Comparison of clinical outcomes of pulmonary sequestration in adults between surgery and non-surgery groups
Introduction
Pulmonary sequestration (PS) is a congenital malformation of the lung characterized as a mass of nonfunctioning lung tissue that is separated from the normal tracheobronchial tree and receives its vascular supply from aberrant systemic arteries (1). PS is rare, accounting for 0.15–6.4% of congenital lung anomalies (2). The most widely accepted hypothesis for the etiology is that PS originates from the formation of an accessory lung bud caudal to the normal lung buds (3). PS is usually classified as either intralobar PS (sharing the visceral pleural covering of a normal pulmonary lobe) or extralobar PS (separated from the normal lung by its own pleural covering). However, many cases involve a mixed type, and the sequestration spectrum includes all anomalous combinations of lung parenchyma, airway, arterial supply, and venous supply (3).
Patients with intralobar PS usually present in late adolescence or early adulthood with recurrent infection in the affected lobe (4). Most patients in adulthood are diagnosed incidentally during imaging studies conducted for other reasons (4). However, adult patients occasionally present with hemothorax, infection, infarction, or hemoptysis (5-7).
Whether asymptomatic PS found in adults should be surgically resected is still the subject of debate because there have been few reports regarding its natural course. Some groups have argued that it should be resected considering its potential infectious complications (8), while others have suggested that it could be followed up without surgery (9). We hypothesized that asymptomatic PS may be observed in adults and not require surgery. In this retrospective study, we compared the clinical characteristics and outcomes between surgery and non-surgery groups of patients diagnosed with PS in adulthood. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-631/rc).
Methods
Study population
This was a retrospective cohort study performed at Samsung Medical Center (a 1979-bed referral hospital in Seoul, South Korea) between 1994 and 2019. The electronic medical records, including patient diagnoses, chest computed tomography (CT), and histopathology reports, were searched with the keyword “pulmonary sequestration” to identify adult patients with PS aged 18 years or older. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the institutional review board of Samsung Medical Center (IRB No. 2019-03-148-001) and the need for informed patient consent was waived due to its retrospective nature.
Diagnosis of PS
For resected cases, diagnoses of PS were confirmed by histopathological findings, which were reviewed by a board-certified experienced pathologist (JH). Non-resected cases were diagnosed based on the presence of anomalous systemic arterial supply and the presence of abnormal lung parenchyma on enhanced chest CT, which were reviewed by board-certified experienced radiologists (SGP and HYL).
Data collection
All available clinical, radiological, and pathological characteristics and follow-up information were obtained by reviewing the electronic medical records in surgery and non-surgery groups. At the time of diagnosis, patient symptoms, medical history, and smoking history were reviewed. Information about patient symptoms at the last follow-up and the development of malignancy during follow-up was also collected. For the surgery group, perioperative information was also reviewed, including surgical records, surgical complications, pathology reports, including PS subtype (intralobar or extralobar) and follow-up data after surgery. The last follow-up data included the patient’s last outpatient or inpatient medical records or radiological findings. Symptom changes between the time of diagnosis and the last follow-up were analyzed for all patients whose follow-up information for 1 month or more was available.
Radiology review
Chest CT at the time of diagnosis, which first revealed the presence of PS, was reviewed by radiologists, who reported the detailed radiological characteristics of PS (maximal size, location, radiological pattern, feeding artery, venous drainage, etc.). In this study, the presence of pneumonia was considered only for the cases which had acute onset (in one month) of symptoms (fever, cough, sputum, or chest or back pain) and active inflammation in PS on chest CT scan. For the non-surgery group, chest CT at the time of diagnosis and the last follow-up were reviewed and size changes were compared. The maximal sizes of the parenchymal lesions in axial, coronal, and sagittal planes were measured.
Statistical analysis
Data are reported as numbers with percentages in parentheses for categorical variables, and as medians with interquartile range (IQR) or mean ± standard deviation (SD) in parentheses for continuous variables. The characteristics of the surgery and non-surgery groups were compared using the t-test or Mann-Whitney test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. In the non-surgery group, the initial PS size and the last PS size were compared using a paired t-test. Symptom changes in the surgery and non-surgery groups according to their initial symptoms (either symptomatic or asymptomatic) were compared using Fisher’s exact test. All tests were two-sided, and P<0.05 was taken to indicate statistical significance. All analyses were performed using SPSS (IBM SPSS Statistics, version 25, USA).
