neutrophil (NEUT) [(4.92±1.38)×109 vs. (4.91±1.17)×109, P=0.654], preoperative lymphocyte (LYMPH) [(1.98±0.68)×109 vs. (2.01±0.57)×109, P=0.216], preoperative mean systolic blood pressure (SBP) (107.26±7.09 vs. 104.70±7.53 mmHg, P=0.071), preoperative mean diastolic blood pressure (DBP) (66.65±5.91 vs. 68.09±6.46 mmHg, P=0.128) and preoperative mean heart rate (76.94±9.71 vs. 77.32±12.59 min, P=0.532) (Table 1).
There were significant differences between the two groups (Figures 7,8) in anesthesia intubation time (2.68±1.24 vs. 5.98±1.25 min, P<0.001), intraoperative mean ETCO2 (56.48±5.83 vs. 37.72±3.49 mmHg, P<0.001) and intraoperative mean heart rate (76.39±9.14 vs. 84.57±14.67 min, P=0.003). However, the operative time (63.60±15.95 vs. 61.91±15.31 min, P=0.902), intraoperative blood loss (16.00±9.61 vs. 15.09±13.10 mL, P=0.109), intraoperative mean SpO2 (97.21%±1.62% vs. 97.02%±1.59%, P=0.064), intraoperative arrhythmia (P=0.911), postoperative drainage tube placement (P=0.376), intraoperative mean SBP (108.44±8.03 vs. 104.17±7.25 mmHg, P=0.071), and intraoperative mean DBP (65.48±6.81 vs. 66.92±5.54 mmHg, P=0.153) of the two groups were not statistically significant (Table 2).
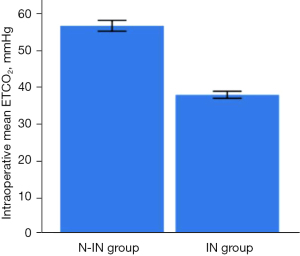
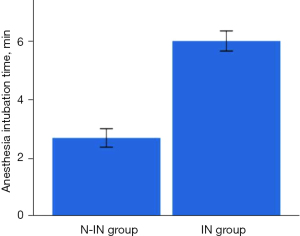
Table 2
| Variable | N-IN group | IN group | P value |
|---|---|---|---|
| Anesthesia intubation time (min) | 2.68±1.24 | 5.98±1.25 | <0.001 |
| Operation time (min) | 63.60±15.95 | 61.91±15.31 | 0.902 |
| Intraoperative blood loss (mL) | 16.00±9.61 | 15.09±13.10 | 0.109 |
| Intraoperative mean ETCO2 (mmHg) | 56.48±5.83 | 37.72±3.49 | <0.001 |
| Intraoperative mean SpO2 (%) | 97.21±1.62 | 97.02±1.59 | 0.064 |
| Intraoperative mean heart rate (min−1) | 76.39±9.14 | 84.57±14.67 | 0.003 |
| Intraoperative arrhythmia (yes/no) | 1 (1.6)/61 (98.4) | 1 (1.9)/52 (98.1) | 0.911 |
| Postoperative drainage tube placement (yes/no) | 3 (5.1)/59 (94.9) | 1 (1.9)/52 (98.1) | 0.376 |
| Intraoperative mean SBP (mmHg) | 108.44±8.03 | 104.17±7.25 | 0.071 |
| Intraoperative mean DBP (mmHg) | 65.48±6.81 | 66.92±5.54 | 0.153 |
Continuous data are presented as mean ± SD and categoric variables as number (frequency and/or %). P<0.05 is considered significant. N-IN, non-intubation anesthesia; IN, intubation anesthesia; ETCO2, End-Tidal Carbon Dioxide; SpO2, peripheral oxygen saturation; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation.
