Time since primary transplant and poor functional status predict survival after redo lung transplant
Introduction
Primary lung transplantation (LTx) offers definitive treatment for patients who have advanced pulmonary disease, with longer survival for patients with many causes of end-stage, life-threatening lung disease and improved quality of life in carefully selected candidates (1,2). However, early graft failure due to primary graft dysfunction (3) or acute cellular rejection (4) and late graft failure due to chronic lung allograft dysfunction (5) both lead to significant morbidity and mortality after LTx. While medical interventions for both early and late lung allograft failure may have limited efficacy, lung re-transplantation is one potential therapy that could definitively treat end-stage lung allograft failure for the appropriate candidates. However, redo LTx comprises a small proportion of overall LTx performed in the US. The number of redo LTx performed annually had initially increased during the lung allocation score (LAS) era (since 2005), but has decreased since the publication of an analysis of the Scientific Registry of Transplant Recipients (SRTR) data from 2005–2010 (6,7). Redo LTx is a complex surgical therapy with a higher risk of perioperative morbidity and mortality compared to primary LTx (6). Early studies utilized various quality of life metrics as surrogates for post-transplant function; however, these demonstrated an inability to predict postoperative outcomes (8). While the LAS predicts outcomes in primary LTx (8), its prognostic accuracy specifically in redo LTx is unknown. In addition, it is unclear whether the individual components of the LAS are predictive of outcomes after redo LTx (6). The Karnofsky Performance Status (KPS) scale, initially developed to quantify functional status of cancer patients after intervention, is a proven predictor of outcomes after primary LTx (9), and a recent study demonstrated that KPS predicts postoperative outcomes after redo LTx (6). However, we hypothesize that early and late lung allograft failure present distinct clinical scenarios correlating with etiology of primary lung allograft failure and functional status before redo LTx. We predict that categorization of redo LTx patients by functional status and time from primary LTx reveals two distinct groups. One group with early allograft failure and poor functional status associated with very poor prognosis after redo LTx. The other group with chronic allograft failure and overall better functional status associated with better survival after redo LTx. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-334/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by University of Minnesota Institutional Review Board (No. STUDY00007767) and informed consent was taken from all the patients. This study used data from the SRTR. The SRTR data system includes all donor, wait-listed candidates, and transplant recipients in the United States (U.S.), submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The SRTR database was used to define the prevalence of redo LTx as well as survival and predictors of survival of patients undergoing redo LTx between 01/01/2005 and 08/30/2019.
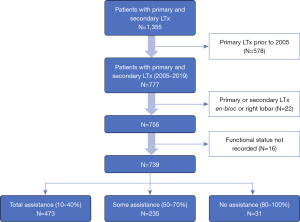
Using the SRTR database, we examined all patients who had a primary and secondary LTx on or before August 31st, 2019 (n=1,355). We excluded those whose primary transplant occurred prior to January 1st, 2005 (578, remaining n=777), and excluded those whose first or second transplant was en-bloc or right lobar (22, remaining n=755). Lastly, we removed those who did not have a functional status recorded for both transplants (16, remaining n=739) (Figure 1). Patient characteristics were generated and displayed in Table 1. These characteristics are at the time of second transplant, unless otherwise noted.