Results
Baseline clinical characteristics
In total, 106 cases of PS in 105 adult subjects with PS were identified during the study period; in the one subject with two cases of PS, one PS was resected and the other PS (in the contralateral hemithorax) was followed up after feeding artery ligation. That subject was excluded and 104 patients with PS were included in the final analyses. Table 1 lists the clinical characteristics of the 104 study subjects in the study population. The median age at diagnosis was 40.5 (30.0–52.8) years. The study population included slightly more female patients (53.8%) than male patients (46.2%). In total, 62 subjects (59.6%) underwent surgery and 42 subjects (40.4%) were followed up without surgery. Among the 42 patients in the non-surgery group, two (1.9%) underwent embolization. One patient in the surgery group underwent embolization of PS, but the lesion was finally resected. The most common presenting symptom was cough (11.5%) and fever (11.5%), followed by hemoptysis (8.7%), sputum (6.7%), and chest or back pain (4.8%). Twenty-two patients (21.2%) had concurrent pneumonia which was confirmed by chest CT scan. Sixty patients (57.7%) had no symptoms or pneumonia at the time of diagnosis.
Table 1
| Characteristics | Number of patients (%) or median (IQR or range) |
|---|---|
| Age at diagnosis (years) | 40.5 (30.0–52.8) |
| Sex | |
| Male | 48 (46.2) |
| Female | 56 (53.8) |
| Smoking history at diagnosis | |
| Never-smoker | 57 (54.8) |
| Smoker (pack-years) | 33 (31.7); 10.0 (0.5–60.0) |
| Unknown | 14 (13.5) |
| Initial presenting symptomsa | |
| Asymptomatic | 69 (66.3) |
| Cough | 12 (11.5) |
| Fever | 12 (11.5) |
| Hemoptysis | 9 (8.7) |
| Sputum | 7 (6.7) |
| Chest/back pain | 5 (4.8) |
| Concurrent pneumonia at the time of PS diagnosis | 22 (21.2) |
| Presence of symptoms or concurrent pneumonia | 44 (42.3) |
| Surgery | 62 (59.6) |
| Embolization only | 2 (1.9) |
| Follow-up duration after diagnosis (months) | 21.3 (0–224) |
a, some patients had more than two symptoms. IQR, interquartile range; PS, pulmonary sequestration.
Radiological manifestations
Table 2 lists the radiological manifestations of the 104 cases of PS. The mean size was 80.4 mm. Left-sided PS was predominant (69.2%), and no PS was found in the upper lobes. The feeding artery arose most frequently from the descending aorta (85.6%) followed by the celiac artery (12.5%), the suprarenal abdominal aorta (1.9%), and the inferior phrenic artery (1.0%). The most common draining vein was the pulmonary vein (89.4%), followed by the azygous vein (8.7%), the renal vein (1.0%), and the portal vein (1.0%). The two most dominant radiological patterns were consolidation (54.8%) and multiple cysts (33.7%) followed by single cyst (12.5%), mass (4.8%), ground glass opacity (GGO; 4.8%), and hyperlucency (4.8%). Figure S1 presents representative radiological patterns.
Table 2
| Characteristics | Number (%) or mean ± SD |
|---|---|
| Maximal size of lesion, mm | 80.4±28.3 |
| Location | |
| Left lower lobe | 72 (69.2) |
| Right lower lobe | 32 (30.8) |
| Feeding arterya | |
| Descending aorta | 89 (85.6) |
| Celiac artery | 13 (12.5) |
| Suprarenal abdominal aorta | 2 (1.9) |
| Inferior phrenic artery | 1 (1.0) |
| Venous drainageb | |
| Pulmonary vein | 93 (89.4) |
| Azygous vein | 9 (8.7) |
| Renal vein | 1 (1.0) |
| Portal vein | 1 (1.0) |
| Unidentifiable | 2 (1.9) |
| Radiological patternc | |
| Consolidation | 57 (54.8) |
| Multiple cysts | 35 (33.7) |
| Single cyst | 13 (12.5) |
| Mass | 5 (4.8) |
| GGO | 5 (4.8) |
| Hyperlucency | 5 (4.8) |
| Others | 3 (2.9) |
a, one case had dual arterial supplies from descending aorta and suprarenal abdominal aorta. Feeding artery was not identifiable by CT scan, but arterial supply from descending aorta was surgically identified; b, one case had dual venous drainage into pulmonary vein and azygous vein, and another case had dual venous drainage into pulmonary vein and portal vein; c, allowed co-dominant imaging findings. Some lesions had two dominant morphological characteristics. PS, pulmonary sequestration; SD, standard deviation; GGO, ground glass opacity; CT, computed tomography.