Postoperative complications in the two groups, such as pneumothorax (P=0.03), pleural effusion (P=0.028), lung infection (P=0.013), postoperative first oral food intake (2.47±0.45 vs. 5.08±0.74 h, P<0.001), postoperative first oral water intake (1.89±0.49 vs. 4.44±0.48 h, P<0.001), postoperative first ambulation (2.52±0.57 vs. 14.23±3.65 h, P<0.001), postoperative first defecation (12.31±2.37 vs. 19.89±2.29 h, P<0.001), and postoperative discharge time (4.84±1.37 vs. 7.58±2.76 days, P<0.001) were all statistically significant (Figure 9), except for plate displacement. In addition, 24-hour postoperative routine blood results, such as WBC [(6.76±1.31)×109 vs. (8.79±1.92)×109, P<0.001], NEUT [(5.04±1.62)×109 vs. (6.46±2.35)×109, P=0.001], and LYMPH [(1.92±0.81)×109 vs. (2.30±0.71)×109, P=0.014] were significantly different (Figure 10). Total hospitalization expenses (22,060.89±6,859.96 vs. 31,923.43±4,642.00 CNY, P<0.001) were also statistically significant (Table 3).
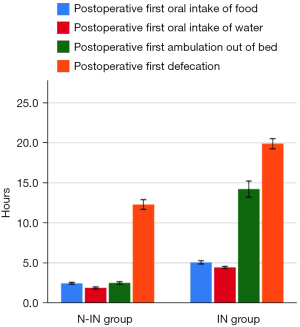
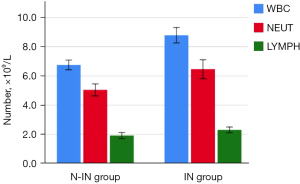
Table 3
| Variable | N-IN group | IN group | P value |
|---|---|---|---|
| Postoperative complications | |||
| Steel bar displacement (yes/no) | 0 (0.0)/62 (100.0) | 1 (1.9)/52 (98.1) | 0.277 |
| Pneumothorax (yes/no) | 1 (1.6)/61 (98.4) | 6 (11.3)/47 (88.7) | 0.03 |
| Pleural effusion (yes/no) | 0 (0.0)/62 (100.0) | 4 (7.5)/49 (92.5) | 0.028 |
| Lung infection (yes/no) | 0 (0.0)/62 (100.0) | 5 (9.4)/48 (90.6) | 0.013 |
| Postoperative first oral food intake (h) | 2.47±0.45 | 5.08±0.74 | <0.001 |
| Postoperative first oral water intake (h) | 1.89±0.49 | 4.44±0.48 | <0.001 |
| Postoperative first ambulation (h) | 2.52±0.57 | 14.23±3.65 | <0.001 |
| Postoperative first defecation (h) | 12.31±2.37 | 19.89±2.29 | <0.001 |
| Postoperative discharge time (days) | 4.84±1.37 | 7.58±2.76 | <0.001 |
| Blood routine results on the third day after surgery | |||
| WBC, ×109/L | 6.76±1.31 | 8.79±1.92 | <0.001 |
| NEUT, ×109/L | 5.04±1.62 | 6.46±2.35 | 0.001 |
| LYMPH, ×109/L | 1.92±0.81 | 2.30±0.71 | 0.014 |
| Total hospitalization expenses (CNY) | 22,060.89±6,859.96 | 31,923.43±4,642.00 | <0.001 |
Continuous data are presented as mean ± standard deviation (SD) and categoric variables as number (frequency and/or %). P<0.05 is considered significant. N-IN, non-intubation anesthesia; IN, intubation anesthesia; WBC, white blood cell; NEUT, neutrophil; LYMPH, lymphocyte.
Discussion
Fast track surgery (FTS) is the latest rehabilitation concept proposed in recent years (21). It is an evidence-based medical approach using surgery, anesthesia, care, nutrition, and cooperation with other departments to optimize the perioperative management of the clinical pathway so as to alleviate perioperative stress reactions, reduce postoperative complications, shorten the length of stay, and promote the recovery of patients (22,23). With the development of the FTS concept, PE repair has evolved from the traditional Ravitch sternal elevation to thoracoscopic-assisted Nuss minimally invasive repair (24).