Table 1
| Recipient characteristic | No assistance (80–100%) | Some assistance (50–70%) | Total assistance (10–40%) | P |
|---|---|---|---|---|
| N | 31 | 235 | 473 | |
| LAS score, mean (SD) | 54.87 (18.97) | 49.27 (14.05) | 66.55 (20.72) | <0.001 |
| Age (years), mean (SD) | 44.48 (19.97) | 48.15 (15.36) | 46.60 (16.92) | 0.347 |
| Gender male, n (%) | 13 (41.9) | 138 (58.7) | 270 (57.1) | 0.206 |
| Caucasian, n (%) | 26 (83.9) | 217 (92.3) | 432 (91.3) | 0.289 |
| BMI, mean (SD) | 22.17 (5.29) | 22.79 (4.52) | 22.85 (5.16) | 0.759 |
| Diagnosis, n (%) | 0.655 | |||
| CF | 10 (35.7) | 64 (27.8) | 134 (29.1) | |
| COPD/A1AT | 6 (21.4) | 48 (20.9) | 74 (16.1) | |
| ILD/IPF | 11 (39.3) | 106 (46.1) | 222 (48.2) | |
| PH | 1 (3.6) | 12 (5.2) | 31 (6.7) | |
| Creatinine, mean (SD) | 1.02 (0.51) | 1.06 (0.36) | 1.04 (0.60) | 0.788 |
| Total bilirubin, mean (SD) | 0.52 (0.64) | 0.50 (0.47) | 0.71 (1.02) | 0.006 |
| Diabetes mellitus, n (%) | 14 (45.2) | 101 (43.3) | 237 (50.4) | 0.199 |
| Mechanical ventilation, n (%) | 1 (3.2) | 8 (3.4) | 192 (40.6) | <0.001 |
| ECMO, n (%) | 0 (0.0) | 2 (0.9) | 82 (17.3) | <0.001 |
| ICU stay, n (%) | 2 (6.5) | 13 (5.5) | 251 (53.2) | <0.001 |
| Never discharge, n (%) | 3 (9.7) | 3 (1.3) | 57 (13.1) | <0.001 |
| Survival at 1-year (%), (95% CI) | 90.3 (80.5, 100) | 84 (79.3, 88.9) | 69.8 (65.7, 74.2) | |
| Survival at 3-year (%), (95% CI) | 40.2 (25.4, 63.7) | 62.4 (55.9, 69.7) | 44.0 (39.4, 49.3) | |
| Survival at 5-year (%), (95% CI) | 31.3 (17.6, 55.8) | 43.9 (36.7, 52.6) | 31.6 (27.0, 36.9) |
LAS, lung allocation score; SD, standard deviation; BMI, body mass index; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; A1AT, alpha-1 anti-trypsin deficiency; ILD, interstitial lung disease; IPF, interstitial pulmonary fibrosis; PH, pulmonary hypertension; ECMO, extracorporeal membranous oxygenation; ICU, intensive care unit; CI, confidence interval.
A histogram was created to show time from first to second transplant by functional status at second transplant, and a bar chart to show whether a patient was still hospitalized from their first transplant at time of re-transplant. Re-transplant survival curves were generated based on time from previous transplant, separately by re-transplant functional status. Curves were also generated based on type of primary-redo transplants, as follows: any-bilateral, bilateral-single, single-single (opposite sides), single-single (same side).
Statistical analysis
A Cox regression model was fit to examine re-transplant survival, adjusting for the following factors: re-transplant functional status, primary transplant functional status, gender, age, estimated glomerular filtration rate (eGFR), forced vital capacity (FVC), and forced expiratory volume in one second (FEV1) at time of re-transplant. We had two variables with severe proportional hazards violations. The first was transplant type, which was used as a stratification variable since it was not of primary interest. For time since previous transplant (<1 vs. 1+ years), we utilized time-dependent coefficients, splitting follow-up into three periods: 0–6, 6–24, and 24+ months based on survival curves. All analysis was performed in R, version 4.0.2.
Results
Redo LTx recipient, donor and operative characteristics with respect to functional status
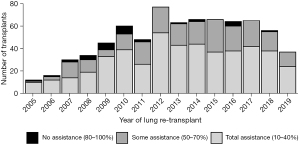
This retrospective study cohort from 01/01/2005 to 08/30/2019 included a total of 739 redo LTx patients identified in the SRTR database. KPS was grouped into 3 categories: 10–40% (total assistance), 50–70% (some assistance), and 80–100% (no assistance). Of the total 739 redo LTx patients, the greatest number, 473 (64%), were in 10–40% group requiring total assistance, 235 (31.8%) were in the 50–70% group requiring some assistance, and very few, 31 (4.2%), were in 80–100% group requiring no assistance (Figure 1). The LAS, calculated prior to redo LTx, was higher among patients requiring total assistance (mean 66.55±20.72) compared to those requiring some assistance (49.27±14.05) or no assistance (54.87±18.97) (Table 1, P<0.001). Demographics including age, sex, and race were comparable among the three groups. Other patient characteristics including body mass index (BMI), etiology of primary lung disease before the first LTx, diabetes mellitus, and serum creatinine were also comparable. Redo LTx recipients who required total assistance had a higher average of serum total bilirubin. Additionally, rates of mechanical ventilation before redo LTx were higher among patients requiring total assistance (40.6%) compared to those that required some (3.4%) or no assistance (3.2%, P<0.001), and bridging to redo LTx with extracorporeal membrane oxygenation (ECMO) was used in 17.3% of patients who required total assistance but in only 2% of those requiring some assistance and none of those requiring no assistance (P<0.001). While 53.2% of those requiring total assistance were in the intensive care unit (ICU) before redo LTx, only 13% of those requiring some assistance and 2% of those requiring no assistance were in the ICU before redo LTx (P<0.001). Redo LTx recipients who required total assistance were also significantly more likely to have never discharged from the hospital after their primary LTx (P<0.001). Donor characteristics were comparable among functional status cohorts (Table 2), except for donor smoking which was more common among recipients requiring no assistance (n=7, 22.6%) compared to those requiring some (6.9%) or no (9.6%) assistance (P=0.017). The redo LTx for 278 (37%) patients was done as a single LTx, including 139 (18.8%) on the contralateral side after primary single LTx, 31 (4.2%) on the same side as the primary single LTx, and 108 (14.6%) with a single following a bilateral transplant. However, the majority of redo LTx were bilateral (461, 62.4%). Recipients requiring total assistance had significantly fewer days on the waitlist and a shorter time interval between the initial LTx and redo LTx compared to the other two groups (P<0.001). The distribution of patients across the primary diagnosis categories was similar between the three functional status groups.