Characteristics of the surgery group
Table 3 lists the details of surgical treatment. Among the 62 patients in the surgery group, the most common surgical indication was past or current pneumonia (50.0%), followed by cough or sputum (14.5%), hemoptysis (9.7%), clinical suspicion of malignancy (9.7%), and chest or back pain (4.8%). Two patients underwent surgery to exclude congenital pulmonary airway malformation (CPAM) and bronchogenic cyst. Two patients (3.2%) were asymptomatic but underwent surgery to prevent complications, such as pneumonia or hemoptysis.
Table 3
| Characteristics | Number (%) or median (range) |
|---|---|
| Indication of surgery | |
| Pneumonia or history of pneumonia | 31 (50.0) |
| Cough or sputum | 9 (14.5) |
| Active or previous hemoptysis | 6 (9.7) |
| Clinical suspicion of malignancy | 6 (9.7) |
| Chest/back pain | 3 (4.8) |
| Not declared | 3 (4.8) |
| Suspicion of other diseasesa | 2 (3.2) |
| Prevention of complication | 2 (3.2) |
| Surgical approach | |
| VATS | 38 (61.3) |
| Thoracotomy | 23 (37.1) |
| Unknown (outside hospital operation) | 1 (1.6) |
| Extent of surgery | |
| Lobectomy | 50 (80.6) |
| Segmentectomy | 3 (4.8) |
| Wedge resection | 6 (9.7) |
| Mass excision | 3 (4.8) |
| Postoperative complications | 18 (29.0) |
| Pneumothorax/prolonged air leak | 6 (9.7) |
| Surgical site problem | 3 (4.8) |
| Atrial fibrillation | 1 (1.6) |
| Cerebral infarct | 1 (1.6) |
| Pneumonia | 1 (1.6) |
| Chylothorax | 1 (1.6) |
| Pleural effusion | 1 (1.6) |
| Rash | 1 (1.6) |
| Prolonged weaning | 1 (1.6) |
| Esophageal tearing | 1 (1.6) |
| Fever | 1 (1.6) |
| Clavien-Dindo classification of complications | 18 (29.0) |
| I | 4 (6.5) |
| II | 7 (11.3) |
| IIIa | 5 (8.1) |
| IIIb | 1 (1.6) |
| IVa | 1 (1.6) |
| IVb | 0 (0.0) |
| V | 0 (0.0) |
| From initial diagnosis to surgery (months) | 1.0 (0.0–58.0) |
a, other diseases included CPAM and bronchogenic cyst. VATS, video-assisted thoracoscopic surgery; CPAM, congenital pulmonary airway malformation.
Video-assisted thoracoscopic surgery (VATS) and open thoracotomy were performed in 38 (61.3%) and 23 (37.1%) patients, respectively. Lobectomy (80.6%) was the most common procedure followed by wedge resection (9.7%) and segmentectomy (4.8%). Three (4.8%) extralobar PS were removed by mass excision.
In total, 18 patients (29.0%) experienced postoperative complications, which were mostly minor, such as pneumothorax (9.7%) and surgical site problems (4.8%). Some significant complications were also reported, including cerebral infarct, prolonged weaning from mechanical ventilation, and esophageal tearing, but these were rare. Grade 3 or higher surgical complication by Clavien-Dindo classification was 38.9% (7/18). The median time interval from diagnosis of PS to surgery was 1 month.