In the past, Ravitch surgery was performed with an 8–15 cm incision in the chest wall depression to cut and dissociate the abnormal costal periosteum, remove bilateral excess costal cartilage, and cut off the sternum in a V-shape (25). Compared with Ravitch sternal elevation, thoracoscopic-assisted Nuss repair has a smaller and more concealed incision, shorter operation time, easier operation, less bleeding, earlier postoperative activity, and no need to remove the costal cartilage or sternum (26,27). In addition, thoracoscopic monitoring can obtain a better surgical field of vision, look directly into the chest, and effectively avoids damage to the pleura, pericardium, liver, or other organs from the guide instrument or steel bar, making the operation safer and more reliable (28). In this study, thoracic surgeons performed the Nuss repair via a single incision on the right side of the patient’s chest wall. Compared with previous bilateral chest wall incisions, there was less trauma, less intraoperative bleeding, a shorter operative time, and a lower risk of exposure to the internal fixation plate (12). Therefore, it can obtain a satisfactory thoracic appearance with less trauma, which is in line with the concept of FTS and has been widely used in clinical practice (29).
Anesthesia is an important part of FTS, so it is necessary to constantly optimize anesthesia management to reduce the significant impact of anesthesia on the body’s physiological function (30). General anesthesia with single endotracheal intubation is the traditional anesthesia method for Nuss PE repair (31). Endotracheal intubation is usually guided by video laryngoscope, and intubation is performed after the glottis is located (32). The laryngeal mask is a new, minimally invasive ventilation device, and since laryngeal mask intubation is relatively easy, an effective airway can be established in a very short time without the guidance of a laryngoscope (33,34). Therefore, the duration of laryngeal mask placement is shorter than that of endotracheal intubation. The results of this study showed that the intubation time of the N-IN group was also shorter than that of the IN group.
The results of this study showed no statistically significant difference between the N-IN and IN groups in terms of surgery time, intraoperative blood loss, and postoperative thoracic drainage tube placement. These results indicate that the two anesthesia techniques have no significant effect on the operation of thoracoscopic-assisted Nuss PE repair, and non-intubated anesthesia does not increase the surgery time, surgical process, or injury to PE patients (35). This study found no significant difference in intraoperative mean SpO2 between the two groups, indicating that airway ventilation via a laryngeal mask can meet patients’ basic oxygen supply needs during surgery, providing the possibility of a safe operation.
Endotracheal intubation needs to make contact with the trachea. After the trachea capsule dilates, it compresses the bronchial mucosa, which strongly stimulates the sympathetic nerve, resulting in increased catecholamine secretion, rapid heart rate, and other cardiovascular reactions (36,37). Therefore, the average intraoperative heart rate of the N-IN group in this study was lower than that of the IN group and showed a statistically significant difference. In addition, endotracheal intubation creates a great burden on the heart and can easily cause blood pressure fluctuations (38). Although increasing the anesthetic dose can alleviate this effect (there was no significant difference in mean intraoperative blood pressure between the two groups), it will deepen the anesthesia and prolong the time to awaken. However, the placement of the laryngeal mask in the N-IN group did not affect the trachea, avoided the stimulation of the sympathetic nerve by tracheal mucosal injury, and was conducive to the protection of the cardiovascular, cerebrovascular, and circulatory systems (39,40), thus resulting in more stable anesthesia hemodynamics in the laryngeal mask group.
From the general clinical data, it can be seen that most of the patients in this study were children or adolescents, and their tracheal mucosa is delicate and easily damaged by tracheal intubation (41). In addition, thoracoscopic-assisted Nuss repair usually involves endotracheal intubation connected to a ventilator for intraoperative ventilation at a low tidal volume, and mechanical ventilation can easily lead to ventilator-related lung injury (42,43). Therefore, the incidence of postoperative complications such as pneumothorax, pleural effusion, and lung infection in the IN group in this study was greater than that in the N-IN group.