Table 2
| Donor or transplant characteristic | No assistance (80–100%) | Some assistance (50–70%) | Total assistance (10–40%) | P |
|---|---|---|---|---|
| N | 31 | 235 | 473 | |
| Donor age (years), mean (SD) | 29.31 (14.23) | 32.86 (13.46) | 34.42 (14.16) | 0.075 |
| Donor gender (male), n (%) | 15 (48.4) | 146 (62.1) | 273 (57.7) | 0.261 |
| Donor race (Caucasian), n (%) | 22 (71.0) | 173 (73.6) | 355 (75.1) | 0.830 |
| Donor BMI, mean (SD) | 24.96 (4.94) | 25.70 (5.09) | 25.42 (5.19) | 0.668 |
| Donor smoker, n (%) | 7 (22.6) | 16 (6.9) | 45 (9.6) | 0.017 |
| DCD (yes), n (%) | 0 (0.0) | 4 (1.7) | 5 (1.1) | 0.624 |
| Transplant type, n (%) | 0.056 | |||
| Bilateral (redo) | 24 (77.4) | 142 (60.4) | 295 (62.4) | |
| Bilateral-single | 2 (6.5) | 31 (13.2) | 75 (15.9) | |
| Single (opposite) | 5 (16.1) | 56 (23.8) | 78 (16.5) | |
| Single (same) | 0 (0.0) | 6 (2.6) | 25 (5.3) | |
| Waitlist time (days), mean (SD) | 91.55 (83.50) | 114.41 (165.07) | 65.43 (123.30) | <0.001 |
| Time difference (years), mean (SD) | 2.71 (2.45) | 3.58 (2.49) | 2.67 (2.62) | <0.001 |
| Primary Tx failure, n (%) | <0.001 | |||
| Acute rejection | 2 (6.4) | 8 (3.4) | 13 (2.7) | |
| Chronic rejection | 15 (48.4) | 145 (61.7) | 211 (44.6) | |
| Other | 8 (25.8) | 23 (9.8) | 88 (18.6) | |
| Primary non-function, n (%) | 3 (9.7) | 10 (4.3) | 84 (17.8) | |
| Average ischemic time (hours), mean (SD) | 4.68 (1.46) | 4.63 (1.67) | 5.02 (1.96) | 0.028 |
| Maximum ischemic time (hours), mean (SD) | 5.35 (1.92) | 5.06 (1.83) | 5.49 (2.20) | 0.037 |
SD, standard deviation; BMI, body mass index; DCD, donor after cardiac death; Tx, transplant.
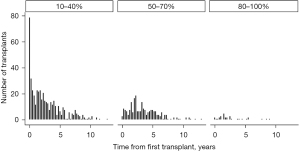
Redo LTx patients were divided into three groups according to functional status as determined by KPS. Then the number of redo LTx in each group was examined with respect to the time since the primary LTx (Figure 2). This comparison demonstrates that most redo LTx were in patients requiring total assistance, and of the redo lung transplants that were done in the first year after the primary transplant, the vast majority (157/193=81.3%) required total assistance. Of redo LTx recipients who required some assistance, most occurred between 1–5 years after the primary LTx, and there were very few redo LTx in patients requiring no assistance.
