PON2 and ATP2B2 gene polymorphisms with noise-induced hearing loss
Introduction
Noise-induced hearing loss (NIHL) is a common sensory deficit which affected more than 10% of adult population, especially in countries with growing industrial activity (1). It is a complex disease induced by a combination of genetic and environmental factors (2). Exposure to noise is the best known environmental factor that causes hearing loss. Other environmental factors include heat, organic solvents, infections and ototoxic agents which have also been demonstrated to contribute to NIHL (3-5). In addition, individual factors such as smoking, high blood pressure, and medical factors may influence the susceptibility to noise (6-9).
Susceptibility to noise damages in fact differed among individuals, which indicated that NIHL was a complex disease resulted from genetic fields. The role of genetic factors in NIHL was confirmed by several animal studies. C57BL/6J mice carrying the mobile sites method-derived Ahl3 gene were more resistant to noise than the regular C57BL/6J mice (10). Some knockout mice studies suggested that the gene coding for plasma membrane Ca2+-ATPase isoform 2 gene (PMCA2) (11), otocadherin23 gene (CDH23) (12), and glutathione peroxidase 1 gene (GPX1) (13) might be involved in the susceptibility of NIHL. However, firm evidence for the involvement of genetic factors in human NIHL was limited. Konings et al. found significant associations between CAT gene single nucleotide polymorphisms (SNPs) and susceptibility to NIHL (14). In Shen’s experiment, individuals with GSTM1 null genotype had a statistically significantly increased risk of NIHL (OR =1.64) compared with those who carrying a wild-type GSTM1 genotype (15). Van Laer et al. identified the genes (including KCNE1, KCNQ1, and KCNQ4) involved in potassium recycling in the inner ear might explain the variability of susceptibility to noise (16). Shen et al. also found that hOGG1 Cys/Cys genotype may be a genetic susceptibility marker for NIHL in Chinese of Han nationality population (17).
One of the major causes of occupational hearing loss was cochlear hair cell damage (18). Noise which induced release of free radicals can damage the cochlear sensorial epithelium, which prompted us with genes involved in the regulation of reactive oxygen species and affecting the vulnerability of the cochlea to NIHL, such as paraoxonase2 gene (PON2). PON gene family consisting of at least 3 genes (PON1, PON2 and PON3) located in the long arm of human chromosome 7 (q21.3–22.1) coding for esterases (19). PON2 expressing in tissues throughout the body may develop its antioxidant effect at a cellular level (20), and its deficiency can increase ROS production, which led to the damage of cochlear hair cell (21). Some researchers have found that PON2 polymorphisms may be associated with diseases, such as ischemic stroke, diabetes, and Alzheimer’s dementia (22-24). An analysis of PON2 polymorphisms in small sample (94 male workers in Italy) exposed to noise revealed a significant association with NIHL (20).
ATPase, calcium-transporting, plasma membrane 2 (ATP2B2), encoding plasma membrane calcium-transporting ATPase isoform2 (PMCA2) is located on human chromosome region 3p25. A unique role of ATP2B2 in hearing was indicated by the high levels of its expression in cochlear outer hair cells, it played an important role in intracellular calcium homeostasis (25). However, disruptions of calcium homeostasis were the base of some diseases such as autism and deafness (25-27). In an animal experiment, Peter J hypothesized that ATP2B2+/− mice may be more susceptible to NIHL (11). Previous researchers who thought ATP2B2 might be a predisposing gene for NIHL also revealed that absence of ATP2B2 which led to defects of auditory systems, may lead to hearing loss (26,28). The biological functions of ATP2B2 and the positive results of previous study made ATP2B2 an attractive candidate gene for NIHL.
Considering the genetic heterogeneity among different ethnicities, we took a case-control study to investigate the association between PON2 and ATP2B2 genes and NIHL in Chinese of Han nationality population. We wanted to analyze the issue whether genetic variability in PON2 and ATP2B2 were associated with high susceptibility to NIHL. Totally, 221 NIHL cases and 233 controls were selected. SNPs in PON2 and ATP2B2 were analyzed to see the differences of noise susceptibility between susceptible and resistant individuals.