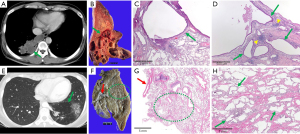
Table 4 lists the pathological findings of 61 subjects; one subject who had undergone surgery at another institution was excluded. A review of pathology revealed that 56 (91.8%) and 5 (8.2%) cases were intralobar and extralobar types of PS. Infection and other pathological findings were observed only in intralobar PS cases (Table 4). Infection was detected in 50.0% of resected intralobar PS specimens, including abscess (19.6%), pneumonia (3.6%), or organizing pneumonia (3.6%). Granulomas (16.1%) and fungal balls (10.7%) were also detected. One case of carcinoid tumorlet (1.8%) was observed. Interestingly, three cases (5.4%) of combined intralobar PS and CPAM were observed. The two cases of combined intralobar PS and CPAM are summarized in Figure 1.
Table 4
| Characteristics | Intralobar PS (n=56; 91.8%) | Extralobar PS (n=5; 8.2%) |
|---|---|---|
| Presence of combined infection, n (%) | 28 (50.0) | 0 (0.0) |
| Abscessb | 11 (19.6) | 0 (0.0) |
| Pneumoniac | 2 (3.6) | 0 (0.0) |
| Organizing pneumoniab | 2 (3.6) | 0 (0.0) |
| Suppurative inflammation | 1 (1.8) | 0 (0.0) |
| Granuloma ± caseation necrosisc | 9 (16.1) | 0 (0.0) |
| TB | 0 (0.0) | 0 (0.0) |
| NTM | 2 (3.6) | 0 (0.0) |
| Aspergilloma/fungal ballb | 6 (10.7) | 0 (0.0) |
| Presence of tumor, n (%) | 1 (1.8) | 0 (0.0) |
| Carcinoid tumorlet | 1 (1.8) | 0 (0.0) |
| Other pathological findings, n (%) | 5 (8.9) | 0 (0.0) |
| CPAM | 3 (5.4) | 0 (0.0) |
| Secondary amyloid deposition | 1 (1.8) | 0 (0.0) |
| Inflammation and fibrosis, honeycombing | 1 (1.8) | 0 (0.0) |
a, one patient underwent surgery at another center; b, one patient had abscess, organizing pneumonia, and positive fungus culture; c, one patient had acute lobar pneumonia with granulomas. PS, pulmonary sequestration; TB, tuberculosis; NTM, non-tuberculous mycobacterium; CPAM, congenital pulmonary airway malformation.
Characteristics of the non-surgery group
Among the 42 patients in the non-surgery group, 33 (78.6%) were followed up after initial diagnosis of PS with regular clinic visits and serial chest CT. Comparison of the axial sizes of PS at the time of diagnosis (61.2±27.1 mm) and at last follow-up (62.3±29.7 mm) revealed no significant differences (P=0.578).
Comparison of clinical characteristics of the surgery group and the non-surgery group
Table 5 compares the characteristics of the surgery and non-surgery groups. The median durations of follow-up from initial diagnosis to last follow-up were 17.5 and 15.0 months in the surgery and non-surgery groups, respectively (P=0.919). Patients in the surgery group (n=62) were significantly younger (38.6 vs. 45.3 years, respectively, P=0.016), more likely to be symptomatic initially (74.2% vs. 47.6%, respectively, P=0.007), and had larger PS (90.0 vs. 66.3 mm, P<0.001) compared with those in the non-surgery group (n=42). Three and one deaths occurred during follow-up in the surgery and non-surgery groups, respectively. During the follow-up after surgery, two patients developed lung cancer more than 10 years after resection of PS; they were 66 and 64 years old at the time of PS resection with smoking histories exceeding 40 pack-years. No cases of primary lung cancer were observed in the non-surgery group during the follow-up period.
Table 5
| Characteristics | Surgery group (n=62) | Non-surgery group (n=42) | P value |
|---|---|---|---|
| Age, years | 38.6±12.8 | 45.3±14.0 | 0.016 |
| Sex | 0.423 | ||
| Male | 31 (50.0) | 17 (40.5) | |
| Female | 31 (50.0) | 25 (59.5) | |
| Size, mm | 90.0±26.4 | 66.3±25.0 | <0.001 |
| Presenting symptoms | 0.021 | ||
| Asymptomatic | 36 (58.1) | 33 (78.6) | |
| Symptomatic | 26 (41.9) | 9 (21.4) | |
| Presenting symptoms or pneumonia | 0.015 | ||
| Asymptomatic and no pneumonia | 30 (48.4) | 30 (71.4) | |
| Symptomatic or pneumonia | 32 (51.6) | 12 (28.6) | |
| Death during follow-up | 3 (4.8) | 1 (2.4) | NA |
| Duration of follow-up from initial diagnosis to last follow-up, months | 17.5 (0.0–224.0) | 15.0 (0.0–183.0) | 0.919 |
| Symptoms at the last follow-upa | NA | ||
| Asymptomatic | 60 (100.0) | 34 (97.1) | |
| Symptomatic | 0 (0.0) | 1 (2.9) | |
| Development of primary lung cancer during follow-up | 2 (3.2) | 0 (0.0) | NA |
Data are presented as n (%) or mean ± SD or median (range). a, patients with last follow-up within 1 month of diagnosis were excluded. NA, not available.