WBC, NEUT, and LYMPH are usually infectious markers in routine blood examinations (44). There was no difference in surgical procedure or operation time between the two groups. However, there were differences in WBC, NEUT, and LYMPH the third day after surgery. It may be that the laryngeal mask in the N-IN group was only placed on the throat, without direct contact with the vocal cords, trachea, and bronchus, and did not stimulate the epiglottis, vocal cords, and tracheal mucosa (45,46). The N-IN group had a lower postoperative inflammatory response than the IN group and a shorter duration of the stress state due to the aseptic inflammatory response caused by mechanical stimulation during tracheal intubation anesthesia (47). In addition, the N-IN group anesthesia process did not require muscle relaxants and only a small dose of analgesic sedation. The reduction in drug dosage can reduce the side effects of narcotic drugs. This allows patients to get out of bed earlier, reduces gastrointestinal reactions, and facilitates the rapid recovery of digestive system function, allowing patients to start drinking and eating as early as possible (48,49). The faster postoperative recovery of patients in the N-IN group resulted in a reduced length of hospitalization and medical costs and was more acceptable to patients and their families, displaying significant social value.
Study limitations
Compared with traditional PE surgery, thoracoscopic-assisted Nuss repair has definite advantages in treating PE, although there are also some complications due to limitations in the design principle (50). The Nuss procedure uses metal fixation, which is nonabsorbable and often requires surgical removal (51). In addition, because the metal cannot expand as the body develops, it limits the development of the rib cage and pushes the bony structure underneath the metal toward the rib cage, creating a new depression (52). Serious complications associated with Nuss repair include heart injury, lung injury, cardiac tamponade, and pericarditis (53). This study found that the mean intraoperative ETCO2 in the N-IN group was significantly higher than that in the IN group, suggesting that non-intubation anesthesia had a certain CO2 accumulation compared with mechanical ventilation due to its special ventilation mode. This may have a detrimental effect on the patient's physiological function (54,55).
Future directions
With the continuous progress of science and technology and the update of ideas, the development of medical technology makes people have higher requirements for surgery and anesthesia. “Holistic minimally invasive” and rapid rehabilitation have become the goal pursued by doctors and patients (56). Therefore, non-intubation anesthesia is increasingly used in thoracic surgery anesthesia (57). Thoracoscopic-assisted Nuss procedure PE repair under non-intubation anesthesia can reduce postoperative complications to a certain extent. However, the effect of elevated interoperative ETCO2 on the physiological function of patients with non-intubation anesthesia is still unclear and needs further exploration.
Conclusions
In conclusion, compared with endotracheal intubation anesthesia, PE repair under non-intubation anesthesia is simpler to perform, with a lower incidence of various intraoperative and postoperative complications, a faster recovery for patients, and without affecting the operation of thoracoscopic-assisted Nuss procedure, all of which are consistent with the concept of FTS.
Acknowledgments
Funding: This study was supported by the Science and Technology Program of Kunming City (No. 2020-1-H-003), the Key Project of Basic Research Program of Yunnan Province (No. 202201AS070009), The Opening Project of Chest Disease Clinical Medical Center of Yunnan First People’s Hospital in 2021 (No. 2021LCZXXF-XB03), the Joint Project of Kunming Medical University (No. 202201AY070001-263), and the Basic Research Special Youth Project of Yunnan Province (No. 202201AU070015).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1150/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1150/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1150/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval for the study was granted by the Ethics Committee of The First People’s Hospital of Yunnan Province (No. KHLL2022-KY012). Written informed consent was obtained from all patients or their legal guardians who participated in the study. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Qadi MO. Disorders of the Chest Wall: Clinical Manifestations. Clin Chest Med 2018;39:361-75. [Crossref] [PubMed]
- Baerdemaeker RD, Geeroms M, Hendrickx B, et al. Correction of Pectus Excavatum Anterior Chest Wall Deformity in Adults Using Custom Made Silicone Implant. Annals of Plastic Surgery 2019;2:1021.