Materials and methods
Participants
One group of Chinese workers occupationally exposed to noise from factories in the cities of Xu Zhou and Yi Zheng in Jiangsu province was selected because of their high workforce stability. The factory working environments were similar, and the workers were commonly exposed to steady noise during their working time. In the first selection round, subjects suffering from conductive or mixed hearing loss were excluded from this study. The Regional Bioethical Committee at Nanjing Medical University approved our study, and informed consent was obtained from all the study participants.
Questionnaire
Subject information gathered by questionnaires was administered through face-to-face interviews by trained interviewers. The following information was collected from all the participants: demographic data, previous and present medical conditions, military history, hereditary factors, smoking and drinking status, noise exposure at previous work factories and during military service. Subjects who drank a bottle of beer or fifty grams of wine per day for at least 1 year were defined as ever drinkers, while the rest were defined as never drinkers. Workers who had one cigarette per day for at least 1 year were defined as ever smokers, and the others were defined as never smokers. Individuals were excluded from the study if they had a history of head injury, previous or present treatment with ototoxic drugs, diseases causing hearing impairment (hereditary deafness, meningitis, mumps, middle ear inflammation, and other viral infections) and potentially harmful noise exposure during military service or leisure time.
Audiometric examination
All the participants in our study underwent an audiometric examination that was performed in a sound isolation cabinet by a trained technician. Both ears were evaluated at 500, 1,000, 2,000, 3,000, 4,000, 6,000 and 8,000 Hz. According to China national criteria for noise in the workplace (GBZ43-2007), a sound pressure individual audiometer should be used in a proper place and proper time to evaluate the real noise level in the working environment. The occupational hygienists provided us with noise measurement data of the selected factories which were normalized to equivalent continuous A-weighted sound pressure from a nominal 8 h weekday (Lex.8 h)
Selection of cases and controls
The NIHL cases were selected without any restriction on age or gender. We defined cases on the basis of Chinese National Occupational Health Standard (GBZ43-2007). Subjects with pure tone audiograms showing sensorineural hearing loss more than 40 dB(A) at high-frequency were included as cases. However, workers with sensorineural hearing loss more than 26 dB(A) at low-frequency and hearing loss on high-frequency worse than that of low-frequency were also defined as NIHL cases. The control group had age, sex, exposure time and exposure level matched with cases, both of whom came from the same company.
SNP selection and genotyping
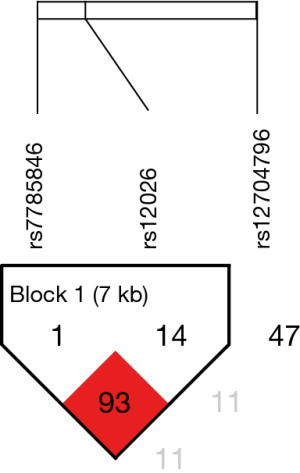
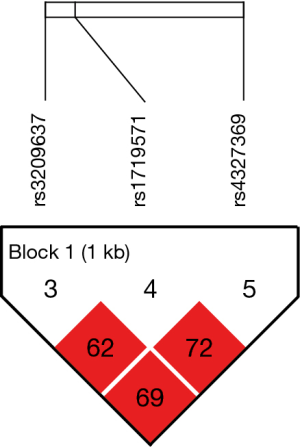
Each subject donated 5 mL venous blood samples for genomic DNA extraction. Genomic DNA was extracted from blood samples using the TianGen DNA extraction kit (Beijing, China). The SNPs information was obtained from dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/) and HapMap phaseII genotype Chinese of Han nationality in Beijing (CHB) dataset (http://www.hapmap.org). We preferred potentially functional regions to intronic regions because of their higher chances of being causative SNPs. Six SNPs included rs12026 (exon), rs7785846 (3'-near gene) and rs12704796 (5'-near gene) within PON2, rs1719571, rs3209637 and rs4327369 located in3'-UTR within ATP2B2 were selected presently based on known heterozygosity and a minor allele frequency (MAF) >0.1.