Clinical courses of the surgery group and the non-surgery group
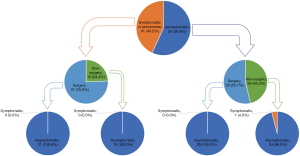
Among the 104 patients included in this study, nine patients who were followed up for less than 1 month were excluded from analysis of symptom changes during follow-up. Therefore, 95 patients were analyzed for symptom changes at the times of diagnosis and last follow-up (Figure 2 and Table 5). At the last follow-up, only one patient in the non-surgery group was symptomatic. The patient was asymptomatic initially but developed blood-tinged sputum at the last follow-up. All other patients were asymptomatic at the last follow-up.
Discussion
In this retrospective study, 36.5% (38/104) of PS subjects were asymptomatic, and those in the surgery group were significantly younger, more likely to be symptomatic initially, and had larger PS compared with those in the non-surgery group. More than 90% of subjects without initial symptoms in the non-surgery group remained asymptomatic at the last follow-up. Among the subjects with initial symptoms in the surgery group, about 20% remained symptomatic at the last follow-up. In the surgery group, about 20% of patients experienced major or minor postoperative complications.
PS was first described in 1946 by Pryce (10); it has since been reported in numerous case series and reports, but its natural course in adults has not been well characterized. To elucidate the disease course of PS in patients with or without resection, this retrospective study collected data from the electronic medical records of 106 patients for a median follow-up period of 17.5 months. This is one of only a few case series of PS involving more than 100 adult patients with follow-up data for more than 1 year in a single tertiary hospital. Only one previous small study compared clinical characteristics of surgery and non-surgery groups in 32 adult patients with PS, and the authors suggested that surgery may not be necessary in asymptomatic adult patients with PS (11).
Baseline clinical and radiological characteristics did not differ significantly from those in previous reports. About one-third of patients in this study were asymptomatic, which is similar to previous reports in which one-third to half of PS patients were asymptomatic (11,12). Radiologically, most PS originated from the descending aorta and drained into the pulmonary vein, which is typical for intralobar PS. The left lower lobe was the predominant location of PS, and no cases of PS were observed in right or left upper lobes. The mean size of PS was 80.4 mm, similar to the median size of 6.6 cm (IQR, 4.4–9.3) reported in a previous study (11). Smoking history was not frequently recorded in previous studies, but we found that two-thirds of patients had a documented smoking history as a previous or current smoker.
The rate of pathologically documented infection in our study population was 23.4%, while in another study, the microbiologically documented infection rate was 8.7% (13). Interestingly, all cases related to infection were intralobar PS, not extralobar PS although the number of resected extralobar PS cases was relatively small (n=5). If the extralobar PS is clinically suspected, although it is very hard to distinguish intralobar and extralobar PS solely based on CT findings, clinicians could consider that the extralobar PS would rarely provoke infectious complications based on our study. Aspergillosis was reported at rates of 10–20% in some case series (14,15). Non-tuberculous mycobacterium (NTM) infection appears to be much rarer; few case reports have been published on NTM infection in PS (16-18). In the present study, some pathology reports revealed granulomas, but culturing of surgical specimens was not commonly done.
In the present study, pathology revealed only one intralobar PS case with carcinoid tumorlet, and the others had no coexisting malignancy. Only seven previous studies have reported malignancies, such as adenocarcinoma and squamous carcinoma, within the sequestered lung tissue (19,20). Carcinoid tumorlet is also a rare malignancy (21).
One study reported that intralobar sequestration is more frequently related to infection in adults than in pediatric patients (22). However, that study was limited to surgically treated patients, so asymptomatic, non-resected PS patients would not have been included. Furthermore, a history of infection would have been more frequent in adult patients because of their longevity. Infection history, as suggested by the patient’s symptoms and childhood history, could be considered an important surgical indication of PS.