- Cao X, Xin H, Zhang H, et al. The Association Between Mycobacteria-Specific Antigen-Induced Cytokines and Host Response to Latent Tuberculosis Infection Treatment in a Chinese Population. Front Microbiol 2021;12:716900. [Crossref] [PubMed]
- Nasr A, Fecteau A, Wales PW. Comparison of the Nuss and the Ravitch procedure for pectus excavatum repair: a meta-analysis. J Pediatr Surg 2010;45:880-6. [Crossref] [PubMed]
- Dingeldein MW, Lu CY, Kim AW, et al. Simultaneous costal cartilage-sparing modified Ravitch procedure and latissimus dorsi transfer for chest wall deformity repair in Poland's syndrome. J Pediatr Surg 2009;44:e29-32. [Crossref] [PubMed]
- Chien HF, Chu SH. Simultaneous Bentall's procedure and sternal turnover in a patient with Marfan syndrome. J Cardiovasc Surg (Torino) 1995;36:559-62. [PubMed]
- Jaroszewski DE, Ewais MM, Lackey JJ, et al. Revision of failed, recurrent or complicated pectus excavatum after Nuss, Ravitch or cardiac surgery. J Vis Surg 2016;2:74. [Crossref] [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- Pilegaard HK, Licht PB. Early results following the Nuss operation for pectus excavatum--a single-institution experience of 383 patients. Interact Cardiovasc Thorac Surg 2008;7:54-7. [Crossref] [PubMed]
- Hebra A, Calder BW, Lesher A. Minimally invasive repair of pectus excavatum. J Vis Surg 2016;2:73. [Crossref] [PubMed]
- Singhal NR, Jones J, Semenova J, et al. Multimodal anesthesia with the addition of methadone is superior to epidural analgesia: A retrospective comparison of intraoperative anesthetic techniques and pain management for 124 pediatric patients undergoing the Nuss procedure. J Pediatr Surg 2016;51:612-6. [Crossref] [PubMed]
- Nuss D, Obermeyer RJ, Kelly RE. Nuss bar procedure: past, present and future. Ann Cardiothorac Surg 2016;5:422-33. [Crossref] [PubMed]
- Liu WL, Yu FL, Yin BL. NUSS procedure by video-assisted thoracoscopy for correction of pectus excavatum. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2008;33:443-7. [PubMed]
- Mao S, Du X, Ma J, et al. A comparison between laryngeal mask airway and endotracheal intubation for anaesthesia in adult patients undergoing NUSS procedure. J Thorac Dis 2018;10:3216-24. [Crossref] [PubMed]
- Starke H, Zinne N, Leffler A, et al. Developing a minimally-invasive anaesthesiological approach to non-intubated uniportal video-assisted thoracoscopic surgery in minor and major thoracic surgery. J Thorac Dis 2020;12:7202-17. [Crossref] [PubMed]
- Shaylor R, Verenkin V, Matot I. Anesthesia for Patients Undergoing Anesthesia for Elective Thoracic Surgery During the COVID-19 Pandemic: A Consensus Statement From the Israeli Society of Anesthesiologists. J Cardiothorac Vasc Anesth 2020;34:3211-7. [Crossref] [PubMed]
- Thoben C, Dennhardt N, Krauß T, et al. Preparation of anaesthesia workstation for trigger-free anaesthesia: An observational laboratory study. Eur J Anaesthesiol 2019;36:851-6. [Crossref] [PubMed]
- Selim J, Maquet C, Djerada Z, et al. Anesthetic Management for Awake Tubeless Suspension Microlaryngoscopy. Laryngoscope 2021;131:E2669-75. [Crossref] [PubMed]
- Njoku MJ. Post-Anesthesia Care: Symptoms, Diagnosis, and Management. critical Care Medicine 2017;45:e1207-8.