Survival of redo LTx recipients with respect to time after transplant and functional status
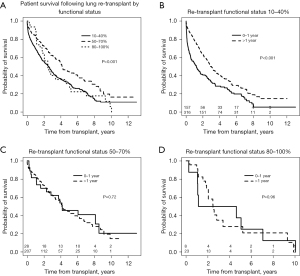
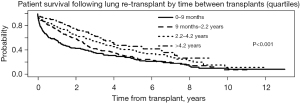
Patients who required total assistance had worse survival after redo LTx compared to those who require some or no assistance (Figure 3A, overall log-rank P<0.001), and the decrement in survival in total assistance patients was most pronounced in the early post-transplant period. For patients requiring total assistance, the survival after redo LTx was significantly worse if the redo LTx was done in the first year after the primary LTx (Figure 3B, P<0.001), and the risk of mortality was most pronounced in the two years after the redo LTx. However, among patients requiring some or no assistance who underwent redo LTx, there was no difference in the survival between those who underwent redo LTx within one year versus after one year after the primary LTx (Figure 3C, P=0.72 and Figure 3D, P=0.96, respectively). To further examine the impact of the time between primary LTx and redo LTx on survival, patients who underwent redo LTx were divided into quartiles with respect to the time since primary LTx. Patients who underwent redo LTx within 9 months of the primary LTx were at significantly increased risk of early post-transplant mortality compared to patients in the other quartiles (Figure 4, P<0.001).
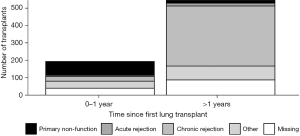
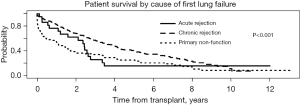
In order to examine the impact of the interval between the primary and redo LTx with respect to the cause of primary lung allograft failure leading to redo LTx, the cause of primary lung allograft failure was compared for patients who underwent redo LTx within one year of primary LTx versus those who underwent redo LTx after one year since the primary LTx. Primary lung allograft non-function and acute rejection were much more common causes of lung allograft failure in the first year after the primary LTx compared to patients who underwent redo LTx more than one year after the primary LTx when chronic rejection was a much more common cause of failure of the primary lung allograft (Figure 5). The survival of patients who underwent redo LTx after failure of the primary lung allograft was compared for different causes of lung allograft failure. Those with chronic rejection had the best survival outcomes (Figure 6, overall P<0.001). After examining pairwise comparisons, we found no difference between acute rejection and chronic rejection (adjusted P=0.08) or acute rejection and primary non-function (adjusted P=0.47). Survival in those whose primary graft failed due to primary non-function was significantly worse than those who failed due to chronic rejection (adjusted P<0.001).
Modeling data
In the multivariate model using the group requiring complete assistance as the reference group, survival after redo LTx for the group requiring some assistance was significantly better than for the total assistance group [Table 3, HR 0.69 (0.54, 0.87), P=0.002]. Also, for every 10% increase in the FVC before redo LTx, there was a statistically significant increase in survival after redo LTx [HR 0.89 (0.82, 0.97), P=0.008]. The survival difference between the group requiring no assistance and the group requiring total assistance was not statistically significant, likely due to the small sample size of the group that did not require assistance. For those who were re-transplanted within one year, survival was significantly worse, but only during the first six months post-transplant [HR 2.73 (1.83, 4.07), P<0.001].
Table 3
| Risk factor | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Functional category 50–70% (1st transplant) | 1.065 | 0.848–1.337 | 0.589 |
| Functional category 80–100% (1st transplant) | 0.962 | 0.68–1.36 | 0.825 |
| Functional category 50–70% (2nd transplant) | 0.688 | 0.544–0.869 | 0.002 |
| Functional category 80–100% (2nd transplant) | 0.982 | 0.626–1.54 | 0.936 |
| Gender (male) | 0.883 | 0.714–1.092 | 0.250 |
| Recipient age (years) | 1.003 | 0.994–1.012 | 0.565 |
| GFR (2nd transplant) | 1.000 | 0.996–1.004 | 0.966 |
| FVC (2nd transplant): 10% increase | 0.889 | 0.815–0.97 | 0.008 |
| FEV1 (2nd transplant) | 1.005 | 0.996–1.013 | 0.280 |
| <1 year since primary transplant (effect during 0–6 months follow-up) | 2.725 | 1.827–4.066 | <0.001 |
| <1 year since primary transplant (effect during 6–24 months follow-up) | 1.493 | 0.954–2.339 | 0.080 |
| <1 year since primary transplant (effect during 24+ months follow-up) | 0.851 | 0.561–1.29 | 0.446 |
CI, confidence interval; GFR, glomerular filtration rate; FVC, forced vital capacity; FEV1, forced expiratory volume in one second.