CPAM is another rare congenital malformation of the lung which is characterized by a proliferation of dilated bronchiolar-like air spaces and its reported incidence was between 1:25,000 and 1:35,000 (23,24). In this study, CPAM and intralobar PS coexisted in 3 patients, which accounted for 5.4% (3/56) of pathologically confirmed intralobar PS. The three CPAM patients had symptoms such as hemoptysis, cough, and pleuritic chest pain, and two of them had symptoms of upper respiratory tract infection and were treated as having community-acquired pneumonia.
The most important finding in comparing the surgery and non-surgery groups was that adult PS patients tended to undergo resection if they were young, symptomatic, and had large PS. A previous report indicated that symptomatic patients were likely to undergo surgery, but the median age and the mean size of PS did not differ significantly between the surgery and non-surgery groups (6). This could be explained by the differences in clinical practices in different countries.
The surgical complication rate of PS is reportedly 4.3–28% (11-13,25), similar to our results (23.4%). Immediate postoperative mortality has rarely been reported in case reports, with the exception of a review of 540 reported cases in 1979 (2). In the present study, no immediate postoperative mortality was observed, but serious complications including cerebral infarct, esophageal tearing, and prolonged weaning were observed in 5.4% of cases. This considerable complication rate should be taken into account when deciding on surgical treatment of PS.
Two patients with intralobar PS in the surgery group developed lung cancer during long-term follow-up of more than 5 years. They had chronic obstructive pulmonary disease (COPD) with a substantial smoking history of more than 50 pack-years at the time of PS diagnosis. However, the development of lung cancer does not seem to be related to resected PS.
In the non-surgery group, the mean size of PS did not change significantly over time (median follow-up period of 15 months). To our knowledge, no long-term studies have reported the size of PS followed up over time. In our study, nearly 70% of asymptomatic patients with unresected PS remained asymptomatic. Furthermore, among 12 asymptomatic patients in the surgery group, one developed symptoms after surgery, which were considered to be related to postoperative changes. Therefore, considering the indolent course of PS, initially asymptomatic patients could be followed up with monitoring of their symptoms and the size of the lesion.
Our study had several limitations. First, it was a retrospective, single-center study. Collection of patient symptoms was dependent on the electronic medical records, so it is possible that the proportion of symptomatic patients may have been underestimated, especially during follow-up. The presenting symptoms seemed to be reasonably investigated in that the proportion of asymptomatic patient was within the range reported previously. Second, this study had a relatively short follow-up duration to investigate the long-term outcome of PS since most asymptomatic patients were lost to follow-up early. However, among 9 initially asymptomatic patients in non-surgery group who were followed up for more than 3 years, 5 (55.6%) patients were still asymptomatic at the last follow-up. Therefore, considering the rarity of PS, our findings provide valuable information regarding the natural course of asymptomatic PS in adults.
Conclusions
In conclusion, adults with PS tended to undergo resection if they were young, symptomatic, and had large PS. More than 95% of subjects who were initially asymptomatic and did not undergo surgery remained asymptomatic at the last follow-up asymptomatic with a median follow-up duration of 15 months. Therefore, the decision to perform surgery for incidentally found PS in adulthood should be made considering the clinical presentation and the risk and benefits of surgery.