SNPs genotyping was determined by using the TaqMan MGB probe assay from Applied Biosystems Inc. (Foster City, CA, USA). Amplifications and analyses were carried out by using the 384-well ABI 7900HT Real Time PCR System, according to the standard protocol (Applied Biosystems). Four blank controls were included in each plate to ensure the accuracy of genotypes. SDS 2.4 automated software was used on allelic discrimination. Quality control was performed by two people in a blinded fashion.
Statistic
All the data were input a computerized database using Epidata3.1. Linkage disequilibrium (LD) between the different polymorphisms in each PON2 and ATP2B2 gene was evaluated by using Haploview software. Associations with continuous variables were tested by one-way ANOVA or Student’s t-tests, and with categorical variables by χ2 test. Interactions with SNPs were tested by χ2 analysis at the genotype level to identify differences between the NIHL patients and controls. The association of genotypes with NIHL was also evaluated by assuming dominant models. If a SNP showed a significant interaction between genotype and noise exposure level, odds ratios (ORs) would be calculated with 95% confidence intervals (95% CIs) with logistic regression analysis to test the level of association between different noise exposure levels. All statistical analyses were performed using SAS (SAS 9.1.3 for Windows, SAS Institute Inc., Cary, NC, USA) and P<0.05 was considered to be statistically significant.
Results
Linkage disequilibrium (LD)
The LD patterns of SNPs were measured with r-squared value using Haploview software. Three SNPs (rs1719571, rs3209637 and rs4327369) in ATP2B2 (Figure 1) and three SNPs (rs12026, rs7785846 and rs12704796) in PON2 (Figure 2) were identified.
Characteristics of the subjects
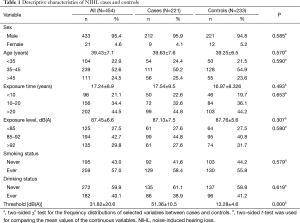
The demographic and occupational characteristics of NIHL workers and controls are shown in Table 1. In total, 221 subjects with hearing loss were compared with 233 subjects without hearing loss (considered to have normal hearing). Among the 454 subjects, 433 were male and 21 were female. The mean age of these subjects at the time of testing was 39.43 years old (range, 19–57 years old).
Full table
There were no significant differences between NIHL cases and controls in respect of sex, age, smoking or drinking status, exposure level and exposure time (Table 1). However, subjects with controls were more likely to have a lower threshold value than NIHLs, whose mean hearing-threshold was about 4 times higher than the controls (P<0.001).
Distributions of PON2 and ATP2B2 SNPs
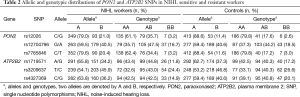
The distributions of PON2 and ATP2B2 SNP genotypes and alleles in our subjects are shown in Table 2. According to NCBI dbSNP, we defined the ancestral alleles wild type for the SNPs. The MAF for all the SNPs was higher than 0.1, which implied that these SNPs were frequent in Chinese of Han nationality population.
Full table
Association of NIHL risk with PON2 and ATP2B2 SNPs
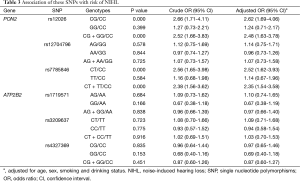
As shown in Table 2 and Table 3, crude and adjusted ORs for genotypic risk of NIHL were estimated separately. The frequencies of the CG and CG + GG genotype with rs12026 (PON2) in the NIHL workers were significantly higher than those in the controls (P<0.01), and they conferred risk factors for NIHL with crude OR values (with genotype CC as reference) of 2.66 (95% CI, 1.71–4.11) and 2.52 (95% CI, 1.66–3.83), respectively. Significant association with NIHL was also found at locus rs7785846, where genotypes CT and CT + TT were the risk types, with crude ORs of 2.56 (95% CI, 1.65–3.98, P<0.01) and 2.38 (95%CI, 1.56–3.62, P<0.01), respectively.