- Durry A, Gomes Ferreira C, Tricard T, et al. Minimally invasive repair of pectus excavatum in children: Results of a modified Nuss procedure. Ann Chir Plast Esthet 2017;62:8-14. [Crossref] [PubMed]
- Chen L, Sun L, Lang Y, et al. Fast-track surgery improves postoperative clinical recovery and cellular and humoral immunity after esophagectomy for esophageal cancer. BMC Cancer 2016;16:449. [Crossref] [PubMed]
- Jiang M, Liu S, Deng H, et al. The efficacy and safety of fast track surgery (FTS) in patients after hip fracture surgery: a meta-analysis. J Orthop Surg Res 2021;16:162. [Crossref] [PubMed]
- Grützner H, Flo Forner A, Meineri M, et al. A Comparison of Patients Undergoing On- vs. Off-Pump Coronary Artery Bypass Surgery Managed with a Fast-Track Protocol. J Clin Med 2021;10:4470. [Crossref] [PubMed]
- Fazzalari A, Srinivas S, Panjwani S, et al. A Fast-Track Pathway for Emergency General Surgery at an Academic Medical Center. J Surg Res 2021;267:1-8. [Crossref] [PubMed]
- Kim HK, Choi YH, Shim JH, et al. Modified Ravitch Procedure. Ann Thorac Surg 2007;84:647-8. [Crossref] [PubMed]
- de Loos ER, Andel PCM, Daemen JHT, et al. Safety and feasibility of rigid fixation by SternaLock Blu plates during the modified Ravitch procedure: a pilot study. J Thorac Dis 2021;13:2952-8. [Crossref] [PubMed]
- Zhu JL. Clinical Analysis of Different Age Groups in Funnel Chest Nuss. China & Foreign Medical Treatment 2019;38:31-2.
- Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002;37:437-45. [Crossref] [PubMed]
- Lee FJ, Lo PC, Wu MY, et al. Modified bilateral thoracoscopy-assisted Nuss procedure for repair of pectus excavatum after previous thoracic procedure. Formosan Journal of Surgery 2020;53:128. [Crossref]
- Jaroszewski DE, Gustin PJ, Haecker FM, et al. Pectus excavatum repair after sternotomy: the Chest Wall International Group experience with substernal Nuss bars. Eur J Cardiothorac Surg 2017;52:710-7. [Crossref] [PubMed]
- Silva PS, Cartacho MP, Castro CC, et al. Evaluation of the influence of pulmonary hypertension in ultra-fast-track anesthesia technique in adult patients undergoing cardiac surgery. Rev Bras Cir Cardiovasc 2015;30:449-58. [Crossref] [PubMed]
- Yu C, Wang K Z, Cui Y B. NUSS procedure for the correction of pectus excavatum. Jilin Medical Journal 2007;28:320-1.
- Karczewska K, Bialka S, Smereka J, et al. Efficacy and Safety of Video-Laryngoscopy versus Direct Laryngoscopy for Double-Lumen Endotracheal Intubation: A Systematic Review and Meta-Analysis. J Clin Med 2021;10:5524. [Crossref] [PubMed]
- Su MP, Hu PY, Lin JY, et al. Comparison of laryngeal mask airway and endotracheal tube in preterm neonates receiving general anesthesia for inguinal hernia surgery: a retrospective study. BMC Anesthesiol 2021;21:195. [Crossref] [PubMed]
- Nishiyama T. Laryngeal Mask. Japanese Journal of Medical Instrumentation 1997;67:225-30. [Crossref] [PubMed]
- Du X, Mao S, Cui J, et al. Use of laryngeal mask airway for non-endotracheal intubated anesthesia for patients with pectus excavatum undergoing thoracoscopic Nuss procedure. J Thorac Dis 2016;8:2061-7. [Crossref] [PubMed]
- Yun LT, Fang H. How to Reduce the Cardiovascular Reaction Caused the Elderly Endotracheal Intubation General Anesthesia. Inner Mongolia Medical Journal 2014;46:1458-61.
- Takita K, Morimoto Y, Kemmotsu O. Tracheal lidocaine attenuates the cardiovascular response to endotracheal intubation. Can J Anaesth 2001;48:732-6. [Crossref] [PubMed]
- Peng SJ, Liu WY, Qin YH, et al. Diltiazem attenuates cardiovascular reaction to endotracheal intubation in general anesthesia cases. Journal of Clinical and Experimental Medicine 2011;10:16-7.