Discussion
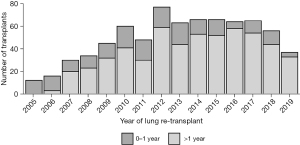
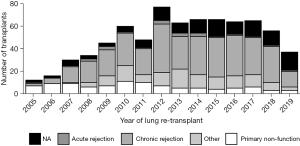
According to the recent OPTN report, primary LTx recipient survival at 1, 3, and 5 years for the period of 2008 to 2015 was 87.9%, 70.5%, and 56% respectively, whereas for redo LTX, recipient survival was 76%, 48.9%, and 33.8%, respectively (7). For primary LTx, most patients require only some assistance (57.7%) or no assistance (12.3%) (8) pre-transplant, while most patients who underwent redo LTx in the present study required total assistance before the redo LTx (64%). Worse functional status has been shown previously to independently predict decreased survival after redo LTx (6,10). However, we proposed that functional status is a surrogate marker for a more specific clinical scenario related to the timing and etiology of primary lung allograft failure. We examined the relationship between functional status before redo LTx and other clinical factors including the time interval between primary and redo LTx, as well as the etiology of allograft failure in patients who underwent redo LTx. Worse survival after redo LTx correlates with lower functional status and is predicted by the interval between primary and redo LTx. Our findings suggest two different clinical scenarios. In the first year after primary LTx, the most common indication for redo LTx was primary non-function, and these patients were more likely to have low functional status, to remain hospitalized until redo LTx, and to require mechanical ventilation or bridging with ECMO. These patients had significantly higher early postoperative mortality after redo LTx. Patients who underwent redo LTx greater than one year after the primary LTx were more likely to be discharged after the primary LTx, have chronic rejection, and only require some assistance prior to redo LTx. This latter scenario was associated with better survival, albeit lower survival than after primary LTx. We found that redo LTx candidates requiring total assistance had significantly fewer days on the waitlist, consistent with the higher LAS values in this group. Also, redo LTx recipients who required total assistance had a shorter time interval between the initial LTx and redo LTx compared to the other two groups (P<0.001). While salvage redo LTx for recipients with a high LAS and very poor functional status is certainly a readily available option, these redo lung transplant recipients may not benefit from LTx to the same degree as primary LTx recipients with a similar LAS. In a previous study, non-overlap in the distribution of potential confounders between primary LTx and redo LTx recipients biased against the direct comparison of mortality after redo compared to primary lung transplant (11). The authors used propensity score matching to adjust for this bias and found no significant difference in survival between first and second transplants. However, the present study suggests that in the past decade, redo LTx recipients greater than a year out from primary LTx with chronic rejection, requiring no assistance, are exceedingly rare. In recent years, the vast majority of redo LTx have required some or total assistance. These findings highlight the need to incorporate multiple factors in the lung transplant candidacy evaluation for redo LTx. This assessment should include the time from primary LTx, cause of lung allograft failure, and functional status in redo LTx candidates in accordance with the fundamental ethical principles of utility and justice pertaining to the OPTN Final Rule. Although the volume of redo LTx peaked in 2012 and then reached a plateau around 60 redo LTx per year, the volume has declined incrementally each year since 2017 (Figure 7). Most patients, requiring total assistance, who undergo redo LTx in the early period after primary lung transplant still require total assistance post-operatively. Overall, there is a trend towards higher numbers of redo lung transplants that occur past one year after the primary lung transplant (Figure 8). In addition, chronic rejection was one of the major indications for patients requiring redo lung transplants in the last decade (Figure 9).
Certainly, other operative factors such as whether a redo LTx is a single ipsilateral, single contralateral or bilateral LTx influence postoperative survival (Figure S1), but these factors were not the focus of the current study. In general, redo LTx recipients carry higher mortality risk because of technically challenging issues including chest wall bleeding due to significant adhesions and higher prevalence of bleeding after the surgery due to coagulopathy possibly related to cardiopulmonary bypass or hepatic or renal insufficiency. In addition, prolonged operating times, increased use of blood products and cardiopulmonary bypass can be major contributing factors to morbidity including primary graft dysfunction and mortality.