Full table
To illustrate the interference of potential confounding factors with our assessment of the relations between these SNPs and NIHL, we performed a multivariate logistic regression analysis. After adjusting to age, sex, smoking and drinking status, the genotypic association with rs12026 remained significant, with adjusted OR values 2.62 (95% CI, 1.69–4.06) of CG genotype and 2.48 (95% CI, 1.63–3.78) of CG + GG genotype. When it came to rs7785846, adjusted OR values seemed also significantly with CT genotype (adjusted OR =2.52, 95% CI, 1.62–3.93) and CT + TT genotype (adjusted OR =2.35, 95% CI, 1.54–3.58).
By contrast, no significantly higher risk was found for any rs12704796 genotypes or any genotypes in ATP2B2 (P>0.05), which may suggest that these SNPs did not have significant effects on noise susceptibility across noise exposure.
Interaction between noise exposure level and SNPs
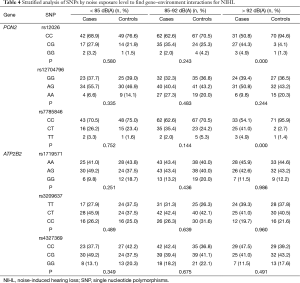
To reveal the interactions between the SNPs and environmental exposure, we performed stratified analysis per noise exposure level. The results are shown in Table 4. For rs12026, the >92 dB(A) exposure groups showed that NIHL workers were more likely to carry the CG genotype, while the control group dominated CC genotype (P<0.01). Similarly, for rs7785846, in the >92 dB(A) noise exposure group, the subjects who carried CT genotype were significantly (P<0.01) higher susceptibility than the CC carrier. However, there was no such trend in the lower noise exposure levels. Unfortunately, in regard to rs12704796 (PON2) and other 3 SNPs located in ATP2B2 gene, there were no significant differences in different exposure levels.
Full table
Discussion
In this present study, we investigated the association between PON2, ATP2B2 polymorphisms and NIHL in Chinese of Han nationality population. We found statistically significant differences between NIHL cases and controls in genotypic distributions of SNPs rs12026 and rs7785846 in PON2. As revealed by multivariate logistic regression analysis, the CG genotype of rs12026 offered risk factors to NIHL (adjusted OR =2.62, 95% CI, 1.69–4.06, P<0.01), and the CT genotype of rs7785846 was associated with higher risk (adjusted OR =2.52, 95% CI, 1.62–3.93, P<0.01). Our findings in Chinese population agreed with the results of the previous analysis of PON2 gene polymorphisms and NIHL, which confirmed positive associations in a case-control study on 28 patients and 61 controls (20). The results of our research indicated that rs12026 and rs7785846 polymorphisms of PON2 may contribute to NIHL.
Table 3 lists the relationship of PON2 and ATP2B2 SNPs with NIHL. We analyzed the dominant models of PON2 and ATP2B2, considering age, sex, and drinking and smoking habits. Workers with rs12026 CG + GG genotypes were more susceptible compared with workers with a CC genotype (OR =2.48, 95% CI, 1.63–3.78), and similar results were found from workers with rs7785846 CT + TT genotypes compared with the CC genotype (OR =2.35, 95% CI, 1.54–3.58). PON2 acted as an antioxidant enzyme, and its over-production was capable of lowering the oxidative state of cells. Therefore, we speculated that the pathogenesis of NIHL may include the release of oxygen species, whose release could damage Corti’s organ, consequently led to neuro-sensorial hearing loss. Recent researchers also confirmed that the concentrations of superoxide radicals in the cochlear fluid increased when exposed to noise (29), and discovered the association between PON2 polymorphism and Alzheimer’s disease and stroke, in which oxidative stress may play a key role in the development (30,31).
In our study, significant gene-environmental interactions between PON2, ATP2B2 gene SNPs and environmental noise exposure were identified (Table 4). There was a significant association with NIHL with rs12026 (PON2) and rs7785846 (PON2) SNPs in the highest noise-exposure group (>92 dB) after grouping the participants by noise exposure level, indicating that polymorphisms in PON2 had a worse result when subjects were exposed to higher noise. Inconsistent genotypic distributions between the case and control group by different noise exposure levels suggested that genotypic effects were dependent on environmental factors. However, this is not elusive that higher noise levels may be more harmful, stimulating the appearance of larger significant effect.