- Qin M, Anesthesiology DO. A comparative study of the cardiovascular responses of patients with hypertension by tracheal intubation and laryngeal mask airway. Chinese Journal of the Frontiers of Medical Science 2015;7:32-4. (Electronic Version).
- Carey MF, Smith J, Cooney CM. Laryngeal mask to aid tracheal intubation. Anaesthesia 1991;46:1083. [Crossref] [PubMed]
- Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567-75. [Crossref] [PubMed]
- He K, Han S, An L, et al. Inhibition of MicroRNA-214 Alleviates Lung Injury and Inflammation via Increasing FGFR1 Expression in Ventilator-Induced Lung Injury. Lung 2021;199:63-72. [Crossref] [PubMed]
- Johnson RF Jr, Gustin J. Acute lung injury and acute respiratory distress syndrome requiring tracheal intubation and mechanical ventilation in the intensive care unit: impact on managing uncertainty for patient-centered communication. Am J Hosp Palliat Care 2013;30:569-75. [Crossref] [PubMed]
- Su QZ, Tang RD, Li JY, et al. Comparative analysis of PCT, hs-CRP and WBC tests results among patients with brucellosis and with other bacteria species infection. International Journal of Laboratory Medicine 2015;36:2505-6.
- Xu JY, Li YJ, Ning XG, et al. SV-VATS exhibits dual intraoperative and postoperative advantages. Ann Transl Med 2021;9:970. [Crossref] [PubMed]
- Peng J, Chen XL, Mao X, et al. Video-assisted thoracoscopic right lower lobectomy for lung cancer using the Harmonic scalpel. J Thorac Dis 2013;5:864-7. [PubMed]
- He J, Gonzalez-Rivas D, Liu H, et al. Tubeless Thoracic Procedures. Cohen E. editor. Cohen's Comprehensive Thoracic Anesthesia. Elsevier, 2022.
- Wang DJ, Qiu JG, Huang WT, et al. Laparoscopic radical cystectomy with tubeless orthotopic ileal neobladder in the treatment of muscle-invasive bladder cancer. Chinese Journal of Endourology 2014;8:5-8. (Electronic Edition).
- He J, Liu J, Zhu C, et al. Expert consensus on tubeless video-assisted thoracoscopic surgery (Guangzhou). J Thorac Dis 2019;11:4101-8. [Crossref] [PubMed]
- Manstein CH. A 10-year minimally invasive technique for the correction of pectus excavatum. Plastic & Reconstructive Surgery 1999;104:600-1. [Crossref]
- Chen XL, Liang FM, Li SY. Nursing of Children with Funnel Chest under Thoracoscopic Treatment (NUSS). Clinical Medical Engineering 2009;16:65-6.
- Kabbaj R, Burnier M, Kohler R, et al. Minimally invasive repair of pectus excavatum using the Nuss technique in children and adolescents: indications, outcomes, and limitations. Orthop Traumatol Surg Res 2014;100:625-30. [Crossref] [PubMed]
- Ceruti S, Dell'Era SA, Ruggiero F, et al. Nasogastric tube in critical care setting: combining ETCO2 and pH measuring to confirm correct placement. 2021.
- Viglino D, Bourez D, Collomb-Muret R, et al. Noninvasive End Tidal CO2 Is Unhelpful in the Prediction of Complications in Deliberate Drug Poisoning. Ann Emerg Med 2016;68:62-70.e1. [Crossref] [PubMed]
- Xi C, Shi D, Cui X, et al. Safety, efficacy and airway complications of the flexible laryngeal mask airway in functional endoscopic sinus surgery: A retrospective study of 6661 patients. PLoS One 2021;16:e0245521. [Crossref] [PubMed]
- Ziegler A, Sagorny C. Holistic description of new deep sea megafauna (Cephalopoda: Cirrata) using a minimally invasive approach. BMC Biol 2021;19:81. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)