Functional outcomes are an important measure of surgical success especially in transplantation where postoperative quality of life plays an important role in allocating organs. As our results have demonstrated, both post-transplant morbidity and mortality appear to be associated with preoperative KPS. Future studies and clinical efforts will need to focus on effective strategies to improve post-transplant conditioning to identify patients in this most disabled cohort who might benefit from LTx. It would be beneficial for future studies to elucidate further information from the group of redo LTx patients requiring total assistance; primarily whether their functional status improves or not. These findings will add weight to the utility of preoperative functional status as a risk stratification tool in assessing candidates for redo LTx.
Study limitations
There are several limitations to our study. Its retrospective study design did not allow to elucidate information of variables not collected in the SRTR database. As with other multicenter registries, it is susceptible to missing data entries or incorrectly entered data. SRTR database does not collect information on the experience of individual surgeons and operative details that would certainly impact the outcomes given the complexity of the surgery. Single institution databases might provide more insight into operative details and the impact on outcomes. Functional status is a highly variable measure that is susceptible to observer bias. In addition, postoperative KPS scores were not included for all the groups, which is in part due to mortality occurring before the follow up. The time for follow up was not standardized and therefore tracking the post-operative performance occurred at varying intervals, which makes it difficult to determine changes over time in an individual patient after transplant. Lastly, waitlist outcomes in total assistance group remains unknown. If the waitlist mortality of total assistance cohort is higher compared to other groups, then the argument can be made that these patients should be transplanted despite high post-transplant mortality risk.
Conclusions
We confirmed our hypothesis that early and late lung allograft failure present distinct clinical scenarios correlating with etiology of primary lung allograft failure and functional status before redo LTx. We found that categorization of redo LTx patients by functional status and time from primary LTx reveals two distinct groups of lung graft failure with disparate clinical trajectories after redo LTx. One group with early allograft failure and poor functional status has a very poor prognosis after redo LTx, and the other group with chronic allograft failure and overall better functional status has better survival after redo LTx. These findings have relevance to individual clinical decision making, as well as donor lung allocation.
Acknowledgments
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and, in no way, should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-334/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-334/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-334/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-334/coif). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by University of Minnesota Institutional Review Board (No. STUDY00007767) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Genao L, Whitson HE, Zaas D, et al. Functional status after lung transplantation in older adults in the post-allocation score era. Am J Transplant 2013;13:157-66. [Crossref] [PubMed]
- Ramsey SD, Patrick DL, Lewis S, et al. Improvement in quality of life after lung transplantation: a preliminary study. The University of Washington Medical Center Lung Transplant Study Group. J Heart Lung Transplant 1995;14:870-7. [PubMed]
- Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004-11. [Crossref] [PubMed]
- Renaud-Picard B, Koutsokera A, Cabanero M, et al. Acute Rejection in the Modern Lung Transplant Era. Semin Respir Crit Care Med 2021;42:411-27. [Crossref] [PubMed]
- Verleden SE, Vos R, Vanaudenaerde BM, et al. Chronic lung allograft dysfunction phenotypes and treatment. J Thorac Dis 2017;9:2650-9. [Crossref] [PubMed]
- Kilic A, Beaty CA, Merlo CA, et al. Functional status is highly predictive of outcomes after redo lung transplantation: an analysis of 390 cases in the modern era. Ann Thorac Surg 2013;96:1804-11; discussion 1811. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. [Crossref] [PubMed]
- Grimm JC, Valero V 3rd, Kilic A, et al. Preoperative performance status impacts perioperative morbidity and mortality after lung transplantation. Ann Thorac Surg 2015;99:482-9. [Crossref] [PubMed]
- Mor V, Laliberte L, Morris JN, et al. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer 1984;53:2002-7. [Crossref] [PubMed]
- Methe H, Zimmer E, Grimm C, et al. Evidence for a role of toll-like receptor 4 in development of chronic allograft rejection after cardiac transplantation. Transplantation 2004;78:1324-31. [Crossref] [PubMed]
- Shuhaiber JH, Kim JB, Hur K, et al. Survival of primary and repeat lung transplantation in the United States. Ann Thorac Surg 2009;87:261-6. [Crossref] [PubMed]