No significant main effect was observed for ATP2B2 gene SNPs (rs1719571, rs3209637 and rs4327369). This finding was not in agreement with the results reported by an earlier study (11). Few reasons here could explain what caused these differences between the results of our study and previous ones. First, there was a large difference between animal trial and human study, which results in different statistical powers achieved. Furthermore, small sample size of our study can also lead to negative results. Alternatively, other unknown environmental factors may explain the lack of difference. Therefore, it remained to be elucidated whether these SNPs in ATP2B2 contributed to NIHL susceptibility or not.
Whatever, current studies had a few limitations. First of all, the aim of the study was similar to previous positive results of association between PON2 and NIHL although with ethnic difference (20). Secondly, when it came to ATP2B2, our research did not explore the association between SNPs in exon and 5'-near gene region between it and NIHL. In addition, rare variants were not investigated in this study. Mutation screening of ATP2B2 in human with NIHL could be performed in further researches. Last but not least, the sample size of our research needs expanding.
In conclusion, our present results provided evidence that PON2 might be relevant to the etiology of NIHL. Follow-up detailed associated study in larger scale and functional researches of PON2 and ATP2B2 are needed.
Acknowledgements
We thank all subjects who participated in this study and Jiangsu Provincial Center for Disease Control and Prevention (JSCDC) that is our cooperating organization helping us recruiting participants in the study.
Funding: This study was supported by the scientific projects of Jiangsu Preventive Medicine (Y2013008); and Medical Science and Technology Development Foundation, Nanjing Department of Health (YKK14169).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Carlsson PI, Van Laer L, Borg E, et al. The influence of genetic variation in oxidative stress genes on human noise susceptibility. Hear Res 2005;202:87-96. [Crossref] [PubMed]
- Sliwinska-Kowalska M, Pawelczyk M. Contribution of genetic factors to noise-induced hearing loss: a human studies review. Mutat Res 2013;752:61-5. [Crossref] [PubMed]
- Chen CJ, Dai YT, Sun YM, et al. Evaluation of auditory fatigue in combined noise, heat and workload exposure. Ind Health 2007;45:527-34. [Crossref] [PubMed]
- Choi YH, Kim K. Noise-induced hearing loss in Korean workers: co-exposure to organic solvents and heavy metals in nationwide industries. PLoS One 2014;9:e97538. [Crossref] [PubMed]
- Fechter LD. Promotion of noise-induced hearing loss by chemical contaminants. J Toxicol Environ Health A 2004;67:727-40. [Crossref] [PubMed]
- Mehrparvar AH, Mirmohammadi SJ, Hashemi SH, et al. Concurrent effect of noise exposure and smoking on extended high-frequency pure-tone thresholds. Int J Audiol 2015;54:301-7. [Crossref] [PubMed]
- Sung JH, Sim CS, Lee CR, et al. Relationship of cigarette smoking and hearing loss in workers exposed to occupational noise. Ann Occup Environ Med 2013;25:8. [Crossref] [PubMed]
- Ni CH, Chen ZY, Zhou Y, et al. Associations of blood pressure and arterial compliance with occupational noise exposure in female workers of textile mill. Chin Med J (Engl) 2007;120:1309-13. [PubMed]
- Chang NC, Yu ML, Ho KY, et al. Hyperlipidemia in noise-induced hearing loss. Otolaryngol Head Neck Surg 2007;137:603-6. [Crossref] [PubMed]
- Morita Y, Hirokawa S, Kikkawa Y, et al. Fine mapping of Ahl3 affecting both age-related and noise-induced hearing loss. Biochem Biophys Res Commun 2007;355:117-21. [Crossref] [PubMed]