Acknowledgments
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C2006282).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-631/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-631/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-631/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Samsung Medical Center (IRB No. 2019-03-148-001) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bolca N, Topal U, Bayram S. Bronchopulmonary sequestration: radiologic findings. Eur J Radiol 2004;52:185-91. [Crossref] [PubMed]
- Savic B, Birtel FJ, Tholen W, et al. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979;34:96-101. [Crossref] [PubMed]
- Clements BS, Warner JO. Pulmonary sequestration and related congenital bronchopulmonary-vascular malformations: nomenclature and classification based on anatomical and embryological considerations. Thorax 1987;42:401-8. [Crossref] [PubMed]
- Walker CM, Wu CC, Gilman MD, et al. The imaging spectrum of bronchopulmonary sequestration. Curr Probl Diagn Radiol 2014;43:100-14. [Crossref] [PubMed]
- Maull KI, McElvein RB. Infarcted extralobar pulmonary sequestration. Chest 1975;68:98-9. [Crossref] [PubMed]
- Avishai V, Dolev E, Weissberg D, et al. Extralobar sequestration presenting as massive hemothorax. Chest 1996;109:843-5. [Crossref] [PubMed]
- Sato Y, Endo S, Saito N, et al. A rare case of extralobar sequestration with hemoptysis. J Thorac Cardiovasc Surg 2004;128:778-9. [Crossref] [PubMed]
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2625 cases in China. Eur J Cardiothorac Surg 2011;40:e39-42. [Crossref] [PubMed]
- Criss CN, Musili N, Matusko N, et al. Asymptomatic congenital lung malformations: Is nonoperative management a viable alternative? J Pediatr Surg 2018;53:1092-7. [Crossref] [PubMed]
- Pryce DM. Lower accessory pulmonary artery with intralobar sequestration of lung; a report of seven cases. J Pathol Bacteriol 1946;58:457-67. [Crossref] [PubMed]
- Alsumrain M, Ryu JH. Pulmonary sequestration in adults: a retrospective review of resected and unresected cases. BMC Pulm Med 2018;18:97. [Crossref] [PubMed]
- Berna P, Cazes A, Bagan P, et al. Intralobar sequestration in adult patients. Interact Cardiovasc Thorac Surg 2011;12:970-2. [Crossref] [PubMed]
- Sun X, Xiao Y. Pulmonary sequestration in adult patients: a retrospective study. Eur J Cardiothorac Surg 2015;48:279-82. [Crossref] [PubMed]
- Berna P. Pulmonary sequestration and aspergillosis. Eur J Cardiothorac Surg 2005;27:28-31. [Crossref] [PubMed]
- Sun X, Xiao Y. A report of seven cases of pulmonary sequestration complicated by aspergillosis and literature review. Zhonghua Nei Ke Za Zhi 2014;53:873-5. [PubMed]
- Koh WJ, Hong G, Kim K, et al. Pulmonary sequestration infected with nontuberculous mycobacteria: a report of two cases and literature review. Asian Pac J Trop Med 2012;5:917-9. [Crossref] [PubMed]
- Miyazaki H, Gemma H, Koshimizu N, et al. Two cases of intralobar pulmonary sequestration associated with nontuberculous mycobacterial infection in a young patient. Nihon Kokyuki Gakkai Zasshi 2004;42:277-83. [PubMed]
- Sekine T, Reshad K, Kosaba S. A case of pulmonary sequestration with atypical mycobacterial infection. Nihon Kokyuki Gakkai Zasshi 1998;36:399-402. [PubMed]
- Lawal L, Mikroulis D, Eleftheriadis S, et al. Adenocarcinoma in pulmonary sequestration. Asian Cardiovasc Thorac Ann 2011;19:433-5. [Crossref] [PubMed]
- Belchis D, Cowan M, Mortman K, et al. Adenocarcinoma arising in an extralobar sequestration: a case report and review of the literature. Lung Cancer 2014;84:92-5. [Crossref] [PubMed]
- Ye Y, Mu Z, Wu D, et al. Carcinoid tumorlet in pulmonary sequestration with bronchiectasis after breast cancer: A case report. Oncol Lett 2013;5:1546-8. [Crossref] [PubMed]
- Van Raemdonck D, De Boeck K, Devlieger H, et al. Pulmonary sequestration: a comparison between pediatric and adult patients. Eur J Cardiothorac Surg 2001;19:388-95. [Crossref] [PubMed]
- Luján M, Bosque M, Mirapeix RM, et al. Late-onset congenital cystic adenomatoid malformation of the lung. Embryology, clinical symptomatology, diagnostic procedures, therapeutic approach and clinical follow-up. Respiration 2002;69:148-54. [Crossref] [PubMed]
- MacSweeney F, Papagiannopoulos K, Goldstraw P, et al. An assessment of the expanded classification of congenital cystic adenomatoid malformations and their relationship to malignant transformation. Am J Surg Pathol 2003;27:1139-46. [Crossref] [PubMed]
- Lin CH, Chuang CY, Hsia JY, et al. Pulmonary sequestration-differences in diagnosis and treatment in a single institution. J Chin Med Assoc 2013;76:385-9. [Crossref] [PubMed]