- Kozel PJ, Davis RR, Krieg EF, et al. Deficiency in plasma membrane calcium ATPase isoform 2 increases susceptibility to noise-induced hearing loss in mice. Hear Res 2002;164:231-9. [Crossref] [PubMed]
- Holme RH, Steel KP. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. J Assoc Res Otolaryngol 2004;5:66-79. [Crossref] [PubMed]
- Ohlemiller KK, McFadden SL, Ding DL, et al. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol 2000;1:243-54. [Crossref] [PubMed]
- Konings A, Van Laer L, Pawelczyk M, et al. Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum Mol Genet 2007;16:1872-83. [Crossref] [PubMed]
- Shen H, Huo X, Liu K, et al. Genetic variation in GSTM1 is associated with susceptibility to noise-induced hearing loss in a Chinese population. J Occup Environ Med 2012;54:1157-62. [Crossref] [PubMed]
- Van Laer L, Carlsson PI, Ottschytsch N, et al. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Hum Mutat 2006;27:786-95. [Crossref] [PubMed]
- Shen H, Cao J, Hong Z, et al. A functional Ser326Cys polymorphism in hOGG1 is associated with noise-induced hearing loss in a Chinese population. PLoS One 2014;9:e89662. [Crossref] [PubMed]
- Liu YM, Li XD, Guo X, et al. Association between polymorphisms in SOD1 and noise-induced hearing loss in Chinese workers. Acta Otolaryngol 2010;130:477-86. [Crossref] [PubMed]
- Primo-Parmo SL, Sorenson RC, Teiber J, et al. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 1996;33:498-507. [Crossref] [PubMed]
- Fortunato G, Marciano E, Zarrilli F, et al. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin Chem 2004;50:2012-8. [Crossref] [PubMed]
- Yang Y, Zhang Y, Cuevas S, et al. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med 2012;53:437-46. [Crossref] [PubMed]
- Lazaros L, Markoula S, Kyritsis A, et al. Paraoxonase gene polymorphisms and stroke severity. Eur J Neurol 2010;17:757-9. [Crossref] [PubMed]
- Andalib S, Vaseghi G, Motavallian A, et al. Association of polymorphism of ser311cys paraoxonase-2 gene with type 2 diabetes mellitus in iran. Int J Prev Med 2013;4:517-22. [PubMed]
- Shi J, Zhang S, Tang M, et al. Possible association between Cys311Ser polymorphism of paraoxonase 2 gene and late-onset Alzheimer's disease in Chinese. Brain Res Mol Brain Res 2004;120:201-4. [Crossref] [PubMed]
- Yang W, Liu J, Zheng F, et al. The evidence for association of ATP2B2 polymorphisms with autism in Chinese Han population. PLoS One 2013;8:e61021. [Crossref] [PubMed]
- Bortolozzi M, Brini M, Parkinson N, et al. The novel PMCA2 pump mutation Tommy impairs cytosolic calcium clearance in hair cells and links to deafness in mice. J Biol Chem 2010;285:37693-703. [Crossref] [PubMed]
- Giacomello M, De Mario A, Lopreiato R, et al. Mutations in PMCA2 and hereditary deafness: a molecular analysis of the pump defect. Cell Calcium 2011;50:569-76. [Crossref] [PubMed]
- Spiden SL, Bortolozzi M, Di Leva F, et al. The novel mouse mutation Oblivion inactivates the PMCA2 pump and causes progressive hearing loss. PLoS Genet 2008;4:e1000238. [Crossref] [PubMed]
- Kurioka T, Matsunobu T, Satoh Y, et al. Inhaled hydrogen gas therapy for prevention of noise-induced hearing loss through reducing reactive oxygen species. Neurosci Res 2014;89:69-74. [Crossref] [PubMed]
- Li BH, Zhang LL, Yin YW, et al. Association between paraoxonase 2 Ser311Cys polymorphism and ischemic stroke risk: a meta-analysis involving 5,008 subjects. Mol Biol Rep 2012;39:5623-30. [Crossref] [PubMed]
- Mu N, Xu SC, Chang Q, et al. Study of lipids, insulin metabolism, and paraoxonase-2-311 polymorphism in patients with different subtypes of Alzheimer's disease (translated version). East Asian Arch Psychiatry 2013;23:114-9. [PubMed]